
15066876
UNITED STATES DISTRICT COURT
EASTERN DISTRICT OF NEW YORK
------------------------------------------------------------------
x
ROCHE DIABETES CARE, INC., ROCHE
DIABETES CARE GmbH, and HOFFMANN-LA
ROCHE, INC.,
Plaintiffs,
-against-
JMD ENTERPRISES d/b/a DKY STORE USA,
JMD INTERNATIONAL, DILEEP KUMAR
YADAV, ABHISHEK JAIN, MEDICAL
HUB_USA STORE, RATNAKAR SHARMA,
AUTHENTIC INDIAN STORE, and ATIKUR
RAHMAN,
Defendants.
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
Case No. _______________________
COMPLAINT
JURY DEMANDED
FILED EX PARTE AND UNDER SEAL
PURSUANT TO 15 U.S.C. § 1116
-----------------------------------------------------------------
x
Plaintiffs Roche Diabetes Care, Inc., Roche Diabetes Care GmbH, and Hoffmann-
La Roche, Inc. (collectively, “Roche”), by and through their attorneys, Patterson Belknap Webb
& Tyler LLP, for their Complaint against Defendants JMD Enterprises d/b/a DKY Store USA,
Dileep Kumar Yadav, JMD International, Abhishek Jain, Medical Hub_USA Store, Ratnakar
Sharma, Authentic Indian Store, and Atikur Rahman (collectively, “Defendants”), allege as
follows:
NATURE OF THE ACTION
1. This is an anti-counterfeiting action against international manufacturers
and sellers of counterfeit versions of Roche’s Accu-Chek
®
diabetes care medical devices. The
Defendants are India-based willful counterfeiters who sell dangerous counterfeits of Roche’s
Accu-Chek
®
medical devices to American patients through Amazon.com and other platforms.
Through this action Roche seeks to put an immediate stop to the sale and distribution of these
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 1 of 43 PageID #: 1
-2-
15066876
dangerous counterfeits in U.S. commerce.
2. Patients throughout the United States and the world rely on Roche’s
trusted Accu-Chek
®
brand medical devices to monitor and treat their diabetes, including by
testing their blood glucose levels daily. Patients know that Roche’s Accu-Chek
®
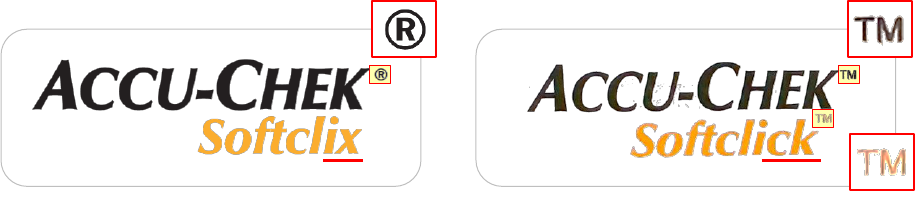
medical
devices are safe, sterile, and accurate.
3. The Defendant counterfeiters make their fake Accu-Chek
®
medical
devices in back-alley Indian apartment buildings. Their counterfeits are low-quality imitations
that give off a strong chemical odor and cannot be trusted to function properly. These
counterfeits are then placed in counterfeit packaging meant to imitate authentic Roche U.S.
packaging, including counterfeit reproductions of Roche’s registered U.S. trademarks. The
counterfeit packaging has salient errors, including misspelling the name of the product or
referring to the manufacturer as “Roche Diabeter Care, Inc.” These counterfeits are then sold to
customers across the nation, including in this District.
4. The Defendants’ counterfeits are likely to give false or inaccurate
measurements of blood glucose levels, putting patients at risk of severe and life-threatening
complications, such as hyperglycemia and over- or under-dosages of insulin. The counterfeits
may also cause infection or other injury when they come into contact with patients’
bloodstreams. The Defendants’ counterfeits pose a serious and ongoing threat to the health and
safety of American patients.
5. By utilizing Amazon’s platform, the India-based Defendant counterfeiters
have immediate and widespread access to the U.S. market and American consumers. The
Defendants have in fact arranged for their dangerous counterfeit medical devices to be stored at
Amazon warehouses across the country, including in this District. The Defendants falsely
advertise their counterfeits as authentic Roche U.S. medical devices on Amazon. When U.S.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 2 of 43 PageID #: 2
-3-
15066876
consumers unwittingly order these counterfeits on Amazon, believing them to be authentic
Roche product, Amazon delivers these dangerous fake medical devices from Amazon
warehouses to U.S. households and businesses, typically within 48 hours.
6. In addition to counterfeits, the Defendants also sell in U.S. commerce,
through Amazon and otherwise, unlawfully diverted international versions of Accu-Chek
®
medical devices, which they falsely advertise to be authentic U.S. products. These international
Accu-Chek
®
medical devices are not intended for sale in the United States and thus do not
comply with U.S. Food and Drug Administration (“FDA”) regulations; they are misbranded
medical devices under federal law and their sale in the United States is a strict-liability federal
crime. Because they differ materially from authentic U.S. Roche products, the international
products that the Defendants divert into the United States violate Roche’s U.S. trademark rights
and are infringing goods under the Lanham Act.
7. To eradicate these dangerous counterfeit medical devices and unlawfully
diverted medical devices from the U.S. marketplace, Roche brings this action for injunctive and
monetary relief for trademark infringement in violation of Section 32 of the Lanham Act (15
U.S.C. § 1114), false descriptions and false designations of origin in commerce in violation of
Section 43 of the Lanham Act (15 U.S.C. § 1125) and New York General Business Law Section
349, trademark dilution in violation of Section 43 of the Lanham Act (15 U.S.C. § 1125) and
New York General Business Law Section 360-1, common law unfair competition and unjust
enrichment, common law fraud and fraudulent inducement, and common law aiding and abetting
fraud. Roche also seeks a Letter of Request directed to the appropriate Indian judicial authorities
supporting Roche’s seeking of a seizure against the counterfeiters under Indian law.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 3 of 43 PageID #: 3

-4-
15066876
PARTIES
Plaintiffs
8. Plaintiff Roche Diabetes Care, Inc. is a corporation organized under the
laws of Delaware, with its principal place of business at 9115 Hague Road, Indianapolis, Indiana
46250.
9. Plaintiff Roche Diabetes Care GmbH is a corporation organized under the
laws of Germany, with its principal place of business at Sandhofer Str. 116 68305, Mannheim,
Baden-Württemberg, Germany.
10. Plaintiff Hoffmann-La Roche, Inc. is a corporation organized under the
laws of New Jersey with a principal place of business at 150 Clove Road, Great Notch, 8th Floor,
Little Falls, NJ.
Defendants
11. Defendant JMD Enterprises d/b/a DKY Store USA (“JMD Enterprises”) is
a corporation that is registered in India under Indian Goods and Services Tax Identification
Number (“GSTIN”) 07ALOPY9462D1ZE, with its principal place of business in New Delhi,
India. JMD Enterprises owns and/or controls the DKY Store USA storefront (“DKY”) on
Amazon.com. Both through DKY and under its own name, JMD Enterprises uses its Amazon
storefronts to sell counterfeit, misbranded, and unlawfully diverted medical devices in U.S.
commerce.
12. Defendant JMD International is company registered in India under GSTIN
07BAGPA4853C1Z5, with its principal place of business in New Delhi, India. Among other
things, JMD International owns and/or operates a storefront under its name on Amazon.com and
uses its Amazon storefront to sell counterfeit, misbranded, and unlawfully diverted Accu-Chek
®
medical devices in U.S. commerce.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 4 of 43 PageID #: 4
-5-
15066876
13. Defendant Dileep Kumar Yadav is a citizen of India and is the founder,
principal, owner, and/or operator of JMD Enterprises and its DKY storefront, and is a moving
and active force behind JMD Enterprises’s sale of counterfeit, misbranded, and unlawfully
diverted Accu-Chek
®
medical devices in U.S. commerce. Yadav founded JMD Enterprises and
is the individual associated with JMD Enterprises’s GSTIN. Yadav is the owner of various real
properties at which JMD and DKY operate their counterfeiting business. Yadav was until recently
listed as the point of contact for DKY on its Amazon storefront.
14. Yadav is also a principal and/or agent of JMD International and is a
moving and active force behind JMD International’s sale of counterfeit, misbranded, and
unlawfully diverted Accu-Chek
®
medical devices in U.S. commerce.
15. Defendant Abhishek Jain is a citizen of India and the founder, principal,
owner, and/or operator of JMD International, and is a moving and active force behind JMD
International’s sale of counterfeit, misbranded, and unlawfully diverted Accu-Chek
®
medical
devices in U.S. commerce. Jain founded JMD International and is the individual associated with
JMD International’s GSTIN.
16. Jain is also a principal and/or agent of JMD Enterprises and is a moving
and active force behind JMD Enterprise’s sale of counterfeit, misbranded, and unlawfully
diverted Accu-Chek
®
medical devices in U.S. commerce. Jain financed the purchase of one or
more properties owned by Yadav at which JMD Enterprises operate their counterfeiting
business.
17. Defendant Medical Hub_USA Store (“Medical Hub”) is a company with
its principal place of business in Rishikesh, Uttarakhand, India. Among other things, Medical
Hub owns and operates an Amazon.com storefront under its name and uses that storefront to sell
counterfeit, misbranded, and unlawfully diverted Accu-Chek
®
medical devices in U.S.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 5 of 43 PageID #: 5

-6-
15066876
commerce.
18. Defendant Ratnakar Sharma is a citizen of India and a principal, owner,
and/or operator of Medical Hub, and is a moving and active force behind Medical Hub’s sale of
counterfeit, misbranded, and unlawfully diverted Accu-Chek
®
medical devices in U.S.
commerce.
19. Defendant Authentic Indian Store is a company with a principal place of
business in India. Authentic Indian Store has on occasion conducted business as Indian
Authentic Store. Among other things, Authentic Indian Store owns and/or operates a storefront
under its name on Amazon.com and uses that storefront to sell misbranded and unlawfully
diverted Accu-Chek
®
medical devices in U.S. commerce.
20. Defendant Atikur Rahman is a citizen of India and a principal, owner,
and/or operator of Authentic Indian Store, and is a moving and active force behind Authentic
Indian Store’s sale of misbranded and unlawfully diverted medical devices in U.S. commerce.
JURISDICTION AND VENUE
21. The Court has subject matter jurisdiction over this action under the
Lanham Act, 15 U.S.C. §§ 1114, 1125; under 28 U.S.C. §§ 1331, 1332, 1338(a), 1338(b), and
1367; and under general principles of ancillary and pendent jurisdiction.
22. The amount of damages at issue exceeds $75,000, exclusive of interest
and costs.
23. This Court has personal jurisdiction over the Defendants because they
regularly transact or have transacted business in New York, including in the Eastern District of
New York, by selling, offering for sale, and marketing what they falsely claim to be authentic
U.S. Roche Accu-Chek
®
medical devices, but which are instead counterfeit, misbranded, and/or
unlawfully diverted Accu-Chek
®
medical devices. Each of the Defendants delivered counterfeit
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 6 of 43 PageID #: 6
-7-
15066876
and/or unlawfully diverted Accu-Chek
®
products into this District both directly and through
Amazon. As alleged in further detail below, each of the corporate Defendants has sold
counterfeit Accu-Chek
®
medical devices and/or unlawfully diverted and misbranded Accu-
Chek
®
medical devices to investigators in the Eastern District of New York. Each of the
individual defendants was a moving and active force behind their respective corporations’ sale of
such counterfeit and infringing Accu-Chek
®
medical devices in the Eastern District of New
York.
24. The Court also has personal jurisdiction over each of the Defendants
pursuant to Fed. R. Civ. P. 4(k) and N.Y. C.P.L.R. 301 and 302. The Court also has personal
jurisdiction over each of the Defendants because the tortious acts described herein (which
occurred in New York, among other places), were conducted and/or directed by each of the
Defendants.
25. The Defendants regularly do or solicit business, engage in a persistent
course of conduct, and derive substantial revenue from goods used or consumed in New York
State.
26. The Defendants intended, knew, expected, or should reasonably have
expected that the tortious acts described herein would have consequences in New York State.
27. The Defendants derive substantial revenue from interstate and
international commerce.
28. Venue is proper in the Eastern District of New York pursuant to 28 U.S.C.
§ 1391(b) and (c) in that a substantial part of the events or omissions giving rise to the claim
occurred in this district, including through Defendants’ delivery of counterfeit and infringing
products delivered into this District, as well as through counterfeit and infringing products that
were sold by Defendants through Amazon storefronts that are stored in, and shipped by, Amazon
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 7 of 43 PageID #: 7

-8-
15066876
from one or more Amazon warehouses located in this District. As set forth above, each
Defendant is subject to personal jurisdiction in this District.
FACTS
29. Roche is the manufacturer of Accu-Chek
®
brand diabetes care medical
devices, including Accu-Chek
®
glucometers, blood glucose test strips, and lancets. Diabetes is a
life-long and potentially deadly disease, and millions of Americans rely on Accu-Chek
®
products
every day to monitor, control, and treat their diabetes.
30. Roche is one of the leading manufacturers of diabetes care medical
devices in the United States and the world. Roche’s Accu-Chek
®
brand diabetes care products
are well-recognized for their high quality, safety, and efficacy.
31. In the United States, Roche’s Accu-Chek
®
blood glucose test strips and
lancets can be purchased directly by consumers with or without a prescription. In addition to
being sold at pharmacies throughout the nation, these Accu-Chek
®
products are also available
through online stores and marketplaces, including Amazon.com.
32. The Defendants, third-party counterfeiters with no connection to Roche,
use their Amazon storefronts to sell counterfeit, misbranded, and/or unlawfully diverted Accu-
Chek
®
test strips and lancets into the United States. The Defendants are willful counterfeiters
who operate out of back-alley apartments in India, and they would remain purely local criminals
if they did not take advantage of Amazon’s platform to falsely advertise, sell, store, and ship
their counterfeits throughout the United States. Instead of being sold in shady local bazaars, the
Defendants’ counterfeits are now being offered to untold numbers of American consumers when
they open Amazon and search for “Accu-Chek.”
33. The Defendants’ counterfeit and misbranded medical devices pose an
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 8 of 43 PageID #: 8
-9-
15066876
immediate danger to the health and safety of American patients.
34. Roche’s authentic Accu-Chek Softclix
®
lancets are high-quality sterile
medical devices designed to pierce the skin, penetrate the blood-skin barrier, and draw blood for
testing. The Defendants’ fake Accu-Chek Softclix
®
lancets are of obviously inferior quality and
are made and stored in unknown conditions, putting American patients at risk of injury and
infection.
35. The Defendants’ counterfeit Accu-Chek
®
test strips are expired or nearly
expired products that are removed from their original authentic packaging under unknown
conditions, have their original labelling stripped by a process that leaves behind a strong
chemical odor, and are re-packaged into counterfeit U.S. packaging with fake expiration dates
that falsely makes the counterfeits appear to be factory-new U.S. product with a long remaining
shelf life. Using Defendants’ counterfeit test strips could provide inaccurate blood glucose
readings to American patients who rely on Accu-Chek
®
strips to give them information they use
to monitor and treat a potentially deadly disease.
36. In addition to selling counterfeit Accu-Chek
®
products, the Defendants
also use their Amazon storefronts to sell unlawfully diverted Accu-Chek
®
test strips that were
manufactured and packaged for international markets, and that the Defendants import back into
the United States. There are several important and material differences between these
international test strips and U.S. test strips – i.e., test strips that Roche manufactures for sale in
the United States and distributes into the United States. Among other things, the international
Accu-Chek
®
test strips do not comply with FDA regulations and requirements and are unlawful
to sell in the United States. Indeed, international Accu-Chek
®
test strips sold in the United States
are misbranded medical devices under the Food & Drug Administration Act, and their sale or
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 9 of 43 PageID #: 9

-10-
15066876
distribution in the United States is a strict-liability federal crime under 21 U.S.C § 333(a).
I. Roche’s Trademarks and Trade Dress
37. Roche is the owner of a family of registered U.S. trademarks including,
among others, the following trademarks that appear on packaging for Accu-Chek SmartView
®
,
Accu-Chek Guide
®
, Accu-Chek Aviva
®
, Accu-Chek Active
®
, and Accu-Chek Instant
®
blood
glucose test strips and Accu-Chek Softclix
®
lancets (collectively referred to herein as the “Accu-
Chek Marks”):
• Roche Diabetes Care GmbH’s “ACCU-CHEK
®
” trademark was registered on the
Principal Register of the United States Patent and Trademark Office on November 14,
2000, as U.S. Registration No. 2,403,536.
• Roche Diabetes Care GmbH’s “ACCU-CHEK SMARTVIEW
®
” trademark was
registered on the Principal Register of the United States Patent Office on October 23,
2012, as U.S. Registration No. 4,230,563.
• Roche Diabetes Care GmbH’s “ACCU-CHEK NANO SMARTVIEW
®
” trademark
was registered on the Principal Register of the United States Patent Office on October
16, 2012, as U.S. Registration No. 4,226,844.
• Roche Diabetes Care GmbH’s “SOFTCLIX
®
” trademark was registered on the
Principal Register of the United States Patent Office on July 6, 1993, as U.S.
Registration No. 1,780,139.
• Roche Diabetes Care GmbH’s “ACCU-CHEK GUIDE
®
” trademark was registered
on the Principal Register of the United States Patent Office on August 1, 2017, as
U.S. Registration No. 5,256,607.
• Roche Diabetes Care GmbH’s “ACCU-CHEK GUIDE ME
®
” trademark was
registered on the Principal Register of the United States Patent Office on April 28,
2020, as U.S. Registration No. 6,042,931.
• Roche Diabetes Care GmbH’s “ACCU-CHEK NANO
®
” trademark was registered on
the Principal Register of the United States Patent Office on September 25, 2012, as
U.S. Registration No. 4,214,217.
• Roche Diabetes Care GmbH’s “ACCU-CHEK AVIVA
®
” trademark was registered
on the Principal Register of the United States Patent Office on March 21, 2006, as
U.S. Registration No. 3,071,846.
• Roche Diabetes Care GmbH’s “ACCU-CHEK AVIVA COMBO
®
” trademark was
registered on the Principal Register of the United States Patent Office on April 7,
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 10 of 43 PageID #: 10

-11-
15066876
2009, as U.S. Registration No. 3,602,826.
• Roche Diabetes Care GmbH’s “ACCU-CHEK AVIVA CONNECT
®
” trademark was
registered on the Principal Register of the United States Patent Office on July 8, 2014,
as U.S. Registration No. 4,561,864.
• Roche Diabetes Care GmbH’s “ACCU-CHEK AVIVA EXPERT
®
” trademark was
registered on the Principal Register of the United States Patent Office on April 7,
2009, as U.S. Registration No. 3,602,825.
• Hoffmann-La Roche, Inc.’s trademark was registered on the Principal
Register of the United States Patent Office on December 26, 2017, as U.S.
Registration Nos. 5,363,165, 5,363,167, and 5,363,168;
38. Roche uses distinctive packaging (the “Accu-Chek Trade Dress”) to
distinguish its Accu-Chek
®
products in the marketplace. Roche has used this distinctive
packaging for over forty years. The Accu-Chek Trade Dress is arbitrary, non-functional, and
distinctive.
39. The Accu-Chek Marks and Accu-Chek Trade Dress have been extensively
and continuously used by Roche, and are inherently distinctive and/or have become distinctive
through the acquisition of secondary meaning.
40. Roche sells over 700 million test strips every year in the United States
alone. The sale of Accu-Chek
®
test strips, lancets, and other Accu-Chek
®
brand products has
been tremendously successful in part due to Roche’s marketing and promotion of the Accu-
Chek
®
brand throughout the country and the world.
41. The Accu-Chek
®
brand is recognized in the United States and throughout
the world as representing high-quality, reliable medical devices manufactured and distributed by
Roche. Accu-Chek
®
test strips and lancets are sold in pharmacies throughout the United States
and world.
42. As a result of Roche’s extensive advertising and promotion of Accu-
Chek
®
test strips and lancets in connection with the Accu-Chek Marks and Accu-Chek Trade
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 11 of 43 PageID #: 11

-12-
15066876
Dress, Roche’s widespread and long-running sale of Accu-Chek
®
test strips and lancets, and the
celebrity that the Accu-Chek Marks and Accu-Chek Trade Dress have achieved, blood glucose
test strips and lancets bearing Accu-Chek Marks and Accu-Chek Trade Dress have been and are
now recognized by the consuming public in the United States and in the trade as originating from
a single source: Roche.
43. Test strips and lancets bearing the Accu-Chek Marks and Accu-Chek
Trade Dress have come to be known by the purchasing public throughout the United States and
abroad as medical devices and blood glucose test strips of the highest quality. As a result, the
Accu-Chek Marks and Accu-Chek Trade Dress and the goodwill associated with them are of
inestimable value to Roche.
44. Roche has used and is currently using the Accu-Chek Marks and Accu-
Chek Trade Dress in commerce and in connection with its sale of Accu-Chek
®
test strips,
lancets, and other products, and plans to continue such use in the future.
II. Roche’s Authentic Accu-Chek
®
Test Strips and Lancets
45. Roche’s Accu-Chek
®
test strips and lancets are industry-leading diabetes
care medical devices relied upon by millions of patients in the United States and worldwide.
46. Roche has manufactured a number of lines of blood glucose test strips
under the Accu-Chek
®
brand, including Accu-Chek SmartView
®
. Accu-Chek SmartView
®
test
strips are high-quality medical devices that give accurate readings with a sample size of just 0.6
microliters of blood.
47. Accu-Chek SmartView
®
test strips are stored in specially designed vials
that bear a label that lists the test strips’ lot number, catalogue number, and expiration date.
Roche warns patients that Accu-Chek
®
test strips should be stored within the vial with the lid
tightly closed, and within certain parameters for air temperature and humidity. Roche also warns
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 12 of 43 PageID #: 12

-13-
15066876
patients never to use expired test strips or test strips where the expiration date is illegible or
unknown.
48. Accu-Chek Softclix
®
lancets are premium, thin-gauge, bevel-cut lancets
specially designed to ensure smooth entry into the skin and precision of insertion to minimize
pain to the patient.
49. Accu-Chek Softclix
®
lancets are also specifically designed to work with
Roche’s Accu-Chek Softclix
®
lancing device, which contains precision guided technology also
designed to reduce pain. Roche warns patients to only use authentic Accu-Chek Softclix
®
lancets with its Accu-Chek Softclix
®
lancing devices, as non-authentic lancets may damage the
lancing device and/or cause it to malfunction.
50. All Roche Accu-Chek
®
medical devices are manufactured in state-of-the
art facilities using specialized equipment, strict specifications, and extensive quality assurance
measures.
III. Roche’s Investigation and Testing of the Defendants’ Counterfeits
51. In late March 2024, a whistleblower reached out to Roche’s online
marketplace monitoring vendor, stating that certain India-based companies were selling
counterfeit Roche test strips in the United States on Amazon.com.
52. Under the direction and supervision of counsel, Roche launched an
investigation, including by making test buys of Accu-Chek
®
products from the identified sellers.
Upon receiving the test purchases, Roche was able to quickly verify the products were not
authentic.
53. Recognizing the counterfeits being sold through Amazon.com as a threat
to American patients, Roche moved quickly to expand its investigation and identify the
counterfeiters. Under the direction and supervision of counsel, Roche arranged for private
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 13 of 43 PageID #: 13

-14-
15066876
investigators to make numerous purchases of Accu-Chek
®
products from Indian sellers on online
marketplaces, including Amazon.com. All of the products that were the subject of these test
buys were advertised and marketed as authentic U.S. Roche Accu-Chek
®
products. The test
purchases were delivered to addresses within this District.
54. Defendants JMD Enterprises, JMD International, and Medical Hub_USA
Store, by and through the actions of their respective individual Defendants, sold multiple
counterfeit Accu-Chek
®
products to Roche’s investigators in this District.
55. Of the test purchases made from Defendants on Amazon.com, most of the
counterfeits were stored in Amazon warehouses and delivered directly by Amazon. In fact, a
number of the counterfeits were delivered by Amazon from Amazon warehouses within this
District, including Queens and Long Island. Amazon currently has untold numbers of these
dangerous counterfeit medical devices in its warehouses across the country, ready to deliver to
unsuspecting American consumers at the click of a button. The Defendants themselves also
directly shipped, from India, a smaller number of counterfeit or unlawfully diverted Accu-Chek
®
products to Roche’s investigators in this District.
56. Roche never gave permission to any of the Defendants to utilize Roche’s
registered trademarks, or to manufacture, market, sell, or distribute these counterfeit and
unlawfully diverted Accu-Chek
®
products in the United States.
A. The Counterfeit Accu-Chek Softclix
®
Lancets
57. Through its examination and testing, Roche has confirmed that all of the
purported Accu-Chek Softclix
®
lancets sold by the Defendants are counterfeit. The lancets
themselves are counterfeit: no aspect of that product was manufactured or licensed by Roche,
and the counterfeit lancets are of obvious lesser quality than authentic Accu-Chek Softclix
®
lancets. Furthermore, the packaging containing the lancets is a counterfeit imitation of Roche’s
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 14 of 43 PageID #: 14
-15-
15066876
authentic packaging, including unauthorized copies of Roche’s registered U.S. trademarks.
58. Although clearly designed to resemble authentic Accu-Chek Softclix
®
lancets, the counterfeit lancets sold by Defendants have numerous differences from authentic
Roche Accu-Chek Softclix
®
lancets.
1. The Counterfeit Lancet Needles
59. Authentic Accu-Chek Softclix
®
lancets are thin, sharp, bevel-cut needles
used to puncture the skin and draw a small amount of blood for testing. These needles come in a
plastic encasement with a removable cap that protects the needle itself. Authentic Accu-Chek
Softclix
®
lancets are specifically designed to be inserted into Accu-Chek Softclix
®
lancing
devices, which among other things allow patients to adjust the length of the needle that punctures
the testing site. The thinness, length, and sharpness of authentic Accu-Chek Softclix
®
lancets are
essential to providing the precision, pain-minimizing blood draw for which the Accu-Chek
Softclix
®
brand has become known.
60. The counterfeit lancets sold by Defendants feature lower-quality needles
that are significantly shorter and less sharp than authentic Accu-Chek Softclix
®
lancets, and thus
will not and cannot provide the precision, pain-minimizing blood draw that authentic Accu-Chek
Softclix
®
lancets provide. The counterfeits will also not function optimally, or function at all, in
Accu-Chek Softclix
®
lancing devices, and may malfunction or damage the lancing device.
61. Authentic Accu-Chek Softclix
®
lancets are manufactured to the highest
standards that, among other things, ensure the lancets are sterile and safe to use to puncture
patients’ skin. The Defendants’ counterfeits are not manufactured by Roche, and are created,
packaged, and stored in unknown conditions. Roche cannot vouch for the sterility, safety, or
efficacy of the counterfeit lancets.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 15 of 43 PageID #: 15
-16-
15066876
2. The Counterfeits’ Plastic Encasements
62. Authentic Accu-Chek Softclix
®
lancets come in a plastic encasement with
a removable circular cap that protects the metal needle itself. In the authentic product, the body
of the plastic encasement has two small circular holes through which the needle itself is visible;
in the Defendants’ counterfeits, there are three such holes in the plastic encasement.
63. In authentic Accu-Chek Softclix
®
lancets, the removable plastic cap sits
atop a small horizontal plastic ridge that aids in the removal of the cap. The Defendants’
counterfeit lancets are missing that horizontal ridge. The encasement of authentic Accu-Chek
Softclix
®
lancets is made of high-quality plastic with smooth edges. The plastic encasement of
Defendants’ counterfeit lancets has sharp out-jutting shards of plastic on the cap and on the base
of the encasement, indicating that the counterfeits were removed or “punched out” of cheap
plastic molding.
64. More generally, the plastic used in the encasement of the counterfeits is of
a noticeably thinner and lower quality as compared to Roche’s high-quality, authentic product.
For example, the plastic cap on the counterfeits is difficult to remove and can leave plastic
residue on the needle itself. This does not occur with authentic Accu-Chek Softclix
®
lancets.
65. The Defendants’ counterfeit lancets also show signs of inconsistent
manufacturing processes. For example, even within the same box of counterfeit lancets, there is
significant variation in the coloring of the plastic encasements: some are different shades of
white, and others have a noticeable blue tint.
3. The Counterfeit Accu-Chek Softclix
®
Packaging
66. The packaging for Defendants’ counterfeit lancets is also intended to
mimic authentic Roche packaging, including the use of unauthorized copies of the Roche
registered trademarks that appear on authentic Roche packaging. However, there are differences
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 16 of 43 PageID #: 16
-17-
15066876
between the Defendants’ counterfeit packaging and authentic Roche packaging that prove the
Defendants’ packaging is fake.
67. Perhaps most obviously, the Defendants’ counterfeit packaging misspells
the name of the product: the counterfeits use the name “Accu-Chek Softclick,” whereas the real
product name is spelled “Accu-Chek Softclix.” The counterfeits also use the ™ symbol after
these brand names, whereas authentic Roche packaging uses the ® symbol, because its
trademarks are registered.
68. The counterfeit lancets also arrive inside the counterfeit box sealed inside
a cloudy plastic bag. There is no such plastic bag used in the packaging of authentic Accu-Chek
Softclix
®
lancets. The plastic bag that holds the counterfeits is often filled with small pieces of
plastic detritus – i.e., additional plastic shards that had broken off the plastic encasement of the
counterfeits.
69. In addition to these differences, there are also differences in font, layout,
and construction of the Defendants’ counterfeit packaging and authentic Roche Accu-Chek
Softclix
®
packaging that confirm the Defendants’ packaging is counterfeit.
4. Roche’s Initial Testing of the Counterfeit Lancets Confirms They Are
Defective Fakes
70. Initial testing of the Defendants’ counterfeit lancets confirmed that they
are low-quality, defective fakes.
Authentic Packaging
Counterfeit Packaging
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 17 of 43 PageID #: 17

-18-
15066876
71. Authentic Accu-Chek Softclix
®
lancets fit snugly into the Accu-Chek
Softclix
®
lancing device, with an audible “click.” The counterfeits do not fit snugly into an
Accu-Chek Softclix
®
lancing device and do not consistently produce the “click.”
72. Authentic Accu-Chek Softclix
®
lancets can be ejected from the lancing
device by pushing a plunger on the device. The counterfeits do not consistently eject from the
lancing device as intended, but instead needed to be pried out of the lancing device by hand,
creating a risk of injury to the patient.
73. Accu-Chek Softclix
®
lancing devices allow the consumer to adjust the
depth of the needle puncture by adjusting a dial on the side of the device. Using one of the same
testing methods that Roche uses to quality-test authentic lancets, Roche tested the counterfeit
lancets by firing them into a silicone block covered with a thin layer of aluminum. Authentic
Accu-Chek Softclix
®
lancets will puncture the aluminum layer and the silicon block at any depth
setting. The counterfeits failed to even leave a visible mark on the aluminum layer, let alone
puncture the silicone, on several different settings – meaning the counterfeits completely failed
to function.
B. The Counterfeit Accu-Chek SmartView
®
Test Strips
74. Roche’s examination of the Defendants’ purported Accu-Chek
SmartView
®
test strips confirms that they are counterfeits.
75. The Defendants’ counterfeits consist of expired or near-expired test strips
removed from their original packaging under unknown conditions and their vial labels removed,
which are then repackaged into a counterfeit reproduction of Roche’s U.S. packaging, but
bearing fake lot numbers, fake serial numbers, and fake expiration dates that falsely give the
appearance of the test strips being factory-new, unexpired U.S. product with a long shelf life.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 18 of 43 PageID #: 18

-19-
15066876
1. The Fake Vial Labels, Expiration Dates, and Serial and Lot Numbers
76. Both the boxes and the vial labels for Defendants’ counterfeit Accu-Chek
SmartView
®
test strips bear fake serial numbers, fake lot numbers, and fake expiration dates.
Using internal records and marks appearing on the bottom of the vials, Roche was able to
determine the actual expiration dates of the test strips originally packed in those vials. The fake
expiration dates on the counterfeit boxes and vial labels make it appear as though the product has
a long remaining shelf life, when in fact the strips are expired or near-expired.
All of the counterfeit Accu-Chek SmartView
®
strips that each of the Defendants sold to Roche’s
investigators bore the exact same serial numbers, lot numbers, and expiration dates. It is clear
the counterfeiters made a single set of counterfeit packaging and reused it for all of their
counterfeits, at least for a significant period of time.
77. The serial numbers on the authentic packaging of Accu-Chek SmartView
®
test strips are unique, per-box identifiers: the serial numbers never repeat from box to box. The
Counterfeit Accu-Chek SmartView
®
packaging with identical serial numbers
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 19 of 43 PageID #: 19
-20-
15066876
fact that the Defendants’ counterfeit Accu-Chek SmartView
®
boxes all bore the same serial
number is proof they are counterfeit. Any distributor of Accu-Chek
®
test strips who received
boxes with identical serial numbers would know they are fakes.
78. The authentic labels originally on the vials containing the test strips were
removed by the counterfeiters. Several of the Defendants’ counterfeits had vials that gave off a
strong, chemical-like odor upon opening the carton. No such odor exists in authentic Accu-Chek
SmartView
®
packaging. The odor from the counterfeits suggests that harsh, currently unknown
chemicals were used to remove the original labelling on the vials. The use of chemicals to
remove the original vial labels could damage the test strips inside, including by causing the
active ingredients of the strips to prematurely degrade.
79. In addition, the counterfeiters may have used heat guns to remove the
original authentic labels from the vials. Use of a heat gun or other heat source can damage the
test strips inside and cause the active ingredients of the strips to prematurely degrade.
80. After the original vial labels were removed, the fake labels applied by the
counterfeiters onto the vials are typically misaligned and show signs of peeling and bubbling.
81. In authentic Accu-Chek SmartView
®
packaging – on both the box and the
vial label – the serial and lot numbers, expiration dates, and other information on the variable-
print area is laser ablated, a high-speed process that leaves a slight but noticeable texture on the
surface. The Defendants’ counterfeits used regular ink printing on the counterfeit boxes and
counterfeit vial labels.
2. The Counterfeit Boxes
82. The counterfeit Accu-Chek SmartView
®
test strips sold by Defendants are
packaged in counterfeit boxes that are intended to duplicate Roche’s authentic U.S. Accu-Chek
SmartView
®
packaging, and include unauthorized copies of Roche’s registered U.S. trademarks.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 20 of 43 PageID #: 20

-21-
15066876
83. There are errors on the Defendants’ counterfeit packaging, some more
obvious than others. For example, the counterfeit packaging bears an obvious typographical
error: on a side panel, it claims the product was manufactured by “Roche Diabeter Care, Inc.”
(emphasis added). The correctly spelled name is, of course, Roche Diabetes Care, Inc.
84. The construction of the counterfeit Accu-Chek SmartView
®
boxes is also
different and of a lower quality than authentic Accu-Chek SmartView
®
packaging. For example,
the top flap of authentic Accu-Chek SmartView
®
boxes is sealed by a machine that puts precise
dots of adhesive on specified areas of the flap. The counterfeit boxes, in contrast, appear to be
glued together by hand, with globs of adhesive that sometimes extend beyond the top flap and
that cause the box to tear upon opening.
Authentic Packaging
Counterfeit Packaging
Counterfeit packaging
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 21 of 43 PageID #: 21

-22-
15066876
3. The Counterfeit Patient Instructional Inserts
85. Finally, the patient instructional inserts included inside Defendants’
counterfeit Accu-Chek SmartView
®
products are also counterfeit, and also make unauthorized
copies of Roche’s registered U.S. trademarks.
86. The Defendants’ counterfeit Accu-Chek SmartView
®
instructional inserts
contain numerous errors that do not and would not occur on Roche’s authentic instructional
inserts. For example, they contain obvious errors demonstrating that these inserts are counterfeit
copies, including: misspellings throughout the insert; missing bullet points omitting instructional
information; incorrect service center telephone numbers; and incorrect markings and symbols.
Authentic Patient Insert
Counterfeit Patient Insert
Authentic Patient Insert
Counterfeit Patient Insert
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 22 of 43 PageID #: 22

-23-
15066876
87. Moreover, the counterfeit instructional inserts are folded incorrectly as
compared to authentic inserts. Roche’s authentic instructional inserts are machine-folded using
specialized machinery, which the counterfeiters were unable to replicate.
IV. The Defendants’ Counterfeit Medical Devices Pose a Serious and Immediate
Threat to Patient Health and Safety
88. The Defendants’ counterfeit Accu-Chek
®
lancets and test strips are being
sold in large quantities to U.S. consumers who are purchasing the products on an extremely well-
known online marketplace, Amazon.com. Consumers have no idea the products are counterfeit
when they order them. The Defendants’ counterfeits put those patients’ health and safety at risk.
89. Roche cannot vouch for the sterility or safety of the Defendants’
counterfeit lancets, which are low-quality fakes meant to imitate Roche’s authentic, high-quality
Accu-Chek Softclix
®
lancets. Lancets are designed to penetrate patients’ skin, break the blood-
skin barrier, and produce a sufficient amount of blood for diabetics to test their blood sugar. The
Defendants’ low-quality counterfeits may injure patients who use them, and more concerningly,
may not be sterile or otherwise safe to penetrate patients’ skin.
90. Roche also cannot vouch for the safety or efficacy of the Defendants’
counterfeit SmartView test strips. The Defendants’ counterfeits were created using expired or
near-expired test strips that will expire well before the fake expiration date on the box; use of
expired strips can lead to inaccurate test results.
91. Moreover, the Defendants’ counterfeits are created from test strips that
were removed from their original packaging under unknown conditions, with the vials containing
the strips appearing to have been subjected to a harsh chemical process and/or concentrated
blasts of high heat to remove the original labelling. There is therefore good cause to be
concerned that Defendants’ counterfeit test strips are damaged or contaminated, which again can
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 23 of 43 PageID #: 23
-24-
15066876
lead to inaccurate test results, as well as posing a contamination risk to patients who put these
counterfeits in contact with blood extracted from a testing site on their body.
92. In addition, authentic Accu-Chek SmartView
®
test strips must be stored at
or below 30 degrees Celsius, or 86 degrees Fahrenheit, as noted on the packaging of the
authentic test strips. When stored above that temperature, the test strips can become damaged,
including by prematurely degrading the active ingredients of the test strips. The counterfeiters
store the counterfeit boxes of Accu-Chek SmartView
®
test strips in back-alley residential
apartments in rooms without air conditioning in New Delhi and elsewhere in India, where
temperatures can routinely exceed 110 degrees Fahrenheit in the summer.
93. Moreover, international shipping regularly exposes goods to high
temperatures for prolonged periods of time. Roche arranges for its authentic Accu-Chek
®
test
strips to be shipped internationally subject to specific requirements for temperature. The
counterfeiters do not follow such rigorous requirements when they import the counterfeits in the
United States, thus potentially exposing the test strips to conditions that could prematurely
degrade the active ingredients.
94. Accu-Chek
®
test strips that are expired, exposed to excessive heat, or
otherwise damaged may contain active ingredients that have degraded to the extent that they will
not give an accurate reading of a patients’ blood glucose levels. When such test strips with
degraded active ingredients do give a reading, they can give a reading that overstates the amount
of glucose in the patients’ blood.
95. Diabetics use Accu-Chek
®
medical devices to monitor their blood sugar
and manage and treat diabetes, a life-long and potentially deadly disease. Patients with diabetes
rely on blood glucose test results to, among other things, determine the levels of insulin they
should self-inject. Inaccurately high test results from the Defendants’ counterfeits could cause
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 24 of 43 PageID #: 24

-25-
15066876
patients to unknowingly inject themselves with an overdose of insulin. Insulin overdoses can
have severe negative consequences for patients, up to and including coma and death. Indeed, the
FDA has publicly warned that using blood glucose test strips that have been previously opened
by someone other than the consumer “may potentially cause infection or lead to inaccurate test
results, which can cause serious harm, including death.”
1
V. The Defendants Also Sell Unlawfully Diverted, Misbranded Accu-Chek
®
Medical Devices in the United States, in Violation of Roche’s Trademark Rights
96. In its investigation and test buys from Defendants, including test buys
made on Amazon.com, Roche learned that in addition to selling counterfeit Accu-Chek Softclix
®
lancets and Accu-Chek SmartView
®
test strips, the Defendants are selling several other once-
authentic but unlawfully diverted international Accu-Chek
®
medical devices. These unlawfully
diverted international Accu-Chek
®
products are manufactured and packaged by Roche for
markets outside the United States, and the Defendants are illegally importing these international
Accu-Chek
®
medical devices into the United States. These unlawfully diverted international
Accu-Chek
®
products are materially different in numerous ways from authentic U.S. Accu-
Chek
®
products – i.e., from the Accu-Chek
®
products that Roche manufactures and packages for
sale into the United States.
97. The only Accu-Chek
®
products that Roche sells and distributes in the
United States are U.S. Accu-Chek
®
products. Roche does not sell or distribute in the United
States any of the international Accu-Chek
®
products – i.e., Accu-Chek
®
products that Roche
manufactures and packages for markets outside the United States.
1
FDA warns about risks of using home use test strips that are pre-owned or not authorized for
sale in U.S., including those for glucose, warfarin, U.S. FOOD & DRUG ADMIN. (April 8,
2019), available at https://www.fda.gov/news-events/press-announcements/fda-warns-about-
risks-using-home-use-test-strips-are-pre-owned-or-not-authorized-sale-us-including.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 25 of 43 PageID #: 25
-26-
15066876
98. In the United States, Accu-Chek
®
medical devices are heavily regulated
by the FDA. All U.S. Accu-Chek
®
products are compliant with FDA regulations, including with
regard to the manufacture and packaging of the products, and their patient instructional inserts.
International Accu-Chek
®
test strips, on the other hand, are manufactured and packaged for sale
in countries outside the United States with their own regulatory authorities and requirements, and
so do not comply with all FDA regulations.
99. Indeed, when sold inside the United States, international Accu-Chek
®
test
strips constitute misbranded medical devices under the Food & Drug Administration Act, and
their sale or distribution in the United States is a strict-liability federal crime under 21 U.S.C §
333(a).
100. The international Accu-Chek
®
products that Defendants are unlawfully
selling in the United States include Accu-Chek Guide
®
, Accu-Chek Aviva
®
, Accu-Chek Active
®
,
and Accu-Chek Instant
®
blood glucose test strips.
101. As noted above, Accu-Chek
®
test strips must be stored within specified
temperature ranges, and the Defendants’ storage practices routinely expose the international
Accu-Chek
®
test strips in their inventory to temperatures well beyond the allowable maximum,
potentially damaging the test strips and rendering them unusable or inaccurate. The same is
likely true with regard to the Defendants’ shipment of the international test strips from India to
the United States, as these shipments are not subject to Roche’s shipping specifications and
requirements.
102. Beyond the damage that the Defendants’ storage and shipping practices
can cause to the products, the international Accu-Chek
®
test-strip products differ in numerous
material ways from the U.S. Accu-Chek
®
test strips that Roche sells domestically.
103. For example, international Accu-Chek
®
test strips have foreign languages
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 26 of 43 PageID #: 26

-27-
15066876
on the box and instructional inserts, whereas U.S. Accu-Chek
®
test strips come in English-
language packaging. Many international Accu-Chek
®
products being sold in the United States
by Defendants do not contain English at all.
104. Moreover, the packaging for international Accu-Chek
®
test strips lacks
numerous aspects present on U.S. Accu-Chek
®
test strip packaging that are material to American
consumers.
105. The packaging for every box of U.S. Accu-Chek
®
test strips also provides
FDA-required written warnings and instructions, including “Do not reuse” and “For in vitro
diagnostic use.” The outer package label of international Accu-Chek
®
test strips does not
provide these warnings.
106. Roche also uses significantly different trade dress for domestic Accu-
Chek
®
products and international Accu-Chek
®
products, including different box colors and
designs. Diabetes is a life-long disease, and American patients who use Accu-Chek
®
test strips
are very familiar with the U.S. Accu-Chek Trade Dress. Those consumers are likely to be
confused upon buying on Amazon.com what is advertised as U.S. Accu-Chek
®
test strips, but is
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 27 of 43 PageID #: 27

-28-
15066876
in reality an unlawfully diverted box of international Accu-Chek
®
test strips.
107. International Accu-Chek
®
test strips also come with instructional inserts
that are materially different from the instructional inserts that are included with U.S. Accu-
Chek
®
test strips. For example, both instructional inserts tell patients where on their body they
may draw a blood sample. However, the international inserts instruct the patients that they may
draw blood from sites on the body that do not appear in the FDA-approved U.S. instructional
inserts.
108. There are myriad other differences between the U.S. and international
patient inserts, including: different units of measurement; an explanation of symbols on the U.S.
insert that does not exist on international inserts; differences in the charts describing the blood
glucose systems’ product characteristics; study references in the U.S. insert that do not exist in
the international inserts; and differences in the safety reporting instructions between U.S. and
international inserts.
109. Individually and as a whole, these differences between U.S. and
international Accu-Chek
®
test strips are material to U.S. consumers and would be confusing to
U.S. consumers who are expecting the languages, regulatory markings, trade dress
measurements, instructional inserts, and other aspects of an authentic U.S. product intended for
sale in the United States. This is particularly so for users of this product who are managing a
lifelong disease and make daily use of Roche’s diabetes care medical devices, and thus look to
Roche for consistency and reliability.
VI. Defendants’ Infringing Sales Interfere with Roche’s Quality-Control Measures
110. Moreover, authentic Accu-Chek
®
test strips and lancets are manufactured
with a catalogue number and lot number. Roche uses catalogue and lot numbers to track where
the test strips are shipped and then to monitor them if any safety or quality issues arise. When a
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 28 of 43 PageID #: 28
-29-
15066876
recall is warranted, for example, Roche notifies the regulatory agencies in the countries that were
authorized to receive the impacted catalogue and lot number, informing them of the particular
issue and seeking their guidance. Roche then issues recall notices, which are only sent to regions
and/or consumers that are authorized to have received the particular subset of product that is the
subject of the recall. By using targeted recalls, Roche spares consumers from seeing inapplicable
recall notices, which can lead to consumer confusion, disposal of non-recalled devices, and
desensitization to recall notices that can result in future recalls being ignored.
111. When Accu-Chek
®
blood glucose testing medical devices are repackaged
into counterfeit packaging and, as such, separated from manufacturing information including
catalogue and lot numbers, it eliminates Roche’s ability to perform a targeted product recall.
112. Furthermore, when international Accu-Chek
®
medical devices are
unlawfully diverted into the United States from the region for which they were manufactured,
Roche’s ability to perform a targeted recall is undermined, because the impacted products are no
longer in the authorized region into which they were distributed.
113. Moreover, U.S. boxes of Accu-Chek
®
test strips prominently display
Roche’s U.S. toll-free consumer hotline, where consumers can call with questions or to report
any quality issues or adverse events. The lack of a U.S. toll-free number on the unlawfully
diverted international test strips further undermines that important quality-assurance function.
114. Finally, as described above, the counterfeit repackaging process also
destroys the integrity of the product, rending it impossible for Roche to vouch for the quality,
safety, or efficacy of the medical device. And the unlawfully diverted international test strips are
subjected to the same storage and shipping conditions that expose the product to unknown
temperatures and storage conditions, potentially damaging the product and rending it inaccurate
or unusable.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 29 of 43 PageID #: 29

-30-
15066876
VII. The Counterfeiters Use Amazon to Take Advantage of American Patients
115. The Defendants are knowing, criminal counterfeiters living and operating
in impoverished areas of New Delhi and elsewhere in India. They operate their counterfeiting
operations out of back-alley apartments and cramped storefronts. Without access to American
consumers through an American sales platform, the Defendants’ counterfeiting would not be
economically viable or would be a purely local problem and could be dealt with locally. But by
opening storefronts on Amazon.com, Defendants are willfully marketing these dangerous
counterfeits directly to American consumers, and benefit by being associated with one of the
most famous American companies in existence. These dangerous counterfeit medical devices
are now stored in Amazon warehouses throughout the United States and delivered by Amazon
delivery vehicles to U.S. customers.
116. The Defendants do directly ship their counterfeit and infringing products
to the United States, but they also participate in Amazon’s Fulfillment by Amazon (“FBA”)
program. Through FBA, Amazon agrees to receive, store, and accept orders on behalf of the
counterfeiters; to pick, pack, and ship the counterfeit goods; and to provide customer service for
the counterfeiters. Through FBA, the counterfeiters ship products directly to Amazon fulfillment
centers, and Amazon stores and ships the products directly to U.S. customers. Amazon, in
return, receives a sizable percentage of the revenue from the counterfeit sales.
117. Indeed, as recently as May 2, 2024, a customer left a negative review on
Amazon’s platform, complaining that he had ordered test strips from DKY, and had received a
different product. Amazon crossed out the language of the negative review—the language
appears on Amazon’s website with a line through it—and replied on behalf of the counterfeiter,
writing: “This item was fulfilled by Amazon, and we [Amazon] take responsibility for this
fulfillment experience.”
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 30 of 43 PageID #: 30
-31-
15066876
118. As another example, a different customer wrote the following review on
Amazon in March 2024 concerning the supposed Accu-Chek Softclix
®
lancets she purchased
from DKY: “These lancets are fake. They will not fit in your device properly and won’t work.
Do not buy!”
119. The Defendants’ purported business model on Amazon makes no sense.
The Defendants claim to purchase U.S. medical devices, import those products to India, and then
turn around and sell those U.S. medical devices back to U.S. customers, re-exporting them back
from India into the United States. And the Defendants claim to do all that at discount pricing,
while also paying sizable fees to Amazon, and still turn a profit. That is an economic
impossibility, and an obvious falsehood.
120. Concerningly, many American consumers who purchased the Defendants’
counterfeits on Amazon did not even realize they are purchasing from a third-party seller, let
alone a seller purporting to import and re-export U.S. medical devices into and out of India.
121. When a U.S. consumer searches Amazon.com for a particular product and
clicks on the corresponding link, the Amazon webpage will feature on the right side of the screen
a large window known as the Amazon “buy box,” which displays the price and shipping
information for that product, along with large “Add to Cart” and “Buy Now” buttons. Those
“Add to Cart” and “Buy Now” buttons are linked to a particular seller on the Amazon
marketplace; Amazon automatically determines which seller will be associated with the “buy
box” based on a proprietary algorithm. The vast majority of Amazon shoppers purchase goods
from whatever seller is associated with the “buy box” when they look up a product on Amazon.
Many of those consumers incorrectly believe that by clicking on those buttons they are buying
from a trusted source, such as the manufacturer or an authorized distributor.
122. In some cases, the seller associated with the Amazon “buy box” for Accu-
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 31 of 43 PageID #: 31

-32-
15066876
Chek
®
products was a Defendant, and the product the U.S. consumer received after clicking on
“Add to Cart” or “Buy Now” was in fact a counterfeit or unlawfully diverted, non-FDA-
approved medical device. Those consumers had no idea that they were being directed to
purchase fake or unlawfully diverted Accu-Chek
®
products from a criminal in India.
FIRST CLAIM FOR RELIEF
Federal Trademark Infringement
15 U.S.C. §1114(1)(a) (Lanham Act Section 32)
123. Roche incorporates each paragraph of this Complaint as if fully set forth
herein.
124. Roche is the owner of all right, title and, interest in and to the Accu-Chek
Marks and Accu-Chek Trade Dress.
125. Defendants, without authorization, have imported into the United States,
and/or distributed and/or sold in the United States, and/or received in interstate commerce, either
a reproduction, counterfeit, copy or colorable imitation of the Accu-Chek Marks and the Accu-
Chek Trade Dress in connection with the sale, offering for sale, distribution, or advertising of
counterfeit Roche products.
126. Defendants, without authorization, have also imported into the United
States, and/or distributed and/or sold in the United States, and/or received in interstate
commerce, either a reproduction, counterfeit, copy or colorable imitation of the Accu-Chek
Marks and/or the Accu-Chek Trade Dress in connection with the sale, offering for sale,
distribution, of diverted international Accu-Chek
®
products featuring the Accu-Chek Marks
and/or Accu-Chek Trade Dress, which products are materially different from the U.S. Accu-
Chek
®
products authorized by Roche for sale in the United States and which are not subject to
and undermine Roche’s quality-control measures.
127. The Defendants’ actions are likely to cause confusion, mistake, or
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 32 of 43 PageID #: 32
-33-
15066876
deception as to the source of origin, sponsorship, or approval of the counterfeit and the diverted
international Accu-Chek
®
products, including in that purchasers and others in this judicial
district and elsewhere in the United States are likely to believe Roche authorizes and controls the
sale of Defendants’ counterfeit and diverted international Accu-Chek
®
products, or that
Defendants are associated with or related to Roche or are authorized by Roche to sell Accu-
Chek
®
products in the United States.
128. Defendants’ actions constitute willful infringement of Roche’s exclusive
rights in the Accu-Chek Marks and Accu-Chek Trade Dress. Defendants are directly,
contributorily, and vicariously liable for their infringement.
129. Defendants’ acts have been committed deliberately and willfully, with
knowledge of Roche’s exclusive rights and goodwill in the Accu-Chek Marks and Accu-Chek
Trade Dress, and with knowledge of the infringing nature of the marks when used in connection
with both the counterfeit and diverted international Accu-Chek
®
products. Defendants’ acts have
been committed with bad faith and the intent to cause confusion, to cause mistake, and/or to
deceive.
130. As a result of Defendants’ trademark infringement, Roche has suffered
and will continue to suffer substantial and irreparable injury, loss and damage to its rights in and
to the Accu-Chek Marks and Accu-Chek Trade Dress, and damage to the goodwill associated
therewith, for which it has no adequate remedy at law. If not restrained, Defendant will have
unfairly derived and will continue to derive illicit income, profits, and business opportunities as a
result of their acts of infringement.
131. As the acts alleged herein constitute infringement of the Accu-Chek Marks
and Accu-Chek Trade Dress under 15 U.S.C. § 1114(1)(a), and as Roche has no adequate
remedy at law, Roche is entitled to injunctive relief as well as to Defendants’ profits, Roche’s
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 33 of 43 PageID #: 33

-34-
15066876
damages, and other remedies provided by 15 U.S.C. §§ 1116, 1117 and 1118, and to reasonable
attorneys’ fees and prejudgment interest pursuant to 15 U.S.C. § 1117.
SECOND CLAIM FOR RELIEF
Federal Trademark Infringement
15 U.S.C. §1114(1)(b) (Lanham Act Section 32)
132. Roche incorporates each paragraph of this Complaint as if fully set forth
herein.
133. Roche is the owner of all right, title and, interest in and to the Accu-Chek
Marks and Accu-Chek Trade Dress.
134. Defendants, without authorization, have imported into the United States,
and/or distributed and/or sold in the United States, and/or received in interstate commerce, either
a reproduction, counterfeit, copy or colorable imitation of the Accu-Chek Marks and the Accu-
Chek Trade Dress in connection with the sale, offering for sale, distribution, or advertising of
counterfeit Roche products.
135. Defendants, without authorization, have also imported into the United
States, and/or distributed and/or sold in the United States, and/or received in interstate
commerce, either a reproduction, counterfeit, copy or colorable imitation of the Accu-Chek
Marks and/or the Accu-Chek Trade Dress in connection with the sale, offering for sale,
distribution, of diverted international Accu-Chek
®
products featuring the Accu-Chek Marks
and/or Accu-Chek Trade Dress, which products are materially different from the U.S. Accu-
Chek
®
products authorized by Roche for sale in the United States and which are not subject to
and undermine Roche’s quality-control measures.
136. The Defendants’ actions are likely to cause confusion, mistake, or
deception as to the source of origin, sponsorship, or approval of the counterfeit and the diverted
international Accu-Chek
®
products, including in that purchasers and others in this judicial
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 34 of 43 PageID #: 34
-35-
15066876
district and elsewhere in the United States are likely to believe Roche authorizes and controls the
sale of Defendants’ counterfeit and diverted international Accu-Chek
®
products, or that
Defendants are associated with or related to Roche or are authorized by Roche to sell Accu-
Chek
®
products in the United States.
137. Defendants’ actions constitute willful infringement of Roche’s exclusive
rights in the Accu-Chek Marks and Accu-Chek Trade Dress. Defendants are directly,
contributorily, and vicariously liable for their infringement.
138. Defendants’ acts have been committed deliberately and willfully, with
knowledge of Roche’s exclusive rights and goodwill in the Accu-Chek Marks and Accu-Chek
Trade Dress, and with knowledge of the infringing nature of the marks when used in connection
with both the counterfeit and diverted international Accu-Chek
®
products. Defendants’ acts have
been committed with bad faith and the intent to cause confusion, or to cause mistake and/or to
deceive.
139. As a result of Defendants’ trademark infringement, Roche has suffered
and will continue to suffer substantial and irreparable injury, loss, and damage to its rights in and
to the Accu-Chek Marks and Accu-Chek Trade Dress, and damage to the goodwill associated
therewith, for which it has no adequate remedy at law. If not restrained, Defendant will have
unfairly derived and will continue to derive illicit income, profits, and business opportunities as a
result of their acts of infringement.
140. As the acts alleged herein constitute infringement of the Accu-Chek Marks
and Accu-Chek Trade Dress under 15 U.S.C. § 1114(1)(b), and as Roche has no adequate
remedy at law, Roche is entitled to injunctive relief as well as to Defendants’ profits, Roche’s
damages, and other remedies provided by 15 U.S.C. §§ 1116, 1117 and 1118, and to reasonable
attorneys’ fees and prejudgment interest pursuant to 15 U.S.C. § 1117.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 35 of 43 PageID #: 35

-36-
15066876
THIRD CLAIM FOR RELIEF
Federal Unfair Competition
15 U.S.C. § 1125(a)(i)(A) (Lanham Act Section 43(a))
141. Roche incorporates each paragraph of this Complaint as if fully set forth
herein.
142. Defendants have, without authorization, imported into the United States
and/or distributed and/or sold in the United States and/or received in interstate commerce, both
counterfeit Accu-Chek
®
products and diverted international Accu-Chek
®
products featuring the
Accu-Chek Marks and/or Accu-Chek Trade Dress, which products are materially different from
the U.S. Accu-Chek
®
test strips authorized by Roche for sale in the United States and which are
not subject to and undermine Roche’s quality-control measures. Such use is likely to cause
confusion, or to cause mistake and/or to deceive as to the affiliation, connection or association of
Defendants with Roche, and as to the origin, sponsorship or approval by Roche of Defendants’
counterfeit and diverted international Accu-Chek
®
test strips and the commercial activities
related to Defendants’ counterfeit and diverted international U.S. Accu-Chek
®
test strips.
143. Defendants’ acts constitute a false representation and a false designation
of origin in violation of Section 43(a) of the Lanham Act, 15 U.S.C. § 1125(a).
144. Defendants’ acts have been committed willfully, with knowledge of
Roche’s exclusive common-law rights and goodwill in the Accu-Chek Marks and Accu-Chek
Trade Dress, as well as with bad faith and the intent to cause confusion or mistake, and/or to
deceive.
145. Roche has suffered and, if Defendants are not enjoined, will continue to
suffer great and irreparable injury, loss, and damage to its rights in and to the Accu-Chek Marks
and Accu-Chek Trade Dress and to the goodwill associated therewith for which Roche has no
adequate remedy at law.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 36 of 43 PageID #: 36

-37-
15066876
146. If not restrained, Defendants will have unfairly derived and will continue
to derive illicit income, profits, and business opportunities as a result of their acts of
infringement.
147. As the acts alleged herein violate Section 43(a) of the Lanham Act, 15
U.S.C. § 1125(a), and as Roche has no adequate remedy at law, Roche is entitled to injunctive
relief and to Defendants’ profits, Roche’s damages, and other remedies provided by 15 U.S.C. §§
1116, 1117 and 1118, and to reasonable attorneys’ fees and prejudgment interest pursuant to 15
U.S.C. § 1117.
FOURTH CLAIM FOR RELIEF
Common-Law Unfair Competition
148. Roche incorporates each paragraph of this Complaint as if fully set forth
herein.
149. Defendants’ acts constitute an infringement of Roche’s trademark rights in
violation of common law, including the common law of the State of New York and elsewhere.
150. As a result of Defendants’ acts Roche has suffered and, if Defendants are
not enjoined, will continue to suffer great and irreparable injury, loss, and damage to its rights in
and to the Accu-Chek Marks and Accu-Chek Trade Dress, and to the goodwill associated
therewith for which Roche has no adequate remedy at law.
FIFTH CLAIM FOR RELIEF
State Law Trademark Dilution
151. Roche incorporates each paragraph of this Complaint as if fully set forth
herein.
152. Roche’s Accu-Chek Marks and Accu-Chek Trade Dress are distinctive
under state law, including New York General Business Law § 360-l.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 37 of 43 PageID #: 37

-38-
15066876
153. Defendants’ acts are likely to cause dilution by blurring and/or
tarnishment, and damage the business reputation of Roche in violation of state law, including
New York General Business Law § 360-l.
154. Through their acts, Defendants have injured and are continuing to injure
Roche’s business reputation and/or have diluted and are continuing to dilute the distinctive
quality of the Accu-Chek Marks and Accu-Chek Trade Dress, in violation of state law, including
New York General Business Law § 360-l.
155. Defendants’ acts greatly and irreparably damage Roche and will continue
to do so unless restrained by this Court. Therefore, Roche is without an adequate remedy at law
and is entitled to, among other things, an order enjoining and restraining Defendants from selling
counterfeit Accu-Chek
®
test strips, and diverted international Accu-Chek
®
test strips.
SIXTH CLAIM FOR RELIEF
State Law Deceptive Business Practices
156. Roche incorporates each paragraph of this Complaint as if fully set forth
herein.
157. Defendants’ acts were consumer-oriented.
158. Defendants’ acts were materially misleading.
159. Defendants’ acts have caused and are continuing to cause injury to Roche
in violation of state law, including New York General Business Law § 349.
160. Defendants’ acts greatly and irreparably damage Roche and will continue
to do so unless restrained by this Court. Therefore, Roche is without an adequate remedy at law
and is entitled to, among other things, an order enjoining and restraining Defendants from selling
Roche products.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 38 of 43 PageID #: 38

-39-
15066876
SEVENTH CLAIM FOR RELIEF
Unjust Enrichment
161. Roche incorporates each paragraph of this Complaint as if fully set forth
herein.
162. The Defendants made false representations and material omissions in
fraudulently selling counterfeit and diverted international boxes of Accu-Chek
®
products,
including by representing the products to be authentic U.S. Accu-Chek
®
products, on consumer-
facing online marketplaces at a lower cost than was legally available in the United States.
163. As a result of these false representations and material omissions,
Defendants wrongfully obtained a monetary benefit to which they were not legally entitled.
164. Defendants have no right to retain these unjust gains.
165. If Defendants are permitted to keep this monetary benefit, it would be
manifestly unjust.
166. By selling both counterfeit and diverted international Accu-Chek
®
products in the United States, Defendants have been unjustly enriched at Roche’s expense in
violation of the common law of New York and elsewhere.
EIGHTH CLAIM FOR RELIEF
Importation of Goods Bearing Infringing Marks
15 U.S.C. § 1124
167. Roche incorporates each paragraph of this Complaint as if fully set forth
herein.
168. By illegally importing both counterfeit Accu-Chek
®
products and diverted
international Accu-Chek
®
products from foreign countries with the intent of inducing consumers
into believing that the products are authentic and authorized for resale in the United States,
Defendants have violated 15 U.S.C. § 1124.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 39 of 43 PageID #: 39

-40-
15066876
169. Roche has been, and continues to be, damaged by Defendants’ activities
and conduct. Defendants have profited thereby and, unless their conduct is enjoined, Roche’s
reputation and goodwill will continue to suffer irreparable injury that cannot adequately be
calculated or compensated by money damages. Accordingly, Roche is entitled to injunctive
relief under 15 U.S.C. § 1116.
NINTH CLAIM FOR RELIEF
False Description and Designation of Origin in Commerce
170. Plaintiffs incorporate each paragraph of this Complaint as if fully set forth
herein.
171. In violation of 15 U.S.C. § 1125(a)(1)(A), Defendants, independently and
in conspiracy with one another, in connection with the counterfeit and the unlawfully diverted
Roche products, used in commerce a slogan, trade dress, word, term, name, symbol or device, or
any combination thereof, or a false designation of origin, false or misleading description of fact
or false or misleading representation of fact, which was or is likely to cause confusion or to cause
mistake, or to deceive as to an affiliation, connection, or association with Roche.
172. Defendants’ actions constitute willful infringement of Plaintiffs’ exclusive
rights in the Accu-Chek Marks and the Accu-Chek Trade Dress.
173. As a direct and proximate result of Defendants’ conduct, Plaintiffs have
suffered irreparable harm to the valuable Accu-Chek Marks and the Accu-Chek Trade Dress, and
to their reputation in the industry. Unless Defendants’ conduct is restrained, Plaintiffs will
continue to be irreparably harmed.
174. Plaintiffs have no adequate remedy at law that will compensate for the
continued and irreparable harm they will suffer if Defendants’ acts are allowed to continue.
175. As a direct and proximate result of Defendants’ conduct, Plaintiffs have
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 40 of 43 PageID #: 40

-41-
15066876
suffered damages to the valuable Accu-Chek Marks and the Accu-Chek Trade Dress, and other
damages in an amount to be proved at trial.
TENTH CLAIM FOR RELIEF
Federal False Advertising
176. Plaintiffs incorporate each paragraph of this Complaint as if fully set forth
herein.
177. In violation of 15 U.S.C. § 1125(a)(1)(B), Defendants, independently and
in conspiracy with one another, in connection with the sale of the counterfeit Roche products and
diverted international Roche products, used a slogan, trade dress, word, term, name, symbol, or
device or any combination thereof, or a false designation of origin, false or misleading
description of fact, or false or misleading representation of fact, which in commercial advertising
or promotion, misrepresents the nature, characteristics, and qualities of the counterfeit Roche
products.
178. Defendants advertised, marketed and promoted the counterfeit Roche
products to the public, and/or to specific segments of the public, using Plaintiffs’ Accu-Chek
Marks and Accu-Chek Trade Dress, as well as other intellectual property belonging to Plaintiffs.
179. Defendants’ actions constitute willful infringement of Plaintiffs’ exclusive
rights in the Accu-Chek Marks and the Accu-Chek Trade Dress.
180. As a direct and proximate result of Defendants’ conduct, Plaintiffs have
suffered irreparable harm to the valuable Accu-Chek Marks and Accu-Chek Trade Dress, and to
their reputation in the industry. Unless Defendants’ conduct is restrained, Plaintiffs will continue
to be irreparably harmed.
181. Plaintiffs have no adequate remedy at law that will compensate for the
continued and irreparable harm they will suffer if Defendants’ acts are allowed to continue.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 41 of 43 PageID #: 41

-42-
15066876
182. As a direct and proximate result of Defendants’ conduct, Plaintiffs have
suffered damages to the valuable Accu-Chek Marks and Accu-Chek Trade Dress, and other
damages in an amount to be proved at trial.
PRAYER FOR RELIEF
WHEREFORE, Roche demands judgment against all Defendants as follows:
(a) an order entering judgment in favor of Roche against Defendants, jointly and
severally;
(b) an order temporarily restraining, and preliminarily and permanently enjoining
Defendants from engaging in the unlawful behavior described above, and from selling, or
distributing, any Accu-Chek
®
or other Roche products;
(c) an order awarding Roche damages in an amount to be determined, including without
limitation actual damages, punitive damages, statutory damages, and an accounting of and/or
disgorgement of Defendants’ profits;
(d) an order awarding Roche a trebling of damages;
(e) an order awarding Roche pre-judgment and post-judgment interest;
(f) an order awarding Roche reasonable attorneys’ fees and other costs; and
(g) for such additional relief as the Court finds just, equitable, and appropriate.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 42 of 43 PageID #: 42

-43-
15066876
JURY DEMAND
Roche hereby requests a jury trial on all claims and issues asserted in this
Complaint.
DATED: May 20, 2024
New York, New York
By:
Geoffrey Potter
Timothy Waters
Rhick Bose
PATTERSON BELKNAP WEBB & TYLER LLP
1133 Avenue of the Americas
New York, NY 10036-6710
Tel: (212) 336-2000
Fax: (212) 336-2222
Attorneys for Plaintiffs Roche Diabetes Care, Inc.,
Roche Diabetes Care GmbH, and
Hoffmann-La Roche, Inc.
Case 1:24-cv-03625-DG-RML Document 1 Filed 05/20/24 Page 43 of 43 PageID #: 43
