
HAL Id: hal-03406814
https://hal.science/hal-03406814
Submitted on 28 Oct 2021
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-
entic research documents, whether they are pub-
lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diusion de documents
scientiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Click and Bioorthogonal Chemistry: The Future of
Active Targeting of Nanoparticles for Nanomedicines?
Ludivine Taiariol, Carole Chaix, Carole Farre, Emmanuel Moreau
To cite this version:
Ludivine Taiariol, Carole Chaix, Carole Farre, Emmanuel Moreau. Click and Bioorthogonal Chem-
istry: The Future of Active Targeting of Nanoparticles for Nanomedicines?. Chemical Reviews, 2022,
122 (1), pp.340-384. �10.1021/acs.chemrev.1c00484�. �hal-03406814�
1
Click and bioorthogonal chemistry: the future
of active targeting of nanoparticles for
nanomedicines?
Ludivine Taiariol, Carole Chaix
*
, Carole Farre and Emmanuel Moreau
*
2
ABSTRACT
Over the years, click and bioorthogonal reactions have been the subject of considerable
research efforts. These high-performance chemical reactions have been developed to meet
requirements not often provided by the chemical reactions commonly used today in the
biological environment, such as selectivity, rapid reaction rate and biocompatibility. Click and
bioorthogonal reactions have been attracting increasing attention in the biomedical field for
the engineering of nanomedicines. In this review, we study a compilation of articles from
2014 to the present, using the terms “click chemistry and nanoparticles (NPs)” to highlight
the application of this type of chemistry for applications involving NPs intended for biomedical
applications. This study identifies the main strategies offered by click and bioorthogonal
chemistry, with respect to passive and active targeting, for NP functionalization with specific
and multiple properties for imaging and cancer therapy. In the final part, a novel and
promising approach for “two step” targeting of NPs, called pretargeting (PT), is also
discussed; the principle of this strategy as well as all the studies listed from 2014 to the
present are presented in more detail.
CONTENTS
INTRODUCTION ................................................................................................................... 3
1. Meta-analysis from the PubMed database using the term “click chemistry and
nanoparticles” ................................................................................................................... 5
1.1 NP type ............................................................................................................... 5
1.2 The type of click chemistry .................................................................................. 7
1.1.1. Copper(I)-catalyzed Azide-Alkyne [3+2] Cycloaddition (CuAAC) ................ 8
1.1.2. Strain-Promoted Alkyne-Azide Cycloaddition (SPAAC) .............................. 9
1.1.3. Photoinitiated thiol-ene reaction ................................................................10
1.1.4. Inverse-electron-demand Diels-Alder [4+2] (IEDDA) .................................10
1.3 The role of click chemistry in NP functionalization ..............................................10
2. Click chemistry and passive targeting NPs .............................................................13
2.1 Passive targeting with payload encapsulation or complexation NPs ...................13
2.1.1 Encapsulation approaches ........................................................................15
2.1.2 Complexation approaches .........................................................................17
2.2 Passive targeting with surface-functionalized NPs .............................................17
2.2.1 Surface functionalization with anticancer drugs .........................................17
2.2.2 Surface functionalization with imaging agents ...........................................21
3. Influence of active targeting using click chemistry on tumor accumulation and
cellular uptake: non-targeting vs targeting NPs ................................................................23
3.1 Vitamins .............................................................................................................28
3.1.1 Folic acid ...................................................................................................28
3.1.2 Biotin .........................................................................................................30
3.2 Carbohydrates or polysaccharides .....................................................................30
3.2.1 Hyaluronic acid .........................................................................................31
3.2.1 Glycosides ................................................................................................32
3
3.3 Aptamer ligands .................................................................................................32
3.4 Peptides .............................................................................................................33
3.4.1 RGD peptides ...........................................................................................33
3.4.2 Cell-penetrating peptide (CPP)..................................................................35
3.4.3 Other peptides ..........................................................................................36
3.5 Protein ligands ...................................................................................................37
3.6 Monoclonal antibodies (mAbs) ...........................................................................37
3.7 Other nano-conjugates .......................................................................................39
4. Bioorthogonal chemistry and pretargeting (PT) systems for NP delivery .................40
4.1 PT approaches with ligands as targeting agents ................................................41
4.2 PT approaches with NPs as a “platform” ............................................................47
5. Click chemistry for multifunctionalized NPs .............................................................50
5.1. NPs using click chemistry for multimodal imaging ................................................54
5.2. NPs using click chemistry for a theranostic approach ...........................................56
5.3. NPs using click chemistry for imaging modality and a theranostic approach .........60
6. Nanoparticles, click chemistry and protein corona ..................................................61
7. CONCLUSIONS AND OUTLOOK ...........................................................................63
AUTHOR INFORMATIONS ..................................................................................................65
Corresponding Authors .........................................................................................................65
Authors .................................................................................................................................65
Author Contributions .............................................................................................................65
Notes ....................................................................................................................................65
Biographies ..........................................................................................................................66
ACKNOWLEDGEMENTS .....................................................................................................66
ABBREVIATIONS ................................................................................................................66
REFERENCES .....................................................................................................................70
INTRODUCTION
Nanotechnologies are an integral part of our daily life, whether at work, at home or on
holiday. An emerging development strategy has made a place for nano-based ingredients
(nanomaterials) in consumer markets, such as paints, building materials, cosmetics, food,
automobiles, electronics, pharmaceuticals, energy and materials, to name but a few.
1
The
ISO/TS 800004-1 (2015) standard defines a nanomaterial as a “material with any external
dimension in the nanoscale (size range from approximately 1-100 nm) or having an internal
structure or a surface structure in the nanoscale” and providing unique physicochemical
properties (e.g. large surface area, high loading capacity, controlled size and shape). We can
distinguish two main categories of nanomaterials in this nanoscale: (i) nano-objects
(nanoparticles (NPs), nanofibers, nanotubes and nano-sheets); and (ii) nano-structured
materials (aggregates and agglomerates of nano-objects, nanocomposites and nanoporous
materials).
2
Over the past two decades, NPs have emerged as promising tools in many
4
scientific fields, including medicine. Nanomedicine has revolutionized the treatment of a
number of pathologies including atherosclerosis,
3
cardiovascular
4
and neurological
disorders,
5
infectious diseases,
6
diabetes
7
and endocrine disorders,
8
arthritis,
9
osteoarticular
pathologies
10
and cancer.
11,12
Moreover, 70% of nanomedical products in the aforementioned
medical specialties concern oncology, with 31% being in phase III clinical trials
2
in the fields
of diagnosis, imaging and treatment.
13,14
However, only 15 anticancer drugs have been
released on the market since 2017.
15
There are a number of reasons for this somewhat
surprising situation. The first is that the results obtained in the preclinical stage can be
different from those of the clinical stages because the EPR effect largely depends on the
tumor microenvironment, which differs between models and individuals. Indeed, several
works from the past decade indicate that only 0.7% of injected NPs are found in the
tumors.
15,16
The second reason is a lack of reproducibility, which can be attributed to multiple
factors, including design study, protocol, material quality and purity.
In parallel to the large-scale development of nanomaterials, another revolution in the field of
biology and medicine has been the advent of click and bioorthogonal chemistries. These
highly effective chemical reactions have been developed to fulfil the stringent rate, selectivity
and biocompatibility requirements for targeting and labelling biomolecules in biological
media.
Click chemistry was first described in 2001 by Sharpless et al. as a highly selective reaction
occurring in mild aqueous conditions, providing good yields and favorable reaction rates
compared with traditional reactions.
17,18
For the development of organic molecules, it
represents a means of obtaining a set of powerful, selective, and modular building blocks,
such as an azide and an alkyne, that work on both small and large scales. Two important
features make the click reaction a new approach in pre-clinical and clinical studies. Firstly,
the functional groups of the reactant and those of the product do not interact with the
functional biomolecules. Secondly, this kind of reaction takes place easily under mild
conditions and in aqueous solvents, providing the best yields and highest rates. Shweta
Verma recently defined click chemistry as "an interesting and novel approach to drug
discovery, materials science, bioconjugation, radiochemistry and nanoscience. It meets an
ever-increasing need for rapid reaction, as it fulfils the criterion of ideal synthesis: efficiency,
versatility, selectivity and high yield with a variety of starting materials”.
19
Historically, the first
so-called bioorthogonal reaction developed in vivo was the Staudinger reaction between an
azide group and a phosphine that occurred under aqueous conditions with no toxic catalyst.
20
However, its reaction kinetics were too slow, which led to the development of strain-
promoted azide-alkyne cycloaddition (SPAAC) and inverse electron-demand Diels-Alder
(iEDDA) reactions, thus allowing for rapid and specific covalent bond formation under
aqueous conditions without the need for toxic catalysts. In the field of biomedical research, in
particular, this has opened a new paradigm, proving that artificial chemical reactions can take
place on cell surfaces, in the cytosol of cells or inside the body, which is not easily achieved
with toxic catalysts.
21
Based on these observations and new advances in chemical synthesis,
we wanted to investigate whether click chemistry has also had a major impact in the field of
the nanoparticle (NP), and particularly nanomedicine for oncology.
To determine the role of click chemistry in the world of NPs, we first used the search term
“bioorthogonal chemistry nanoparticles” in the PubMed database, because bioorthogonal
5
reactions are normally defined as copper-free and therefore facilitate nanomedicine
applications. Surprisingly, we identified only 167 articles for the period 2010-2020 and only
139 from 2014 to recently. Hence, we used the search term “click chemistry nanoparticles”
and identified 1,170 articles, including 848 from 2014. We suggest that this major difference
between search terms might be attributed to inappropriate use of the term “click” by authors
whose articles concern bioorthogonal chemistry. Indeed, several articles were common to
both searches while the other 37, included in this analysis, did not appear in the “click
chemistry” search.
Finally, the most representative panel of 848 articles was classified according to several
criteria such as NP type (liposomes, dendrimers, quantum dots, silica NPs, gold NPs, etc.),
the nature and role of click chemistry (“decoration” or molecule grafting, passive targeting,
and in case of active targeting, the nature of the targeting molecules used). This resulted in a
selection of 319 articles; the others were excluded because they did not fall within the field of
oncology or because of a lack of information. In this review, we describe new modification
possibilities for NPs and highlight the contribution of click chemistry (i.e. copper catalyst and
copper catalyst-free reactions) to the registration of functional organic and inorganic NPs for
cancer imaging and therapy. This includes recent applications in which the reactions
themselves have been used for functionalization and for coupling ligands to NPs. Finally, we
discuss the impact and future of click and bioorthogonal chemistries in the area of NPs.
1. Meta-analysis from the PubMed database using the term “click chemistry and
nanoparticles”
In order to gain a better insight into the possible impact of click chemistry in nanomedicine,
we conducted a detailed analysis of these 319 articles. Firstly, we analyzed whether all types
of nanoparticles were concerned by click chemistry and which class of click chemistry was
the most used (CuAAC, SPAAC or IEDDA). We also analyzed the different articles to
determine the different applications of click chemistry in the world of nanomedicine.
1.1 NP type
Trends may be observed in data concerning the different types of nanomaterials used for
click chemistry (Figure). Polymeric NPs and dendrimers tend to be the most commonly used,
representing approximately 72% of the references when combined as organic nanomaterials,
unlike micelles and liposomes, which represent only 16%. This is probably due to the wide
use of polymers to coat NPs in order to make them biocompatible and easily functionalizable
on the surface. Polymers are soft materials that circulate more easily in blood vessels and
prevent aggregation and clogging risks. Furthermore, dendrimers are excellent agents for in
vivo diagnostics involving imaging and therapy because they have a large number of reactive
groups to be functionalized and high solubility in the aqueous phase. This results in a high
functionalization capacity for targeted cancer treatments and bioimaging devices.
22–25
The
high solubility of liposomes and their rapid elimination from systemic circulation could be an
advantage for biological applications.

6
Figure 1. Analysis of the different types of NPs using the search term “click chemistry and nanoparticles” in
articles published from 2014. (a) Organic materials, (b) Inorganic materials.
Silica (SiO
2
), gold (Au) and iron oxide (Fe
3
O
4
) NPs account for 75% of inorganic NPs and
offer an interesting click chemistry modification platform for both imaging and therapy.
AuNPs are widely used as multifunctional NPs because they can be decorated by targeting,
therapeutic and fluorescent molecules and, at the same time, they can be used for
photothermal (PTT) or photodynamic therapy (PDT) thanks to their surface plasmon
resonance properties
26
and as contrast agents for CT imaging.
27
Silica is a material of choice
for biomedical applications because of its stability, versatility and biocompatibility. This
material can also be applied as a coating to a number of metallic systems, such as Fe
3
O
4
NPs and AuNPs, to improve their biocompatibility.
28–31
The stable SiO
2
NP category includes mesoporous silica NPs (MSNs), which are commonly
employed for their capacity to encapsulate or absorb cargo (e.g. anticancer drugs, siRNA,
plasmid DNA and peptides) into large porous volumes (> 0.9 cm
3
/g) for stimuli-responsive
drug release.
32
MSNs are notably used to improve drug accumulation and the therapeutic
effects of insoluble hydrophobic anticancer drugs.
33
They provide adaptable pore size, a wide
surface area for functionalization (> 900 m
2
/g), and have high thermal and chemical
stability.
34,35
These diverse properties highlight the advantages of this gatekeeper for imaging,
36
targeting
37
and therapy.
38
Fe
3
O
4
NPs, also referred to as “Ultra Small SuperParamagnetic
Iron Oxide” NPs (USPION, diameter < 50 nm) or “SuperParamagnetic Iron Oxide” NPs
(SPION, diameter with a range of 50-500 nm), have attracted a great deal of interest in the
field of click chemistry due to their numerous biomedical applications. These nanomaterials
have magnetic properties that have been largely exploited for magnetic-resonance imaging
(MRI),
39
sometimes combined with positron-emission tomography (PET)
40
and/or optical
imaging.
41
In therapeutic approaches, they are used either for the delivery of conventional
gene
42
or chemotherapies,
43
or for the hyperthermal destruction of cancer cells.
44
Their
surface, usually coated with a matrix of silica, polysaccharides (e.g., dextran) or polymers
(e.g., polyethylene glycol, PEG), is suitable for functionalization with a wide variety of
targeting or therapeutic molecules.
45,46
CdSe
ZnS
Silica/MSNs
20%
Gold
25%
Iron/MNPs/SPION
27%
QDs
7%
MOF
4%
Diamond
4%
UCNPs
4%
Others*
9%
Polymeric
38%
Dendrimers
29%
Micelles
13%
Liposomes
8%
Hydrogels
5%
Others*
7%
*Ag, Pt, Zr, Cu, CeO
2
, TiO
2
, CoFe
3
O
4
, ZnO, Carbon nanotubes, silicon,
silsesquioxane based NPs.
Inorganic nanomaterials
*Cationic polypeptide, graphene, chitosan, cellulose, cyclodextrin, gelatin oleic
and lipid based NPs.
Organic nanomaterials
(a) (b)
Fe
3
O
4

7
1.2 The type of click chemistry
The surface chemical modification of a wide variety of organic and inorganic nanomaterials
has been extensively investigated over the past few years. The incorporation of chemical
functions onto the surface of the NPs allows subsequent functionalization to gain either
stability or specific properties. Oligonucleotides, monoclonal antibodies (mAbs), proteins and
peptides, for instance, can be attached by electrostatic interactions or by covalent linkage.
Thiol, phosphine oxide, phosphonate and carboxylate groups are employed too, particularly
in metal oxide NPs.
47
Surface modification can usually be carried out by either multi-step or
one-step functionalization.
48
Chemical reactions, such as carbodiimide-mediated coupling
between carboxyl and amine, succinimidyl ester-amine and maleimide-thiol are commonly
used standard bioconjugation reactions (Figure).
49,50
More detailed information on
functionalization strategies for the incorporation of multiple functions and/or biomolecules on
the surface of nanomaterials is presented in the review by De Crozals et al.
34
Figure 2. Traditional reactions for NP bioconjugation. (a) Carbodiimide coupling, (b) Succinimidyl ester-amine
reaction, (c) Maleimide-thiol reaction. In the schemes, the symbols R, R
1
, R
2
and R
3
refer to any substituent.
Conventional reactions, such as amine-activated ester acylations, are widely used for
derivatizing NPs with proteins in vitro. Nevertheless, reaction conditions such as temperature
and pH must be considered. For instance, the use of carbodiimide coupling reactions can be
subject to hydrolysis after formation of the reactive intermediate o-acylisourea using 1-ethyl-
3-(3-dimethylaminopropyl)carbodiimide (EDC), thus limiting overall yields.
51
Furthermore,
since the latter is only slightly soluble, the number of activated carboxyl groups present on
the NPs must be controlled to avoid the risk of losing colloidal stability and encouraging
aggregation. The agent N-hydroxysuccinimide (NHS) or the more hydrophilic agent N-
hydroxysulfosuccinimide (Sulfo-NHS) can be used in conjunction with EDC to improve the
amino-group coupling yield to the activated ester. Coupling chemistries are not specific
enough to ensure the binding strength of conjugations and biomolecule orientation, which are

8
essential for biological functions. For instance, mAbs and proteins possess many different
amino acid functional groups (e.g., aspartic acid, glutamic acid) which can react with each
other, producing undesirable byproducts and competing with NP-protein binding. The
development of more specific reactions could help to control specific binding sites. Similarly,
multifunctional NPs with several functional molecules often generate non-specific adsorption
and uncontrolled grafting, possibly causing unexpected hydrophobic or electrostatic
interactions.
34
Reactions may occur between the functional group and the particle surface, for
instance back-bonding of amino or ammonium groups with negatively charged NPs.
48
It is
therefore necessary to develop simple but more efficient reactions to produce heteroatom-
linked molecular systems in a specific manner. In this context, click chemistry has
demonstrated its superior ability to provide all these properties, compared with traditional
chemical methods. This attractive chemistry is the focus of this review, and the different
types of click chemistry commonly used are presented in more detail in the following
sections.
1.1.1. Copper(I)-catalyzed Azide-Alkyne [3+2] Cycloaddition (CuAAC)
Our meta-analysis reveals that the most documented click reaction for NPs in the literature is
copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC), representing more than the half of
the listed publications (Figurea).
Figure 3. Graphic representations of CuAAC, SPAAC, thiol-ene and IEDDA applications for NPs according to
publications from 2014 to 2020. (a) Total number of publications for each type of click chemistry, (b) Evolution
over time of the total number of publications for each type of click chemistry, (c) Proportion of publications
concerning active and passive targeting NPs.
In recent years, a number of studies have investigated Cu-catalyzed cycloaddition and
demonstrated its effectiveness for the surface modification of organic and inorganic NPs with
molecules and biomolecules. CuAAC between an azide and a terminal alkyne generates a
0
20
40
60
80
100
120
140
160
CuAAC SPAAC Thiol ene IEDDA
Number of publications
(c)
(b)(a)
*Aptamers and small organic molecules (estradiol,
arginine, acetazolamide, afatinib).
Conception/Coating
of NPs 65%
Conjugation of
non-
targeting
molecules
35%
Active targeting
NPs
41%
Passive
targeting NPs
59%
Vitamins
19%
Carbohydrates/
Polysaccharides
13%
Peptides
38%
Proteins
4%
Monoclonal
antibodies
18%
Others*
8%
0
10
20
30
40
50
2014 2015 2016 2017 2018 2019 2020
Number of publications
CuAAC
SPAAC
Thiol-ene
IEDDA

9
stable 1,2,3 triazole
17
with a fast reaction speed (second-order rate constant k
2
up to 10
4
M
-
1
s
-1
)
52
and reproducible results in physiological conditions (Figure).
18
Modification of the NP
surface for specific cell interactions generally involves a first step allowing chemical binding
of an alkyne or azide on the surface, followed by a second step to add the biomolecules.
However, the use of CuAAC has been in decline since 2016 (Figureb). This could be due to
the metal catalyst of the reaction, Cu(I), which strongly limits its use in biological settings
because of the cytotoxicity induced by possible interaction or chelation with biomolecules
53
or
in the preparation of imaging tools because of the fluorescence reduction for certain proteins
(e.g. green fluorescent protein, GFP) and quantum dots (QDs).
52,54,55
CuAAC can also form in
situ complexes with some compounds, possibly making it more difficult to quantify the
reaction product and purity.
56
For example, Williams and co-workers describe a reduction of
cell viability due to inadequate methods for removing all the Cu ions after functionalizing
nanoporous silica NPs with polysialic acid.
57
Moreover, Cu(I) may be unstable in aqueous
solutions at this degree of oxidation. This reaction is not suitable for in vivo coupling.
Figure 4. Copper(I)-catalyzed azide-alkyne [3+2] cycloaddition (CuAAC) reaction scheme. In the scheme, the
symbols R and R
1
refer to any substituent.
1.1.2. Strain-Promoted Alkyne-Azide Cycloaddition (SPAAC)
The drawbacks of copper have led many research projects to turn to biocompatible catalyst-
free click reactions, such as SPAAC, developed in 2010 by Bertozzi and coworkers.
58
Between 2014 and 2020, approximately 20% of the studies recorded concern SPAAC
cycloaddition (Figurea), which consists in using strain-stable carbocyclic alkynes, called
cyclooctynes, to allow the reaction with azide moieties (Figure). SPAAC does not require a
catalyst and is inert towards biomolecule functional groups and other functions found in
biological environments. However, the relatively slow reaction rate of azides (N
3
) with
dibenzocyclooctyne groups (DBCO) (k
2
= 0.2-0.5 M
-1
s
-1
)
52
can limit in vivo applications.
Attention therefore turned to another stable aliphatic cyclooctyne called bicyclo[6.1.0]nonyne
(BCN), which offers accelerated reaction rate constants up to 2.9 M
-1
s
-1
.
59
Figure 5. Strain-promoted alkyne-azide cycloaddition (SPAAC) reaction scheme. In the scheme, the symbols R
and R
1
refer to any substituent.
CuAAC
Azide (N
3
) + Alkyne
SPAAC
Azide (N
3
) + Dibenzocyclooctyne (DBCO)
Azide (N
3
) + Bicyclononyne (BCN)

10
1.1.3. Photoinitiated thiol-ene reaction
The thiol-ene reaction has also been investigated by scientists for NP functionalization.
60,61
Thiol-ene involves a reaction between a thiol (SH) and an alkene group to form an alkyl
sulfide and can be initiated by free-radical addition (photoinitiation, thermal or redox)
(Figure). This reaction is mainly involved in the synthesis of dendrimers and polymers
62
due
to the fact that it can effectively perform radical-based photopolymerization reactions for
staged growth (propagation and chain transfer) and chain growth (polymerization), combining
all the benefits of click chemistry.
63
Figure 6. Photoinitiated thiol-ene reaction scheme. In the scheme, the symbols R and R
1
refer to any substituent.
1.1.4. Inverse-electron-demand Diels-Alder [4+2] (IEDDA)
IEDDA cycloaddition occurs between an electron-rich dienophile, such as trans-cyclooctene
(TCO) or norbornene, and an electron-poor diene called 1,2,4,5-tetrazine (Tz). This is
another click reaction of interest for nanomaterials with an excellent reaction rate (k
2
= 10
4
M
-
1
s
-1
). Although IEDDA is the fastest click chemistry reaction, it is rarely described for NP
functionalization (Figurea,b and 7), remaining most commonly used for two-step protocols
(i.e. pretargeting strategies) to deliver NPs to the cells (see Part 4 of this manuscript).
64,65
Figure 7. Inverse-electron-demand Diels-Alder [4+2] (IEDDA). In the scheme, the symbols R, R
1
and R
2
refer to
any substituent.
1.3 The role of click chemistry in NP functionalization
Several trends can be observed in the six-year analysis concerning the field of
nanomedicines (Figurec). First of all, it should be noted that 41% of the click chemistry
reactions described above are used for active targeting, allowing NPs to achieve specific cell
targeting prior to functionalization by several ligands/biomarkers of interest such as peptides,
vitamins, mAbs, carbohydrates and proteins.
66,67
59% of publications describe click reactions
for passive targeting. 65% of the articles describe proofs of concept (no particular
applications) for NP design (e.g. dendron assembly for dendrimers, polymer assembly for
polymeric NPs) and coating (for example with polymers or polyethylene glycol units). The
hν
Thiol-ene
Thiol (SH) + Alkene
IEDDA
Trans-cyclooctene (TCO) + Tetrazine (Tz) Norbornene + Tetrazine (Tz)
11
conjugation of non-targeting molecules, firstly for encapsulation (e.g. polymers, polyethylene
glycols, stimuli-responsive molecules) or complexation (e.g. amylose and carboxylic
functions), and secondly for surface functionalization (e.g. anti-cancer drugs, contrast agents
and stimuli-responsive linkers) constitutes the remaining 35% of the listed articles (Figure).

12
Figure 8. Different applications of click chemistry reactions for NP functionalization.
13
2. Click chemistry and passive targeting NPs
Two main strategies are reported to achieve site-specific delivery of NPs: active targeting
and passive targeting, which is also called the EPR effect, described for the first time in 1986
by Maeda and co-workers.
68
Most nanomedicine research has been devoted to passive
targeting. Indeed, the tumor microenvironment (TME) has numerous functional abnormalities
which enable NPs in the 20-200 nm size range to diffuse into tissues and gather in the tumor
region. Tumor tissue includes an unnatural vasculature barrier that is poorly organized and
irregular in shape; once the NPs have passed through the intercellular gaps in this barrier
(i.e. extravasation), weak lymphatic drainage allows them to remain in the tumor.
16,69,70
For
most of the articles investigated, click chemistry was used to develop passive targeting NPs
to incorporate therapeutic agents either (i) by encapsulation/complexation and/or (ii) by
adding them to the surface of the nanoparticles. These two aspects are more
comprehensively developed in the following sections.
2.1 Passive targeting with payload encapsulation or complexation NPs
Based on the EPR effect for effective transfer into tumors, NPs were first exploited because
of their capacity to encapsulate drugs. The first passively targeted nanomedicine approved
by the FDA in 1995 for clinical use, now considered to be one of the most competitive
chemotherapeutic systems, was PEGylated liposome embedding doxorubicin (DOXIL™ in
the US, Caelyx™ in other countries). This nanocarrier has been found to increase blood
circulation time and has the potential to enhance tumor accumulation in a broad range of
cancers (sarcoma, breast, myeloma and ovarian).
71
Other successful liposomal drug delivery
methods are also now used in clinics, such as Abraxane™, an albumin-bound-particle, for
metastatic breast cancer and pancreatic adenocarcinoma.
72
Liposomal encapsulation of
paclitaxel produces more effective response rates than the free drug. Similarly,
DaunoXome™ and Myocet™, non-PEGylated 50 nm liposomal daunorubicin and 150 nm
liposomal doxorubicin, respectively, have also been developed as NPs to enhance the EPR
effect. Among cancer therapies, paclitaxel-loaded polymeric micelles (i.e. Genexol®-PM)
have been approved in Korea for breast, lung and pancreatic cancers.
73
As mentioned
above, paclitaxel (PTX), daunorubicin, doxorubicin (DOX) are the most commonly embedded
anti-cancer drugs; however, others have recently emerged, such as cisplatin (CDDP),
methotrexate (MTX) and docetaxel. Over the past two decades, various nanocarrier systems
encapsulating chemotherapeutic drugs have been developed to improve anti-cancer effects,
while reducing their potential toxicity. There are major advantages to encapsulation systems:
i) they extend the half-life of the loaded drug, ii) they increase drug exposure to tumors by
exploiting the EPR effect, iii) they improve bioavailability and therefore the therapeutic index.
The increasing number of preclinical in vitro and in vivo studies describing passively and
non-targeted nanocarriers is encouraging in terms of transfer to the clinical stage.
In this study, the articles listed highlight the fact that click chemistry contributes to the
encapsulation of therapeutic agents by entrapping with PEG polymers, or to the formation of
dendrimers and polymeric NPs, and to drug complexation by incorporating molecules such
as amylose or carboxylic functions (Table 1). Generally speaking, the encapsulated
therapeutic drugs are released by endogenous or exogenous stimuli, such as pH,

14
temperature, redox, enzyme, or by a combination of triggers for efficient tumor
accumulation.
74
MSNs, polymeric NPs and micelles are the most commonly employed NPs described in the
publications as pH-responsive drug carriers using click chemistry. They are usually designed
to encapsulate therapeutic molecules and to be taken up by an endocytosis route, then
hydrolyzed by endosomes or lysosomes, resulting in higher toxicity for tumor cells than free
drugs.
Table 1. Overview of passively targeting NPs (as drug carriers) using click chemistry for the encapsulation and
complexation of drugs, from 2014 to nowadays.
Type of click
chemistry and use
(grafted click
function)
Type of NPs
(grafted click
function)
Drug loading
Release
mechanism
Effects
In vitro/In
vivo studies
Refs.
CuAAC, formation of
micelles
Nanomicelles
(Alkyne/N
3
)
DOX, MPLA
adjuvant
n.d
Cytotoxicity and
anticancer
activity
yes/yes
75,76
CuAAC, synthesis of
dendrimers
β-cyclodextrin
dendrimers
MTX
Acidic release
Cytotoxicity
yes/no
77
CuAAC, ring-opening
metathesis
copolymerization
(ROMP)
Polymeric
Vorinostat
Acidic release
Cytotoxicity and
anticancer
activity
yes/yes
78
CuAAC, incorporation of
labile Schiff base for
acidic control release
Polymeric
DOX
Acidic release
Cytotoxicity
yes/no
79
CuAAC, incorporation of
Pt(IV) prodrug (Alkyne)
Nanomicelles (N
3
)
Pt(IV), DOX
Endosome
degradation
Cytotoxicity
yes/no
80
CuAAC, incorporation of
photo-responsive
molecules (N
3
/Alkyne)
MSNs (N
3
/Alkyne)
DOX
Photo-
responsive
release
Cytotoxicity
yes/no
81
CuAAC, modification of
side chain polymeric
NPs with PEG (Alkyne)
Polymeric (N
3
)
CPT
Ester linker
hydrolysis
Cytotoxicity and
anticancer
activity
yes/yes
82
CuAAC, surface
incorporation of PEG
(Alkyne)
NMOFs (N
3
)
Dichloroacetic
acid
pH-
responsive
release
Cytotoxicity
yes/no
83
CuAAC, incorporation of
amylose (N
3
)
Cationic amylose-
based dendrimers
(Alkyne)
Plasmid DNA
n.d
Gene
transfection
efficacy
yes/no
84
CuAAC, conjugation of
dextran (N
3
)
Dextran-based
PAMAM dendrimers
(Alkyne)
Plasmid DNA
n.d
Gene
transfection
efficacy
yes/no
85
CuAAC, incorporation of
amylose (N
3
)
Cationic amylose-
based dendrons
(Alkyne)
Thrombin
n.d
Hemostatic
activity
yes/yes
86
Thiol-ene, incorporation
of silyl ether produg
(Allyl)
MSNs (SH)
CPT
Acidic release
Cytotoxicity
yes/no
87
Thiol-ene, incorporation
of carboxyl groups for
CDDP chelation
Dendrimers (Allyl)
CDDP, PTX
n.d
Cytotoxicity and
anticancer
activity
yes/yes
88
Thiol-ene, incorporation
of carboxyl groups
(maleic anhydride) for
drug chelation
MSNs (SH)
DACH-Pt
n.d
Cytotoxicity
yes/no
89

15
n.d: not determined, N
3
: azide, SH: thiol, DOX: doxorubicin, MTX: methotrexate, CPT: camptothecin, CDDP: cisplatin, Pt(IV):
Platinum(IV), DACH-Pt: dichloro(1,2-diaminocyclohexane)platinum(II), MSNs: mesoporous silica nanoparticles, NMOFs: metal-organic
framework nanoparticles.
“Effects” indicate the NP effects on cancer cells in vitro and/or in vivo.
“In vitro/in vivo studies” indicate whether in vitro or in vivo tests were performed (yes) or not (no) in the article.
2.1.1 Encapsulation approaches
Examples of CuAAC
In a recent study, Mei et al. described azide and alkyne micellar nanocarriers embedding
paclitaxel PTX, demonstrating an improvement of tumor retention and anti-tumor effects for
the treatment of lymph node metastasis of breast cancer.
75
After the 25 nm micelles (S-PTX)
were accumulated in the tumor, catalysts (copper sulfate and sodium ascorbic acid) were
directly injected intratumorally to initiate CuAAC between the micelles. This process enabled
the formation of larger micelles (S-PTX (+), 100 nm) which were better retained in the tumor
by the EPR effect 12 hours after injection compared with S-PTX and pre-formed larger
micelles (L-PTX) (p<0.1). These S-PTX (+) nanomicelles showed significant antitumor
activity with 76% tumor suppression vs 49% for L-PTX and 57% for S-PTX.
The same authors also applied this strategy for immunochemotherapy with co-delivery of
doxorubicin DOX and monophosphoryl lipid A (MPLA), and demonstrated effective tumor
volume suppression in combination with anti-PD-L1 (M-DOX/MPLA(+) + anti-PD-L1),
contrasting with M-DOX/MPLA(+) without anti-PD-L1 for which tumor progression restarted
10 days after administration.
76
In 2015, Toomari and co-workers developed β-cyclodextrin (β-
CD) dendrimers based on CuAAC click chemistry.
77
The encapsulation properties of β-CD
enable the loading of numerous MTX drugs which are better released over time in acidic (pH
3) rather than physiological pH conditions.
Other research has also been carried out on the use of NPs for encapsulating bioactive
molecules to improve their pharmacokinetics. pH-responsive polymeric NPs have been
developed to embed Vorinostat.
78
This molecule is known to treat cutaneous T-cell
lymphoma by inhibiting the protein histone deacetylases (HDAC) overexpressed in cancer
cells. The CuAAC reaction has been used to incorporate alkyne Vorinostat on
azidomacromonomer before ring-opening metathesis copolymerization, a process for chain-
growth polymerization, using norbornene to drive the reaction. The authors thus developed a
successful triggered-delivery system, which enhanced delivery in tumors thanks to the EPR
effect and cellular internalization by endocytosis in an acid environment (pH < 6).
In the same year, Yu et al. developed another biodegradable pH-responsive polymeric NP
encapsulating DOX.
79
CuAAC was of interest in this study because it does not involve any
protection/deprotection of the aliphatic polyester (polylactide, PLA) scaffolds typically used
after polymerization. Alkyne-functionalized PLA was prepared for the incorporation of an
azido-acid-labile Schiff base permitting controlled release of the DOX (pH 5.5).
Nanoprecipitation, followed by addition of a biocompatible PEG surfactant enabled the
formation of dispersible DOX-loaded NPs presenting maleimide functions available for the
potential incorporation of targeting moieties via thiol-maleimide chemistry. In vitro studies on
MCF-7 breast cancer cells showed a slightly larger decrease in cell viability compared with
the free drug, suggesting promising results in vivo using passive tumor targeting. In another
study, pH-responsive micelles were synthesized for the co-delivery of chemotherapeutic
drugs. Although the Pt(IV) prodrug was anchored using CuAAC, DOX was embedded in the
core by physicochemical interactions.
80
Internalized nanomicelles were then deteriorated in

16
endosomes allowing DOX release (pH 5.5). The synergistic effect of both drugs released
resulted in better cytotoxic activity (IC
50
up to 0.02 µM at 72 hours post-incubation in A357
cells) compared with the free drug (IC
50
free DOX: up to 0.13 µM at 72 hours post-incubation
in A357 cells) or micelles with only one drug (IC
50
DOX-loaded micelles: up to 0.14 µM at 72
hours in A357 cells). This system showed improved therapeutic efficacy of the drugs (IC
50
MSN-Pt: 25.95 µM vs IC
50
oxaliplatin: 39.29 µM) thanks to a higher cellular uptake (80.11 ng
Pt/mg protein at 4 hours post-incubation) than with the free drug (0.69 ng Pt/mg protein at 4
hours post-incubation) in HepG-2 cells.
In 2015, Noureddine and co-workers emphasized the benefits of developing the CuAAC
reaction to obtain homogenous multi-functionalizable NP structures. This method resulted in
structures carrying azide and alkyne groups, unlike conventional grafting methods for which
uncontrolled loading and functionalization are often obtained.
81
Click chemistry was used to
develop light- and consequently pH-triggered MSNs containing DOX. Under blue irradiation
(at 365 nm), the two moieties interact by energy transfer (FRET), inducing, upon protonation,
dissociation of the acceptor, decluttering of the pores and finally DOX release.
In another study, Cai el al. demonstrated the utility of the CuAAC reaction for modifying side
chain polymeric NPs with PEG-containing alkynes.
82
Drug polymers (SS-CSPT) were
covalently assembled with PEG using CuAAC, followed by self-assembly by
nanoprecipitation to form particles. These thiol redox-responsive polymeric NPs provided an
efficient controlled drug release system dependent on ester linker hydrolysis. They afforded
cytotoxicity on HeLa cells that was significantly higher than irinotecan but similar to the SN-
38 (IC
50
: 19 nM, 14 nM and 2800 nM, respectively) usually used in clinics, and efficient
anticancer efficacy in vivo on MCF-7 cells (8.6% vs 5.3% of irinotecan).
CuAAC has also been employed with pH-responsive NMOFs to attach PEG to the surface to
improve stability and cellular uptake. This also allowed the incorporation of fluorescent
calceins for endocytosis studies or for therapeutic studies investigating anticancer drugs (e.g.
dichloroacetic acids).
83
The resulting PEGylated NPs (i.e. PEG550 and PEG2000) enhanced
cellular uptake and therefore decreased cell viability at 0.75 mg/mL on HeLa cells compared
with non-PEGylated NPs (50% vs 125%).
Examples of thiol-ene chemistry
In 2017, camptothecin (CPT) prodrug-functionalized MSNs obtained by thiol-ene click
reactions demonstrated efficient release of the drug in acidic conditions (pH 4.0) thanks to
the presence of cleavable silyl ether bonds, with tumor cell inhibition being similar to that
achieved with the free drug (40% cell growth inhibition) (Figure).
87

17
Figure 9. Illustration of an acid-responsive silyl prodrug being from an MSN-SH nanocarrier. Reprinted with
permission from reference
87
. Copyright
2017
Elsevier.
2.1.2 Complexation approaches
Examples of CuAAC
Natural linear polysaccharides are widely used in the biomedical field for their
biodegradability and biocompatibility properties.
90
In 2015, Mai et al. performed CuAAC
chemistry to synthesize a safe dendrimer structure based on amylose from potatoes. The
positively-charged nanocarriers enabled the complexation of anionic plasmid DNA and in
vitro studies confirmed efficient transfection (up to 70% transfection efficiency) without the
use of a targeting ligand.
84
The same authors achieved effective gene transfection in serum
with high generation of PAMAM dendron nanocomplexes conjugated with dextran by
CuAAC.
85
Another team implemented this strategy with clicked amylose dendrons for hemostatic action
through thrombin complexation.
86
The nanocomplexation of thrombin offered better stability
than with native thrombin (activity retention percentage > 50% vs < 5% after 60 days,
respectively). Moreover, it had the same hemostatic effect; firstly, in vitro with fibrinogen
coagulation tests and in vivo on an artery hemorrhage rat model.
Examples of thiol-ene chemistry
The heterogeneity of tumors and increased drug resistance in cancer cells has made
combination chemotherapy a common strategy to treat tumors. Cai et al. described
dendrimer micelles containing two types of anti-tumor drugs: CDDP and PTX.
88
The first was
chelated by carboxyl groups incorporated by thiol-ene chemistry and inhibited DNA
synthesis; while the second was only encapsulated and inhibited cell cycle mitosis. In vitro
studies on ovarian cancer cells (SKOV-3) demonstrated a decrease of IC
50
by combining a
2:1 ratio of CDDP/PTX (IC
50
: 39/19.5 ng/mL) compared with free CDDP and PTX (IC
50
:
1354 and 32 ng/mL, respectively). In vivo, this synergistic effect significantly improved tumor
growth inhibition (relative tumor volume (RTV
CDDP/PTX
) = 2 vs RTV
CDDP
= 4 and RTV
PTX
= 11)
and medium survival time (40 days) compared with just PTX (23 days) or CDDP (34 days)
for the same drug dosage (6 mg/kg CDDP and 3 mg/kg PTX).
Thiol-ene chemistry has also been investigated to design MSNs containing 1,2-bidentate
carboxyl groups for efficient anticancer drug chelation of dichloro(1,2-
diaminocyclohexane)platinum(II) (DACH-Pt).
89
2.2 Passive targeting with surface-functionalized NPs
As well as encapsulation and complexation, some articles concern the use of click chemistry
for: (i) conjugating anticancer drugs and chemotherapeutic agents on the surface of NPs; (ii)
adding stimuli-responsive linkers; and (iii) incorporating reporter molecules or contrast agents
onto the surface of NPs (Table 2).
2.2.1 Surface functionalization with anticancer drugs

18
In general, passive targeting NPs are subsequently delivered intratumorally after EPR
accumulation and drugs are specifically released by pH or enzyme-responsive linkages.
Nanomaterials with enzyme-responsive linkages are ideal for selective delivery of drugs into
tumor cells because of their up-regulation and cellular actions in tumor tissue. Tumor-
associated enzyme matrix metalloproteinase (MMP) is an enzyme of interest that is
overexpressed in cancer cells and able to cleave peptide sequences selectively in the TME
for controlled drug delivery.
91
The specific peptide substrates are usually deposited onto
nanoscale vehicles and act as linkers for the conjugation of antitumor drugs.
Table 2. General overview of passive targeting NPs using click chemistry to incorporate drugs on the surface,
from 2014 to nowadays.
Type of click
chemistry and use
(grafted click function)
Type of NPs
(grafted click
function)
Drug loading
Release
mechanism
Effects
In vitro/In vivo
studies
Refs.
CuAAC, incorporation of
bile acid (Alkyne)
Dendrimers (N
3
)
Bile acid
n.d
Cytotoxicity
yes/no
23
CuAAC, incorporation of
MMP-2 peptide (Alkyne)
and formation of
nanoclusters
AuNPs (N
3
)
DOX
Enzymatic
cleavage (MMP
cleavage)
Anticancer activity
no/yes
27
CuAAC, formation of
nanoclusters between
DOX-AuNPs and MMP-
QDs
AuNPs (N
3
) and
QDs (Alkyne)
DOX
Enzymatic
cleavage
(MMP cleavage)
Cytotoxicity and
anticancer activity
yes/yes
92
CuAAC, incorporation of
gemcitabine
Dendrimers
(Alkyne)
Gemcitabine (N
3
)
Enzymatic
cleavage
(Cathepsin-
sensitive linker)
Cytotoxicity and
anticancer activity
yes/yes
93
CuAAC, incorporation of
DOX on the surface
Dendrons
(Alkyne)
DOX (N
3
)
Enzymatic
cleavage
(Cathepsin-
sensitive linker)
Cytotoxicity and
anticancer activity
yes/yes
94
CuAAC, incorporation of
the neuroprotective drug
(minocycline)
Dendrimers
(Alkyne)
Minocycline (N
3
)
Ester linker
hydrolysis
Treatment of
neuroinflammation
yes/yes
95
CuAAC, incorporation of
oligonucleotides (Alkyne)
during nanogel formation
Nanogels (N
3
)
DOX
Hybridization
reaction and
oligonucleotide
degradation
Cytotoxicity
yes/no
96
CuAAC, incorporation of
HDACi (Alkyne)
Polymeric (N
3
)
HDACi
Acid-responsive
release
Cytotoxicity and
anticancer activity
yes/yes
97,98
SPAAC, Incorporation of
immune cells and
possibility of incorporating
drugs (N
3
)
Dendrimers
(DBCO)
Immune cells
(RAW 264.7)
n.d
n.d
yes/no
99
n.d: not determined, N
3
: azide, DBCO: dibenzocyclooctyne, DOX: doxorubicin, HDACi: histone deacetylase inhibitor, MMP: matrix
metalloproteinase, AuNPs: gold nanoparticles.
“Effects” indicate the NP effects on cancer cells in vitro and/or in vivo.
“In vitro/in vivo studies” indicate whether in vitro or in vivo tests were performed (yes) or not (no) in the article.
Examples of CuAAC
In 2017, bile acid was efficiently grafted by CuAAC click reactions on dendrimers and
demonstrated higher cytotoxicity towards glioma cells with second generation dendrimers (up
to 99.31% inhibition at 50 µM; IC
50
: 10.68 µM) compared with first generation dendrimers (up
to 94.1% inhibition at 50 µM; IC
50
: 14.86 µM).
23

19
Mao et al. recently used click chemistry to form nanoclusters (DOX@AuNCs) between azide-
modified AuNPs bearing DOX (DOX/N
3
@AuNPs) and MMP-2 cleavable peptides with an
alkyne group at each end (Figure).
27
Intravenous injection of DOX@AuNCs in vivo
demonstrated efficient tumor suppression 28 days after injection on human lung carcinoma
tumors compared with DOX@AuNPs and free DOX (tumor volume for DOX@AuNCs,
DOX@AuNPs and DOX: 50mm
3
, 250mm
3
and 280mm
3
, respectively).
Figure 10. Fabrication and anticipated in vivo behaviors of DOX@AuNCs. (A) Fabrication of DOX@AuNCs by
copper(I)-catalyzed azide-alkyne cycloaddition of DOX/N3@AuNPs and APA. Azido groups were immobilized on
AuNPs using N3-PEG-SH and DOX was conjugated to AuNPs by a pH-sensitive hydrazone bond. (B)
Intravenous injection of DOX@AuNCs in tumor-bearing mouse. The DOX@AuNCs could target tumor sites by
EPR effect. Following by entering tumor sites, the APA between AuNPs are digested by abundant MMP-2, and
the digested particles are endocytosed by tumor cell, and finally release DOX in low pH lysosome. The released
DOX could target nucleus and induce cell apoptosis thus suppress tumor growth. Reprinted with permission from
re
27
. Copyright 2018 Elsevier.

20
This anticancer effect was evaluated in vivo with CT images of tumors after accumulation of
Gd-functionalized nanoclusters (Gd@AuNCs). Due to their size (250-300 nm), DOX@AuNCs
were 150 times more retained in the tumor site compared with DOX@AuNPs alone (20-30
nm). This suggests preferential accumulation of DOX@AuNCs at the tumor site due to EPR
effects facilitating the release of DOX (pH < 5.5) after nanocluster dissociation by MMP
cleavage.
Similarly, Kim and colleagues described clicked DOX nanoclusters composed of AuNPs and
QDs for imaging and therapy.
92
They also used a MMP-cleavable peptide to cleave the
particles selectively and release DOX onto AuNPs under reducing conditions using
glutathione. They highlighted the advantage of using passively targeted NPs in vivo for a
prolonged anticancer effect. After 15 days, the nanoclusters demonstrated greater tumor
regression compared with DOX alone (relative tumor volume: 0.8% at 15 days to 0.4% at 28
days vs 0.8% at 28 days).
A similar study also used CuAAC reactions to synthesize PEGylated dendrimer-gemcitabine
(dendrimer-GEM) conjugates to control the release of gemcitabine by cathepsin B,
93
a
cystein protease known to be involved in cancer and metastatic cell progression and to
activate MMPs.
100
They demonstrated higher tumor growth inhibition of 89.92% in vivo on
4T1 murine breast cancer cells compared with free GEM, which gave only 44.59% inhibition.
This was due to the local concentration of dendrimer-GEM in tumors under the EPR effect
and enzyme-responsive drug release.
The same observations were made in 2017 on PEGylated dendrons conjugated with DOX
(mPEGylated dendron-GFLG-DOX) via cathepsin-sensitive linkers using CuAAC chemistry.
94
An efficient antitumoral effect on breast cancer was observed compared with the free drug
with much higher tumor growth inhibition (80.3% vs 57.3%, respectively).
Sharma and co-workers demonstrated the ability of fluorescent PAMAM dendrimers known
to cross the impaired BBB for the targeting of specific neuroinflammation mediators
95
. A
neuroprotective drug (i.e. minocycline) was introduced using CuAAC (D-mino) and
selectively released in acidic conditions (pH 5.5) by cleaving the amide bond by esterase. D-
mino showed superior anti-inflammatory and antioxidant actions in microglial cells by
significantly inhibiting inflammatory cytokine tumor necrosis factor α (TNF-α) and nitric oxide
(NO) production compared with free minocycline at the same 500 µM concentration (TNF-α
released: 566.5 pg/mL vs 1150 pg/mL (no reduction); NO released: 0.12 µM vs 2.92 µM).
In another report, CuAAC enabled controlled release of DOX after hybridization with a
complementary ODN duplex-modified hydrogel and nuclease degradation of the
oligonucleotides.
96
The reversible hybridization platform demonstrated a 10% decrease in
cell viability compared with the same platform and unloaded DOX nanogels.
CuAAC has also been used to introduce histone deacetylase inhibitor (HDACi) acid-
responsive prodrug on polymeric NPs.
97,98
The NPs exhibited a positive antitumor effect in
vivo on AK7 murine mesothelioma cells with 80% tumor weight regression thanks to the
passive targeting induced by NPs.
Examples of SPAAC
SPAAC chemistry has also been investigated for the design of cell-dendrimer hybrids.
99
SPAAC occurs between N
3
-modified immune cells (RAW264.7) obtained by metabolic glycol-

21
engineering and clickable DBCO-dendrimers. More importantly, the newly functionalized
RAW cells preserve their viability and intracellular pathway, suggesting potential future
applications for these cell-NP hybrids as drug delivery systems. These dendrimers also offer
the possibility of including other molecules of interest as drugs and imaging agents.
2.2.2 Surface functionalization with imaging agents
The emergence of nanomedicines has opened new perspectives for the use of imaging
probes for both molecular and cellular imaging.
101
Click chemistry developments in recent
years have enabled the introduction of contrast agents for imaging, as well as
functionalization with a variety of molecules.
50
In the articles of this study, several
nanomaterials are combined with multiple imaging methods to improve diagnostic efficiency.
Single-photon emission computed tomography (SPECT), computed tomography (CT),
magnetic resonance imaging (MRI), positron emission tomography (PET) and optical
imaging (fluorescence) are the main techniques used for multimodal cancer imaging.
102–104
In
particular, light in the near infrared (NIRF: 650-900 nm) is frequently combined with PET or
MR functionalities because it allows deeper tissue penetration thus improving in vivo
detection of tumors (Table 3).
Table 3. General overview of passive targeting NPs using click chemistry to incorporate imaging agents on the
surface, from 2014 to nowadays.
Type of click
chemistry and use
(grafted click
function)
Type of NPs
(grafted click
function)
Imaging agent
(grafted click function)
Type of
imaging
In vitro/In vivo
studies
Refs.
CuAAC,
incorporation of NIR
dye
Fe
3
O
4
(N
3
)
IR675 (Alkyne)
MR, NIRF
yes/yes
105
CuAAC,
incorporation of
dyes
Polymeric
(Alkyne)
Dyes (Cy3, ATTO-740,
coumarin 343) (N
3
)
Optical
yes/no
106
CuAAC,
incorporation of
metalloporphyrins
Fe
3
O
4
(N
3
)
Metalloporphyrin
complexed with Zn
(Alkyne)
Photonic,
optical
no/no
107
SPAAC,
incorporation of
imaging probes
Glycol chitosan
(N
3
)
64
Cu-DOTA, Cy5.5-MMP
(DBCO)
PET, NIRF
yes/yes
108
SPAAC,
incorporation of
chelated
89
Zr
Liposomes
(DBCO)
89
Zr-CLL (N
3
), Cy5
PET, NIRF
no/yes
109
SPAAC,
incorporation of
imaging probes
AuNPs (DBCO)
125
I (N
3
)
PET
no/yes
110
SPAAC,
incorporation of IR
dye
Gold nanorods
(N
3
)
IRDye 800CW (DBCO)
Optical
no/no
31
N
3
: azide, DBCO: dibenzocyclooctyne, Fe
3
O
4
: iron oxide nanoparticles, AuNPs: gold nanoparticles, MMP: matrix metalloproteinase, MR:
magnetic resonance, NIR: near infrared, PET: positron emission tomography.
“In vitro/in vivo studies” indicate whether in vitro or in vivo tests were performed (yes) or not (no) in the article.
Examples of CuAAC

22
This type of click chemistry has been explored for the efficient preparation of bimodal NPs
combining by MR and NIRF imaging to improve in vivo imaging.
105
The use of CuAAC for the post-functionalization of copolymeric NPs with diverse organic
dyes (Cy3, ATTO-740 or coumarin 343) allows fluorescence signal location.
106
The mild
conditions of copper-free click reactions enhance the stability of dye-labelled SPION, by
reducing aggregation and allowing in vivo detection, which was not possible with the
conjugation processes used previously.
Hollingsworth et al. synthesized metalloporphyrin-modified superparamagnetic silica-coated
Fe
3
O
4
NPs by CuAAC for photonic and optical applications.
107
Examples of SPAAC
Bimodal nanotherapeutics combining two types of imaging, such as PET/NIRF, have been
demonstrated. Lee and co-workers developed glycol chitosan NPs (CNPs) as imaging
probes combining PET and NIRF.
108
SPAAC was used for both functionalizing azide CNPs
with
64
Cu through radiolabeling of DOTA-Lys-PEG
4
-DBCO and for Cy5.5 labeling with MMP
activable peptide (Figure11). The latter was conjugated with a dark quencher (BHQ3) and
released in the presence of specific MMPs (MMP-2, 13 and 9 particularly) to enhance
fluorescence signals. The bimodal imaging probe thus formed was able to provide
information on the biodistribution and accumulation of NPs in the tumor site, and on the
activity of the MMP biological marker overexpressed in cancer cells. Thanks to dual-modality
imaging, NPs can provide high sensitivity associated with a good depth of penetration via
PET as well as specific molecular detection through optical imaging.
Figure 11. Schematic representation of MMP-sensitive NPs labeled using copper free click chemistry (SPAAC)
with AMP-DBCO (comprising MMP-specific peptide, Cy5.5 NIRF dye and BHQ-3 (dark quencher) and 64Cu-
DOTA-Lys-PEG4-DBCO. CNP: chitosan nanoparticles, MMP: matrix metalloproteinase, AMP: Activatable MMP-
specific peptide probe, Lys: Lysine, DOTA: 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid, NIRF: near
infrared, PET: positron emission tomography. Figure modified from ref
108
.
In 2015, SPAAC was employed to link DBCO-modified AuNPs with
125
I-labeled azide giving
an excellent radiochemical yield (> 95%) for potential use an as imaging probe.
110
SPAAC
23
has also been used to develop
89
Zr-labeled liposomal NPs (
89
Zr-CLL) using in vivo dual PET/
NIRF imaging.
109
In another study, silica-coated gold nanorods combining fluorescence and surface plasmon
resonance (SPR) showed attractive fluorescent properties for future biomedical
applications.
31
The benefits of passive targeting NPs obtained by click chemistry enable good therapeutic
and imaging efficacy. However, a recent meta-analysis by Wilhelm et al. found that a median
of only 0.7% ID of NPs are accumulated in tumors after injection.
16
In fact, numerous studies
have demonstrated that the EPR effect is highly dependent on TME heterogeneity between
models and individuals, and reliant on the physical and chemical properties of the
NPs.
74,111,112
Various studies have thus tried to improve tumor accumulation and biodistribution by taking
into account these parameters and TME heterogeneity.
69,113,114
The effects of size, shape and
surface properties have been investigated to design smart and effective therapeutic NPs.
115
For example, rod-shaped NPs induce a higher cell uptake than cubes, cylinders or even
spheres. Positively-charged NPs are also preferred because of a possible electrostatic
interaction with negatively charged cell-membranes.
116
Another promising strategy to overcome TME heterogeneity consists in developing active
targeting NPs. Unlike passive targeting, active targeting relies on the conjugation of tumor-
specific biomarkers onto nanoparticles that are generally overexpressed in the TME. An
important question remains: could the NPs be considered as a “miracle recipe” if they could
be addressed specifically by grafting the targeting molecule using click chemistry?
3. Influence of active targeting using click chemistry on tumor accumulation and
cellular uptake: non-targeting vs targeting NPs
It has been widely reported that active targeting can facilitate specific uptake in cancer cells,
although very few active targeting nanomedicines are under clinical trial (e.g. MCC-465,
SGT-53, CALAA-01 in phase I and BIND-014 in phase II).
117
Many different kinds of targeting
moieties overexpressed on tumor cells can be introduced by click chemistry for specific
cellular interaction. Click chemistry is therefore considered to be a carefully engineered
approach for the development of new molecular delivery systems and represents 38% of the
articles listed in this review. The molecules most investigated for targeting are folates,
carbohydrates, cell-binding peptides (e.g. integrin ligands and cell-penetrating peptides),
proteins, mAbs and oligonucleotides. Many studies describing click chemistry for this
purpose highlight efficient preparations of NP-conjugates to target cancer cells and compare
the effects of active targeting NPs on cancer cells (Table 4).

24
Table 4. General overview of articles comparing active targeting NPs and passive targeting NPs functionalized by click chemistry, from 2014 to nowadays
Type of click chemistry and
use (grafted click function)
Targeting
molecule
Target
Type of NPs
(grafted click
function)
Drug loading
Effects compared to passive targeting
NPs
Refs.
VITAMINS
CuAAC, incorporation of folic
acid
Folic acid (N
3
)
Folate
receptor
Cerium oxide
(Alkyne)
DOX and
Ganetespib
Higher cellular uptake
118
CuAAC, incorporation of folic
acid
Folic acid
(Alkyne)
Folate
receptor
Polymeric (N
3
)
MTX
17% higher cellular uptake
3-fold higher cytotoxicity
119
CuAAC, incorporation of folic
acid
Folic acid (N
3
)
Folate
receptor
Micelles (Alkyne)
DOX
2.2-fold higher cellular uptake
120
CuAAC, incorporation of folic
acid
Folic acid
(Alkyne)
Folate
receptor
MSNs (N
3
)
DOX
7% higher cytotoxicity
38
CuAAC, incorporation of PEG-
biotin on PLGA polymer
Biotin (N
3
)
Biotin receptor
Polymeric (Alkyne)
DOX
2-fold higher cellular uptake
1.4-fold higher cytotoxicity
2.2-fold higher tumor uptake
2.3-fold higher antitumor activity
121
SPAAC, incorporation of folic
acid
Folic acid (BCN)
Folate
receptor
AuNPs (N
3
)
Up to 4.3-fold higher cellular binding
122
SPAAC, incorporation of folic
acid
Folic acid (BCN)
Folate
receptor
Polymeric (N
3
)
2.5-fold higher cellular binding
123
IEDDA, incorporation of folic
acid
Folic acid (Tz)
Folate
receptor
MSNs
(Norbornene)
Actinomycin D
Higher cellular uptake
37

25
Table 4: to be continued
Type of click chemistry and
use (grafted click function)
Targeting molecule
Target
Type of NPs
(grafted click
function)
Drug loading
Effects compared to passive targeting NPs
Refs.
CARBOHYDRATES
CuAAC, incorporation of HA
polymer
Hyaluronic acid
(Alkyne)
CD44 receptor
Lipidic (N
3
)
siRNA
1.8-fold higher cellular uptake
10% higher cytotoxicity
15% higher gene-silencing in vitro
2-fold higher tumor uptake and antitumor activity
30% higher gene-silencing in vivo
124
APTAMERS
SPAAC, incorporation of DNA
AS14111 (DBCO)
Nucleolin receptor
NMOFs (N
3
)
DOX
10% higher cytotoxicity
125
SPAAC, incorporation of DNA
DNA (DBCO)
Class A scavenger
receptor
NMOFs (N
3
)
5-fold higher cellular uptake
126
PEPTIDES
CuAAC, incorporation of iRGD,
RGDS and poly(glutamic acid)
iRGD, RGDS and
poly(glutamic acid)
(N
3
)
Integrin β
3
and
γ-glutamyl
transpeptidase
PSi (Alkyne)
Higher cellular uptake
127
CuAAC, incorporation of RGE
RGE (N
3
)
Neuropilin-1
Exosomes (Alkyne)
embedding SPION
NPs
Curcumin
60% higher cellular targeting
20% higher cytotoxicity
3-fold higher tumor targeting
1.34-fold higher antitumor activity
128
CuAAC, incorporation of RGD
RGD (Alkyne)
Integrin β
3
Micelles (N
3
)
PTX
3-fold higher cellular uptake
14-fold higher cytotoxicity
Higher antitumor activity
129
CuAAC, incorporation of RGD
RGD (N
3
)
Integrin β
3
Fe
3
O
4
(Alkyne)
1.4-fold higher cellular binding
130
CuAAC, incorporation of cRGD
cRGD (N
3
)
Integrin β
3
NDs (Alkyne)
12-fold higher cellular binding
131
CuAAC, incorporation of cRGD
and BODIPY
cRGD (Alkyne)
Integrin β
3
Fe
3
O
4
(N
3
)
Higher cellular uptake
132
CuAAC, incorporation of F3-
peptide
F3-peptide (Alkyne)
Nucleolin
Polymeric (N
3
)
DOX
10 to 20-fold higher cellular uptake
133

26
Table 4: to be continued
Type of click chemistry and use
(grafted click function)
Targeting molecule
Target
Type of NPs
(grafted click
function)
Drug loading
Effects compared to passive targeting
NPs
Refs.
PEPTIDES
SPAAC, incorporation of iRGD and
DOTA
iRGD (N
3
)
Integrin β
3
PSi (DBCO)
Sorafenib
No difference in cytotoxicity
1.63-fold higher tumor accumulation
No difference in tumor growth inhibition
134
SPAAC, incorporation of RGDS
iRGD and RGDS (N
3
)
Integrin β
3
PSi (BCN)
Sorafenib
20% higher cellular uptake for RGDS
15% higher cytotoxicity for RGDS
135
SPAAC, incorporation of GLA
GLA peptide (N
3
)
PSMA receptor
Dendrimers
(Cyclooctyne)
MTX
8-fold higher cellular uptake
136
SPAAC, incorporation of LHRH
LHRH peptide (DBCO)
LHRH receptor
Polymeric (N
3
)
p53
Up to 70% higher cytotoxicity
137
MONOCLONAL
ANTIBODIES
CuAAC, incorporation of anti-HER-2
Anti-HER-2 (N
3
)
HER-2
Dendrimers
(Alkyne)
Higher cellular uptake
138
CuAAC, incorporation of anti-HER-2
Anti-HER-2 (Alkyne)
HER-2
Polymeric (N
3
)
DOX
1.5-fold higher cellular uptake on SK-BR-3
and 1.3-fold higher on MCF-7
20% higher cytotoxicity on SK-BR-3 and
MCF-7
139

27
Table 4: to be continued
n.d: not determined, N
3
: azide, DBCO: dibenzocyclooctyne, RGD: Arginine-Glycine-Aspartic acid, iRGD: Cystein-Arginine-Glycine-Aspartic acid-Lysine-Glycine-Proline-Aspartic acid-Cystein, RGDS: H-Arginine-
Glycine-Aspartic acid-Serine-OH , LHRH: luteinizing hormone releasing hormone, siRNA: Small interfering RNA, DNA: deoxyribonucleic acid, PSMA: prostate-specific membrane antigen, HER2: human
epidermal growth factor receptor 2, GLA: glutamate urea, DOX: doxorubicin, MTX: methotrexate, PTX: paclitaxel, pOA-GFP; green fluorescent protein, MMP: matrix metalloproteinase, PSi: porous silicon, MSNs:
mesoporous silica nanoparticles, AuNPs: gold nanopaticles, NMOFs: metal-organic framework nanoparticles, ICP: infinite‐ coordination‐ polymer, QDs: quantum dots.
Type of click chemistry and
use (grafted click function)
Targeting molecule
Target
Type of NPs
(grafted click
function)
Drug
loading
Effects compared to passive targeting
NPs
Refs.
OTHERS
CuAAC, incorporation of arginine
Arginine (N
3
)
n.d
Hydogels
(Alkyne)
pMMP-9
13% higher transfection efficiency
20% higher MMP-9 protein expression
inhibition
No difference in antitumor activity
140
CuAAC, incorporation of arginine
Arginine 8 (Alkyne)
n.d
Mesoporous
bioactive glass
(N
3
)
pOA-GFP
No difference in transfection efficiency
141
CuAAC, incorporation of nuclear
localization sequences
Nuclear localization
sequences (Alkyne)
Nucleus
QDs (N
3
)
Up to 44% higher nucleus targeting
142
SPAAC, incorporation of
acetazolamide
Acetazolamide
(DBCO)
Carbonic anhydrase IX
Micelles (N
3
)
PTX
1.22-fold higher cellular uptake in MDA-MB-
231 and 1.28-fold higher in MDA-MB-468
28.5% higher cytotoxicity in MDA-MB-231
and 26.1% MDA-MB-468
143
SPAAC, incorporation of DNA
Anti-HER2 DNA
(DBCO)
HER2
ICP (N
3
)
6-fold higher gene knockdown
144

28
3.1 Vitamins
Vitamins are vital nutrients internalized by the metabolic activity of cancer cells due to the
presence of specific overexpressed receptors on the cell surface.
145
Vitamin-functionalized
NPs constitute an attractive strategy for targeting tumor cells thanks to the specific
recognition of vitamins by cell surface receptors, and have been employed in several
targeted drug delivery approaches.
3.1.1 Folic acid
Folic acid (FA), also called folate or vitamin B9, is a non-immunogenic water-soluble vitamin
involved in the synthesis of purines and pyrimidines of DNA as well as in cellular growth.
146
This molecule, once attached to its folate receptor (FR), is internalized by endocytosis. As
FRs are overexpressed in a variety of tumors (e.g. ovarian, colorectal, breast, brain, lung,
etc.) and only slightly expressed in normal cells, they are one of the ligands most widely used
for the active targeting of nanomaterials. Click chemistry has been used to conjugate many
ligands to nanomaterials, and this approach has also been adapted to attach other frequently
used cancer cell biomarkers, such as FA.
Examples of CuAAC
Several FA-based drug nanocarriers conjugated by CuAAC click reactions have been found
to improve cellular uptake and therefore therapeutic efficacy in a number of studies. For
instance, the folate-decorated nanoceria (FNC) co-encapsulated DOX and Ganetespib (GT)
(FNC-Doxo-GT), inducing a higher rate of A549 cell death within 48 hours of incubation due
to the synergistic effect compared with FNC-Doxo and FNC-GT (90%, 70% and 75%
cytotoxicity, respectively) (Figure).
118
Moreover, fluorescence microscopy images
demonstrated specific cellular uptake of FNC-Doxo-GT compared with the non-targeted
PNC-Doxo-GT, for which no fluorescence was detected.
Figure 12. “Click chemistry as used to synthesize FNC, whereas a combination of drugs encapsulated using a
solvent diffusion method. Reprinted with permission from ref
118
. Copyright 2017
.
American Chemical Society.
Likewise, folate conjugated NPs decorated with MTX on poly(ε-caprolactone)-co-
methoxypoly(ethyleneglycol) (P(MTXCLCL)-mPEG) demonstrated better cellular uptake than

29
the non-targeted P(MTXCLCL)-mPEG (51.8% vs 34.7% uptake after 120 min of incubation)
and a greater cytotoxic effect on MCF-7 cells (IC
50
: 0.053 µg/mL vs 0.167 µg/mL).
119
In another article, Wu and co-workers synthesized FA-modified core-shell nanomicelles
encapsulating DOX and P-glycoprotein siRNA simultaneously.
120
Fluorescent images of the
micelles confirmed the FR-mediated pathway in MCF-7 cells and a significant decrease in
cellular uptake when the cells were treated with FA (95.4% vs 65.1% of DOX positive cells).
Moreover, the synergistic cytotoxic effects of DOX and siRNA obtained in vitro were greater
than with nanomicelles alone or free DOX (85.3%, 41.3% and 15.7%, respectively).
Furthermore, these nanomicelles induced a significant improvement in tumor growth
inhibition in vivo compared with free DOX (p<0.05). siRNA delivery results in downregulation
of P-gp protein and therefore inhibits DOX efflux from the cells.
Other FA-conjugated hollow ZnO NPs delivering PTX (FCPZnO) exhibited a higher level of
cytotoxic activity than free PTX on MDA-MB-231 cells (IC
50
: 11.84 nM vs 7.21 nM) and MCF-
7 cells (IC
50
: 14.02 nM vs 8.06 nM).
147
In murine MDA-MB-231 tumor xenografts, FCPZnO
also demonstrated 4-fold higher tumor growth regression compared with PTX alone after 35
days.
Another study reports FA-modified multiblock polyurethane micelles loading DOX (SSPHPU-
FA-DOX) demonstrating a 2.2-fold improved cellular uptake on HeLa cells compared with
SSPHPU-DOX as determined by both confocal microscopy images and flow cytometry.
148
In
vivo, DOX/SPION@SSPHPU-FA decreased tumor weight by up to 98.6% thanks to
magnetically targeted drug delivery.
Zhang et al. synthesized FA and Cy7-modified chitosan NPs (CF7Ns) capable of both PDT
and NIRF imaging.
149
In vitro, HeLa cells showed higher fluorescence intensity with CF7Ns
than with non-targeted C7Ns on fluorescence microscopy images and in flow cytometry. The
cytotoxic effect in vitro was also significantly enhanced with NIR laser irradiation cells
compared with C7Ns (75% vs 65% for 24 h at 2.8 µg/mL) and improved apoptosis cells for
48 hours (70.4% vs 27.8%).
In 2016, FA-MSNs delivering DOX (NP2-BZ-FA-DOX) upon photoactivation (by UV or blue
irradiation) exhibited superior cytotoxic activity against MCF-7 cells compared with non-
targeted NP2-BZ-DOX (37% vs 30%).
38
These results suggest that the targeting abilities of
FA directly influence drug efficiency because of superior internalization and therefore
photoactivation in the cells.
Examples of SPAAC
Similarly, BCN-FA anchored on hollow AuNPs (HAuNP-DNBA-FA) improved SERS (Surface-
Enhanced Raman Scattering) imaging on FR-positive cancer cells (i.e. KB, HeLa and A549
cell lines) compared with non-targeted HAuNP-DNBA-N
3
(Raman intensity: up to 650 vs 150
on KB cells).
122
Another study using BCN-FA for SPAAC with N
3
-polymeric NPs (NPs-PEG-FA) also
revealed a higher rate of binding on HeLa cells compared with untargeted NPs-PEG in a
fluorescence microscopy study (mean fluorescence intensity (MFI): 25 vs 10).
123

30
Example of IEDDA
Other MSNs anchoring FA (MSN-pHSA-CA-FA) by norbornene/tetrazine click chemistry have
produced in vitro results for specific cell recognition and carbonic anhydrase-based pH-
responsive drug delivery.
37
The controlled drug release is ensured by the detachment in
acidic conditions (pH=5.5) of carbonic anhydrase during endosomal internalization.
Fluorescence microscopy images of KB cells treated with MSN-pHSA-CA-FA showed a
higher cellular uptake than with MSN-CA.
3.1.2 Biotin
Biotin is one of the vitamins required for tumor cellular growth. This molecule internalizes
cells by binding to the sodium dependent multivitamin transporter (SMVT) on the cell surface,
and can therefore be used for targeted drug delivery. The biotin receptor is overexpressed on
the tumor cells due to the high demand of biotin for rapid tumor growth. A few examples of
conjugation onto NPs for tumor-targeting exist in the literature.
Examples of CuAAC
T2-weighted magnetic resonance images of A549 cell phantoms incubated with Pyrene-
Biotin-Fe
3
O
4
NPs revealed specific cell internalization. In this case, click reactions were used
to develop biofunctional Fe
3
O
4
NPs. Pyrene and biotin were grafted onto the NP surface
through Diels-Alder and azide-alkyne cycoladdition respectively, with strict control over
reactivity.
Moreover, a proof-of-concept for pyrene release by external magnetic field showed potential
as a controlled drug delivery platform.
150
In 2017, biotinylated-PEG-PLGA NPs containing DOX (BPNP) improved 4T1 cellular uptake
in flow cytometry compared with non-biotinylated DPNP (MFI of 214 vs 113) and were more
cytotoxic after 24 hours (IC50: 91.5 nM vs 131.8 nM).
121
In vivo, BPNP demonstrated higher
tumor uptake on mice bearing 4T1 breast tumor cells than DPNP (maximum tumor
concentration: 49.4 ng/mg vs 22.45 ng/mg); the level of antitumoral activity was higher,
leading to increased tumor volume regression (99.36 to 29.74 mm
3
) compared with DPNP
(102.72 to 68.43 mm
3
) and free DOX (100.43 to 76.38 mm
3
).
Examples of SPAAC
Other articles mention biotin-clicked NPs by SPAAC for biosensing applications to amplify
RNA and DNA detection with AuNPs
151
or UCNPs.
152
3.2 Carbohydrates or polysaccharides
One possibility for the preparation of drug delivery targeting NPs is to use carbohydrates
specifically directed against cell surface receptors (i.e. lectins). There are different classes of
targeting molecules for binding lectins including monosaccharides (e.g., galactose, mannose,
fucose, sialic acid), disaccharides (e.g., lactose and N-acetyllactosamine), and
polysaccharides (e.g., hyaluronic acid, pullulan, dextran, chitosan). Lectins are known to be
involved in the growth and metastasis of tumors and constitute an interesting targeting
approach using the endocytosis process.
153,154
Several carbohydrate NPs have thus been

31
synthesized using click chemistry for specific targeting therapies and, for example, have
recently been reported to have produced glyco-gold NPs.
155
Functionalization using CuAAC
click chemistry is only reported for the studied period.
3.2.1 Hyaluronic acid
NPs containing hyaluronic acid (HA) are attractive candidates for therapeutic applications.
HA is a natural anionic polysaccharide, an extracellular constituent of connective tissues and
is able to bind the CD44 receptor overexpressed in several tumor cells. Furthermore, HA is
often used as a carrier for intracellular controlled drug release through degradation by
hyaluronidase (HAase).
156
Examples of CuAAC
Sun et al. investigated this strategy for engineering tumor-targeted siRNA delivery
nanosystems (Figure13).
124
CuAAC enabled easy conjugation of alkyne-HA polymers (alk-
HA) onto lipid-based complexes encapsulating siRNA (RSC) using the N
3
-modified
cholesterol present on the surface (RSC-HA).

32
Figure 13. (A) Schematic illustration of the “collaborative assembly” strategy for construction of an siRNA delivery
system consisting of a lipidpolymer hybrid nanocarrier via a combination of an electrostatically driven physical
assembly and a click reaction-mediated chemical assembly. (B) Schematic illustration of tumor-targeted siRNA
delivery by RSC-HA. (i) Accumulation of RSC-HA at the tumor site; (ii) endocytosis of RSC-HA into the tumor
cells; (iii) HAase-mediated degradation of the HA shell of RSC-HA and endosomal escape into the cytoplasm; (iv)
GSH-triggered disassembly of RSC-HA and release of the complexed siRNA in the cytoplasm; and (v) gene
silencing induced by the released siRNA. Reprinted with permission from ref
122
. Copyright 2017 American
Chemical Society
Flow cytometry revealed a significant improvement in the cellular uptake of RSC-HA
compared with RSC in vitro on A549 cells (MFI 140 vs 80). The efficiency of siRNA gene-
silencing also improved significantly with 55% downregulation of mRNA expression for RSC-
HA compared with 40% for RSC, and a higher rate of anticancer activity was observed in
vitro (total apoptotic ratio: 23.48% vs 13.36%). Moreover, vectors with HA polymers grafted
onto the liposome by click chemistry showed greater in vivo stability in the blood, resulting in
efficient tumor targeting, compared with RSC/HA in which HA was physically adsorbed on
the complex. 2-fold higher tumor uptake and tumor growth inhibition were obtained after 10
days of treatment compared with non-targeted RSC, with approximately 30% superior gene-
silencing efficiency.
3.2.1 Glycosides
Sugars can enhance binding affinity with lectin receptors thanks to a phenomenon called the
“cluster glycoside effect”, which causes diverse copies of glycoside clusters to interact
together to enhance recognition.
157
Dendritic multivalent glycosides are widely used in a
number of biomedical approaches.
158
Only examples of CuAAC click chemistry during the
studied period are therefore described herein.
Examples of CuAAC
Rajakumar et al. incorporated glucose into dendrimers by CuAAC and showed cardio-
protective and anti-diabetic properties.
159
Interestingly, Kong et al. synthesized mannose and galactose-modified SiO
2
NPs, and
demonstrated the concept of a quantitative fluorine NMR method to determine the density of
carbohydrates incorporated by CuAAC.
160
CuAAC was also used to incorporate thiosialoside
on fullerenes directed against the influenza virus neuraminidase, despite a moderate antiviral
activity.
161
3.3 Aptamer ligands
Nucleic acid aptamers are single-stranded oligonucleotides (DNA or RNA) widely used as
therapeutic molecules. They can target a large variety of specific proteins overexpressed by
cancer cells leading to possible internalization. Aptamers can be chemically synthesized and
easily modified with a broad range of chemical functions.
162
Aptamer-decorated nanocarriers
thus show promise as drug delivery systems. Nevertheless, most of the strategies employed
for surface functionalization are based on electrostatic interactions and suffer from possible
instability. In this context, click chemistry was able to ensure DNA/RNA surface
functionalization of nanomaterials. For instance, CuAAC demonstrated effective
bioconjugation of DNA on polymeric QDs for hybridization.
163
Examples of SPAAC

33
Among the aptamers currently employed, AS14111 is specific to the nucleolin receptor.
125
In
one study, AS14111 was conjugated by SPAAC on metal-organic framework NPs (NMOFs)
for specific DOX release in acidic conditions (pH 5.0).
164
Targeted NMOFs demonstrated
higher cytotoxicity than non-targeted NMOFs in vitro on MDA-MB-231 cancer cells after three
days (45% vs 35%).
In another study, SPAAC was also investigated to conjugate DNA on NMOFs (MOF-DNA)
and achieved a 5-fold increase in the cellular uptake of 14 nm MOF-DNA, compared with
MOF alone.
126
In 2016, UCNPs-MB/Dox was developed with the conjugation of a TK1 mRNA-specific
molecular beacon (MB) bearing a quencher (BHQ-1) and an alkene handle modified UCNP
through click reaction. These original UCNPs showed 57% specific cytotoxicity on MCF-7
cancer cells compared with no cytotoxicity for LO2 normal cells.
165
Due to the high affinity and specificity of aptamers, they have been widely investigated for
protein detection assays. For instance, Chen et al. designed double-stranded DNA-MSNs
embedding FITC (dsDNA-FITC-MSN) to elaborate thrombin biosensors. FITC was released
from the pores only after specific dsDNA-thrombin recognition, suggesting potential use as a
stimulus responsive system to detect thrombin in serum samples.
36
Akiel et al. used SPAAC to functionalize N
3
-NDs with DBCO-single strand DNA for
subsequent hybridization to complementary strands.
166
Specific DNA association can be
characterized by a different X-band EPR spectroscopy profile compared with the non-
hybridized nanomaterial, making them promising sensor platforms.
Examples of thiol-ene
Other authors have anchored aptamers on NPs through thiol-ene click reactions in order to
detect and capture bovine serum-albumin (BSA).
29
NPs were combined with a molecularly
imprinted polymer layer able to entrap molecules such as proteins in a complex matrix. Thiol-
ene click chemistry was also successfully applied to colloidal NPs for aptamer
bioconjugation.
46
3.4 Peptides
3.4.1 RGD peptides
Among the various types of peptides, integrins are a large class of cell adhesion receptors
comprising 24 different peptides. They are very useful for targeting tumor sites due to their
role in disease progression.
167
The tripeptide sequence Arg-Gly-Asp (RGD) is often used as
a ligand owing to its affinity with a wide variety of integrins that promote cellular adhesion and
internalization.
168
Examples of CuAAC
In 2018, Jia and co-workers improved the ability of exosomes embedding RGE-modified
SPION (Super Paramagnetic Ion Oxide Nanoparticle) and Curcumin (RGE-Exo-SPION/Cur)
to target glioma cancer cells.
128
They demonstrated a much higher targeting ability with RGE-
Exo in vitro on U251 cells compared with free-Exo (close to 100% vs 40%) and 3-fold higher

34
tumor targeting in vivo on glioma tumor-bearing mice 4 hours after intravenous injection.
Cytotoxicity was found to be significantly higher with RGE-Exo than with free-Exo (60% cell
inhibition vs 40%), as was tumor volume suppression (70mm
3
to 3mm
3
vs 70mm
3
to 20mm
3
)
with no tumor regression for 28 days.
CuAAC conjugation of RGD peptides is usually performed using polymers and PEG-coated
NPs with alkyne peripheral functional groups. In a recent study, the potential of RGD
targeting was reported on micelles loaded with PTX comprising polymers and N
3
-PEG.
129
RGD achieved 3-fold higher 4T1 cellular uptake compared with non-targeted NPs thanks to
MMP-2 proteolytic cleavage, and superior cytotoxicity (IC
50
: 79 ng/mL vs 1130 ng/mL). In
vivo, RGD-NPs exhibited higher tumor growth inhibition with a 1.9-fold volume increase after
24 days compared with the 16-fold increase for non-targeted NPs.
In 2016, Arriortua et al. investigated the conjugation of N
3
-RGD with amino-alkyne polymeric
iron NPs (Fe
3
O
4
@PMAO_RGD).
130
The hyperthermia properties of Fe
3
O
4
@PMAO_RGD
NPs significantly increased the necrosis of liver and colorectal tumors (up to 16%), compared
with saline groups, under a magnetic field without damaging the hepatic tissues.
Fluorescent probes have been successfully used in the field of targeting NPs and afford new
opportunities in biomedicine such as specific cancer diagnosis. In this context,
nanodiamonds (NDs) constitute interesting fluorescent systems for bioimaging applications
because of their optical crystal properties responsible for NIRF. Slegerova et al. described
fluorescent NDs coated with an alkyne-copolymer allowing incorporation of both N
3
-cyclic
RGD peptides and fluorescent molecules (Alexa Fluor 488) (FNDs-cRGD) for glioma cancer
cell imaging.
131
These FNDs-cRGD demonstrated 12-fold higher U-87 MG cellular binding
compared with FNDs. A similar strategy to introduce N
3
-labeled
125
I-RGD or FITC probes for
prostate cancer cell imaging was applied by Rehor et al. using NDs coated with alkyne-PEGs
and polymers.
169
In 2017, multifunctional RGD-Fe
3
O
4
NPs also showed 1.4-fold higher cellular binding
compared with non-targeted NPs on BT-20 cells.
132
Another study demonstrated enhanced cellular uptake of porous silicon (PSi) NPs loaded
with three different molecular weight targeting peptides (i.e. N
3
-functionalized iRGD, RGDS
and poly(glutamic acid)) on endothelial EA.hy926 and U87 MG cells.
127
Oz et al. synthesized NPs conjugated with cRGD and BODIPY fluorescent dye by CuAAC
and thiol-maleimide click chemistry for the imaging of MDA-MB-231 breast adenocarcinoma
cells.
170
They demonstrated higher cellular uptake in fluorescence microscopy with the
targeting cRGD peptide.
Examples of SPAAC
Wang et al. compared active and passive cellular targeting to improve retention and augment
the efficacy of the therapy at the target site. They reported a new theranostic and
multifunctional porous silicon (PSi) NP suitable for both imaging and delivery of a therapeutic
agent (i.e. sorafenib).
134
The surface was modified using SPAAC to introduce the DOTA
chelators necessary for
111
In-radiolabeling for SPECT/CT imaging. In a second step, the
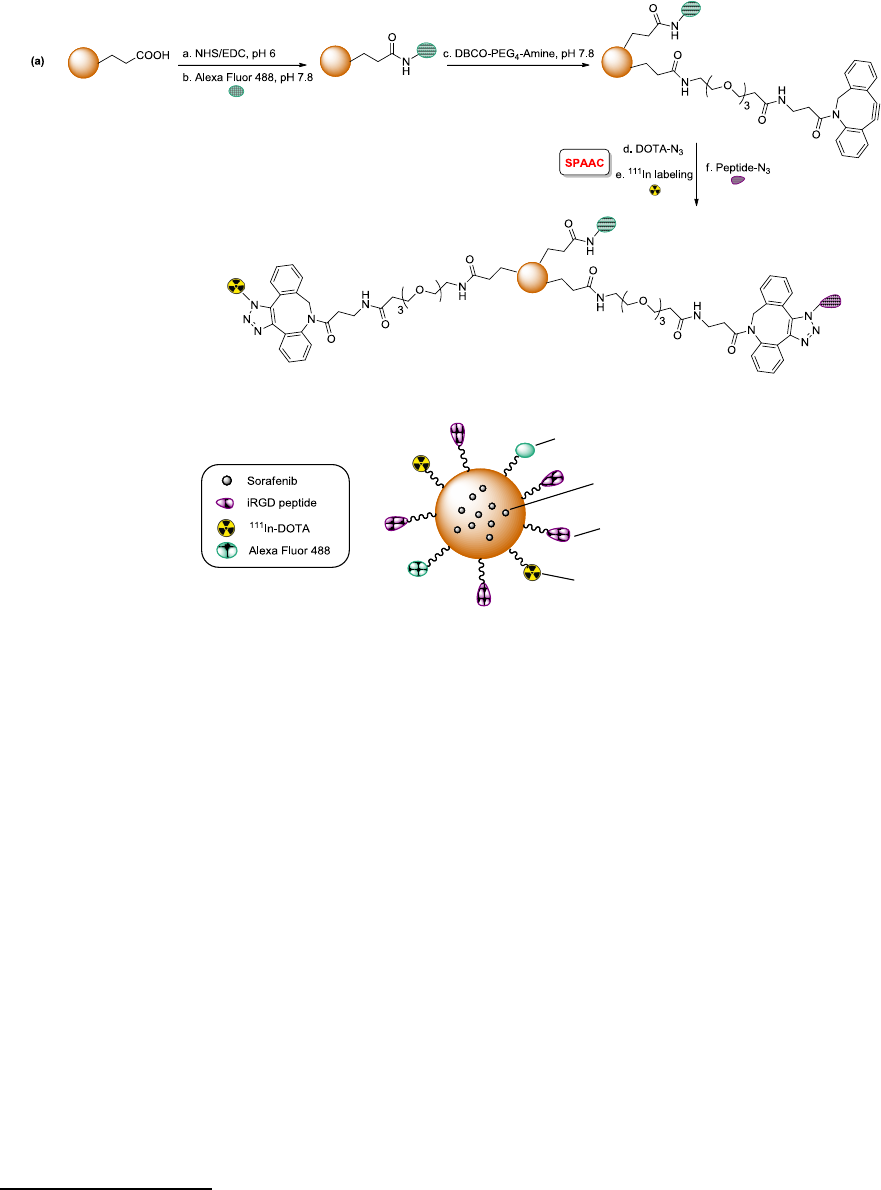
35
targeting peptide iRGD was inserted to form PSi-iRGD-NPs (Figure). The PSi-iRGD-NPs
exhibited superior tumor-specific accumulation in prostate cancer cells (PC3-MM2)
compared with PSi-NPs 27 hours after intravenous injection (4.4 vs 2.7 tumor-to-muscle
ratios of the radioactivity). However, the NPs demonstrated similar levels of tumor growth
inhibition after intravenous and intratumoral injection.
Figure 14. (a) Synthesis and (b) schematic structure of the multifunctional nanocarrier encapsulating Sorafenib,
functionalized with Alexa Fluor 488 and labeled
111
In-DOTA and iRGD peptide via SPAAC click chemistry. Figure
modified from ref
134
.
An efficient drug delivery system was also created by using BCN-functionalized PSi-NPs to
conjugate N
3
-RGD derivatives (TCPSi-RGDS/iRGD).
135
This approach led to a significant
improvement (20% higher) in cellular uptake for TCPSi-RGDS and 15% higher cell growth
inhibition with sorafenib on EA.hy926 endothelial cells compared with non-targeted NPs.
3.4.2 Cell-penetrating peptide (CPP)
Cell penetrating peptide (CPP) was recently identified as a promising ligand for targeting
cancer therapy. Natural and synthetic peptides are widely used to enhance cellular uptake
via the endocytosis process and are conjugated to deliver cargo-based nanomedicines in
particular.
171
Trans-activating transcriptional activator (Tat) is one of the CPPs used for
targeting delivery systems. CPP is a positively-charged peptide derived from the Human
Immunodeficiency Virus 1 (HIV-1) and can access the cell nuclei to exercise its translocation
activity.
172
Examples of CuAAC
In 2015, Liu et al. described PSi nanocomposites constituted of an acetylated dextran matrix
(AcDX), degradable in acidic conditions, embedding the three therapeutic molecules (i.e.
SPECT/CT imaging
Fluorescence imaging
Targeting molecule
Therapeutic agent
(b)
36
Sorafenib, MTX and PTX) currently used to treat breast cancer.
173
They used oxime click
chemistry to include cell-penetrating peptides (CPP) as receptor internalization pathways to
finally obtain PSi@AcDX-CPP NPs.
Experiments carried out at two different levels of pH (i.e. pH 7.4 and pH 5.0) indicated
controlled release of the cargos only at pH 5.0 because of hydrolysis of the polymeric matrix.
The advantage of adding CPP-targeting moieties to improve internalization was confirmed by
cell uptake studies on two breast cancer cell lines comparing PSi@AcDX-CPP and
PSi@AcDX NPs (MCF-7: MFI of 2000 vs 250; MDA-MB-231: MFI of 1550 vs 250).
PSi@AcDX-CPP significantly improved proliferation inhibition compared with the non-
targeting PSi@AcDX (cell viability for MCF-7 and MDA-MB-231: ~ 60% vs no cytotoxic
effect).
In the same year, N
3
-Tat was used to functionalize micelles loaded with DOX via CuAAC.
174
Specific release of the drug was achieved by two types of amide hydrolysis. The first was
carried out by the nanocarrier’s positively-charged amphiphilic diblock copolymer poly(L-
lysine)-block–poly(L-leucine) (PLL–PLLeu). This polymer became negatively charged at pH
~6.5 in the extracellular microenvironment of the tumor, allowing internalization by the tumor
cells. The second was carried out by the Tat peptide amidated by succinyl chloride (Tat(SA)).
After uptake by the cells, the peptide was activated at pH 5.0 in the endosome through SA
hydrolysis. This pH-sensitive amide allowed subsequent activation of nuclear targeting and
DOX release. Confocal images of the HeLa cells showed more red fluorescence of DOX with
targeting NPs than with non-targeting NPs and demonstrated higher nuclear delivery of DOX
for 24 hours (400 ng vs 140 ng of DOX/10
6
cells respectively).
One robust nanomedicine application, the delivery of small interfering RNA (siRNA), is a
promising tool for silencing genes overexpressed in cancer. In vitro studies on the
neurotropic rabies virus confirmed the higher level of delivery of CPP anandamide and
siRNA-functionalized dendrimers to neural stem cells.
175
They efficiently transfected siRNA
(up to 2-fold higher relative mRNA concentration) leading to a 2-fold higher decrease in
virality compared with the non-targeting anandamide system.
3.4.3 Other peptides
Other bioactive peptide ligands are used for a variety of pathologies and applications. Wan’s
group demonstrated highly efficient and highly chemoselective peptide conjugation with
dendrimers using SPAAC, associated with no alteration of in vitro and in vivo bioactivity.
176
Alkyne-functionalized polylysine dendrons were prepared for click chemistry with N
3
-PEG-
chain-modified χ-MrIA analogues, disulfide-rich χ-conotoxin peptides implicated in the
treatment of neurological disorders, by selectively inhibiting the human norepinephrine
transporter.
177
Other alkyne-functionalized lysine dendrons using a solid-phase Cu(I)-
catalyzed for azide peptide conjugation have shown great potential for future
immunoassays.
178
The targeting potential of peptide for vectorized drug delivery into prostate
cancer cells using PSMA-targeted PAMAM dendrimers has also been reported by other
investigators.
136
These nanocarriers were obtained by SPAAC conjugation of N
3
-glutamate
urea (GLA) targeting peptides and N
3
-MTX. They demonstrated approximately 8-fold higher
cellular uptake compared with non-targeted NPs. In another study, synthesis of luteinizing
hormone releasing hormone (LHRH) peptide-functionalized NPs encapsulating recombinant

37
human tumor suppressor proteins (p53) showed enhanced anticancer effects in vitro on
MDA-MB-231 cells compared with the non-targeted version (almost 100% vs up to 30% cell
growth inhibition).
137
Another article also showed enhanced cellular uptake with polymeric DOX-NPs conjugated
with a nucleolin-targeting F3-peptide overexpressed in tumor cells (9L, MCF-7 and NCI/ADR-
RES cells).
133
A review published by Tang and Beker
179
contains more examples of click
reactions for peptide-functionalized nanomaterials prior to 2014.
3.5 Protein ligands
Glycoprotein transferrin is one type of protein used as a targeting ligand because human
transferrin receptors are abundantly expressed in tumor cells.
180
Examples of SPAAC
After bioconjugation with quantum dots
181
and magnetic nanogels
182
using SPAAC, protein
ligands reduce side effects and improve the delivery of therapeutic drugs thanks to the
receptor-mediated endocytosis pathway. Epifluorescence images of HeLa cells show that
bioconjugation of N
3
-QDs with DBCO-transferrin promotes cellular uptake. In the second
study, SPAAC between N
3
-PEG-transferrin and bicyclononyne-NPs (BCN-NPs) resulted in
up to 90% conjugation efficiency. Magnetic NPs were thus used to detect and capture
circulating tumor cells overexpressing transferrin receptors.
Example of IEEDA
Tz-norbornene click chemistry has been successfully applied to hydrogel NPs to anchor
proteins such as fluorescent ovalbumin (Tz-Oval), alkaline phosphatase (Tz-ALP) and
glucose oxidase (Tz-GOx) for potential applications in tissue engineering and glucose
biosensing.
183
These protein-functionalized NPs showed higher bioactivity than their non-
functionalized analogs NF-Oval, NF-ALP and NF-GOx (5-fold, 12-fold and 9-fold increase in
bioactivity, respectively) at higher concentrations.
3.6 Monoclonal antibodies (mAbs)
Monoclonal antibodies are immunoglobulin (Ig) proteins whose IgG form remains a key
element of many targeted therapies. The growing interest in using mAb-conjugated NPs for
active targeting is mainly due to their ability to improve tumor accumulation. Full mAbs, as
well as mAbs fragments, such as F’(ab)
2
, F’(ab) or diabody structures, can be used to target
tumors. Many strategies are employed to attach these mAbs or mAbs fragments to NPs; they
must ensure optimal orientation for subsequent functional binding and antigen (Ag)
recognition. The thiol-maleimide reaction is easily employed with mAb because of the thiol
groups that naturally exist in cysteine residues.
184
However, the main restriction to the use of
this reaction is the potential hydrolysis of the maleimide into an unreactive maleic acid amide
that may reduce conjugation efficiency. In this context, click chemistry has been widely used
for covalent linkage by prior mAb derivatization with click functions.
185–187
This conjugation
strategy offers the possibility of generating stable, efficient interactions between the two
entities with minimal purification steps and high yields.
30,188
Among the mAbs conjugated by

38
click chemistry, anti-EGFRs have been most widely studied.
189
They are used to target and
treat tumors thanks to their involvement in cancer progression.
Examples of CuAAC
In 2016, N
3
-anti-HER-2 mAb was covalently introduced on alkyne PAMAM dendrimers by
CuAAC; AuNPs and Gd were also entrapped in the dendrimer and DOTA, respectively (Au-
G5-Gd-Herceptin) (Figure).
138
Confocal images of A549 cells exhibited higher internalization
with Au-G5-Gd-Herceptin than with Au-G5-PEG-Alkyne-DOTA-Gd-NHAc.
The same observations were made with PLGA-Phis-PEG NPs conjugated with anti-HER-2
(PNH) by CuAAC and encapsulating DOX.
139
PNH showed approximately 1.5-fold and 1.3-
fold higher cellular uptake on SK-BR-3 and on MCF-7 cell lines respectively compared with
PN after 120 min of incubation. They also demonstrated higher cytotoxicity effects (SK-BR-3:
70% vs 50%; MCF-7: 65% vs 45% cell growth inhibition) at 0.5 µg DOX/well.
Figure 15. Schematic structure of the multifunctional NP-imaging agent Au-G5-Gd-Herceptin. Figure modified
from ref
138
.
Examples of SPAAC
Kotagiri et al. demonstrated that the SPAAC reaction produced anti-EGFR-QDs with a higher
yield than anti-EGFR-QDs obtained by traditional thiol-maleimide conjugation which depends
on hydrolysis (88% vs 60%).
55
Moreover, the stability of DBCO and azide functions in
aqueous solution resulted in a larger number of mAbs per QD (up to 8.4 vs 3.9). Confocal
images of BxPC-3 and MDA-MB-231 cells showed higher fluorescence intensity for the click-
conjugated mAb-QDs. This was also the case for SiO
2
NPs, where a larger amount of anti-
HER-2 (Trastuzumab) was conjugated by SPAAC than by EDC/NHS coupling (50 to 70% vs
1 to 20%) resulting in a targeting capacity 8-fold higher on MDA-MB-231 breast cancer
cells.
190
In 2016, Ma et al. described efficient synthesis of polymeric-coated QDs bioconjugated with
anti-EGFR (QD-EGFR Ab) by SPAAC; selective attachment of the membrane region was
achieved compared with the QD-IgG control.
163
SPAAC was also performed with anti-
VEGFR-functionalized QDs for in vitro imaging of angiogenic receptors.
191
Qiao and his team
synthesized NaGdF
4
:Yb,Er@NaGdF
4
upconversion NPs (UCNPs) conjugated with the
antigastric tumor antibody, MGB
2
, and demonstrated both specific optical and MR imaging
properties in vivo on implanted SGC7901-Luc cells, thanks to their targeting capacity.
192
(AcHN)
m
Alexa Fluor 647
DOTA-Gd
Anti HER2-mAb
AuNPs
CuAAC binding

39
mAb-conjugated NPs have also been investigated for biosensing applications because of
their high affinity and specificity towards biomarkers of interest. Click chemistry has proved to
be a promising strategy for immobilizing such probes for immunoassays.
30,193
3.7 Other nano-conjugates
Examples of CuAAC
In another study, the authors synthesized dendrons and α-cyclodextrin-based hydrogels,
including arginine conjugated by CuAAC, for pMMP-9 plasmid delivery (MPEG-PLLD-
Arg/pMMP-9 and MPEG-PLLD-Arg/pMMP-9/α-CD).
140
They demonstrated significantly
higher transfection of HNE-1 cells with MPEG-PLLD-Arg/pMMP-9 than with PEI-25k/pMMP-9
(up to 62% vs 49%) and consequently higher inhibition of MMP-9 protein expression (70% vs
50%).
In vitro transfection assays with MPEG-PLLD-Arg/pMMP-9/α-CD transfected up to 72% of
cells at 12 hours and induced superior HNE-1 cell apoptosis compared with PEI-25k/pMMP-9
(46% vs 31%). However, tumor growth inhibition in vivo was similar with both formulations. In
2017, Li et al. also conjugated octaarginine 8 on mesoporous bioactive glass (MBG-PG-
Arg8) by CuAAC for gene transfection but found no significant difference in transfection
efficiency with MBG-PG-Arg8 compared with MBG-NH
2
(32% vs 29%).
141
Targeting efficiency and intracellular internalization have also been achieved by Maity and
Stepensky.
142
They designed efficient QDs decorated with nuclear localization sequences
(QD-NLS) by CuAAC, conferring specific nucleus accumulation. The intracellular efficiency of
three different QD formulations was compared on HeLa cells: QD-COOH, QD-azide and QD-
NLS. QD-NLS improved cell targeting efficiency by up to 44.2% compared with QD-COOH
and QD-N
3
(MFI of 11.4, 3.60 and 6.07, respectively).
Examples of SPAAC
In 2018, Tatiparti et al. described PTX-loaded nanomicelles (HSA-PTX-AZT) targeting
carbonic anhydrase IX (CA IX) directed against triple negative breast cancer cells.
143
The
NPs functionalized with acetazolamide (AZT, hypoxia marker of CA IX) by SPAAC showed
higher apoptosis compared with non-targeted NPs in vitro on MDA-MB-231 and MDA-MB-
468 (MDA-MB-231: 74.8% vs 46.3%; MDA-MB-468: 75.3% vs 49.2%) due to receptor-
mediated cell uptake. Fluorescence spectroscopic studies also demonstrated significantly
higher cellular uptake (MDA-MB-231: MFI ~ 120 vs 98; MDA-MB-468: MFI superior to 100 vs
~ 78) at 16 hours. In 2015, anti-HER2 DNA-labeled ferric nitrate infinite-coordination-polymer
NPs (HER2-ICP) were developed and found to reduce HER2 expression by up to 80% with
10 µM DNA on SKOV-3 cells compared with no efficient gene knockdown with the
NonTarget-ICP.
144
40
4. Bioorthogonal chemistry and pretargeting (PT) systems for NP delivery
This section concerns the term bioorthogonal chemistry and not click chemistry.
Bioorthogonal chemistry means that the formation of the covalent link between the two
entities does not need a catalyst and may occur in physiological conditions. The previous
sections of this paper cite several articles mentioning SPAAC and IEDDA systems, which are
characteristic of bioorthogonal chemistry. In these previous examples, the click reaction was
used to build the NP for both passive and active targeting. In this fourth section,
bioorthogonal chemistry is described for pretargeting (PT) systems. The PT approach using
bioorthogonal chemistry is another extremely promising solution to improve tumor targeting.
Due to the limited number of cellular receptors and TME heterogeneity, bioorthogonal
chemistry has been attracting growing interest over the past few years. The delivery of NPs
onto the surface of living cells has improved the targeting efficiency and delivery of both
imaging agents and drugs.
50,64,194
This two-step approach offers several advantages over the
conventional, direct covalent coupling of targeting ligands. It prevents NP aggregation and
cross-linking between macromolecular entities (mAbs, avidin, etc.). NPs with bioorthogonal
functions are often easier to synthesize and purify, requiring only removal centrifugation
unlike direct conjugates, which often require optimization processes to maximize the ligand-
to-NP ratio without compromising colloidal stability. Direct labeling of mAbs/avidin on NPs
can decrease Ag recognition because of steric hindrance compared with mAb. Moreover, for
larger NPs this can lead to massive conjugates that are less easily delivered to the TME.
This strategy could also improve the number of NPs per mAb and therefore amplify the
signal per biomarker. Compared with traditional antibody-NP conjugation, this method can be
applied to a broader range of pathologies depending on the suitable mAb.
PT approaches applied to NPs rely on specific, covalent interactions between two reactive
groups (i.e. bioorthogonal entity or tag), one usually carried by the nanomaterial reacting with
another incorporated onto the molecule of interest (e.g. imaging, therapeutic agent or
targeting molecule). This two-step strategy consists in injecting a tagged targeting agent (i.e.
NP or molecule of interest), which will accumulate in sufficient quantities on the cells, then
injecting a second tagged agent (i.e. NP or molecule of interest), which specifically and
covalently binds through the bioorthogonal reaction. The different PT approaches for
potential imaging, diagnosis and drug delivery applications reported from 2010 to the present
are summarized in Figure and detailed in the section below. In most studies, mAbs are
widely used as primary targeting agents (Figurea), but NPs are also increasingly used as
platforms for imaging and therapeutic agents (Figureb).

41
Figure 16. Different pretargeting strategies for imaging, diagnosis and therapy. (a) Firstly, targeting ligands are
injected to enable specific accumulation in tumors. Secondly, NPs are administered and will covalently bind with
the suitable tag on the targeting ligands. (b) Firstly, NPs are administered to accumulate in cancer cells either by
passive or active targeting. Secondly, imaging or therapeutic agents are injected and will bind with the reactive,
complementary tag previously deposited on the NPs.
4.1 PT approaches with ligands as targeting agents
An overview of recent reports using PT approaches with ligands as targeting agents is
presented in more detail in this section and summarized in Table 5.
Table 5. General overview of PT approaches using ligands as pretargeting agents.
Bioorthogonal
system
Primary tumor-targeting agent
for the first step of the PT
approach
Complementary agent for the
second step of the PT
approach
Application
Refs.
SPAAC
DBCO/N
3
Ac
4
MAnNAz (N
3
)
Liposomes (DBCO)
In vitro/in vivo optical imaging
and drug delivery
195
Ac
4
MAnNAz (N
3
)
Gelatin-oleic-DOX NPs (DBCO)
Drug delivery and anticancer
effect
45
Ac
4
MAnNAz (N
3
)
Liposome DBCO or liposome
TCO (2 step-targeting)
In vivo imaging and therapy
196
Ac
4
ManDBCO (DBCO)
QDs (N
3
)
In vitro optical imaging
197
Rituximab (DBCO)
Dendrimers (N
3
)
In vivo imaging and therapy
198
Metabolic lipid (N
3
)
RBCG (DBCO)
In vivo therapy
199
BCN/N
3
Ac
4
MAnNAz (N
3
)
Chitosan NPs (BCN)
In vivo optical imaging, MR and
CT imaging
200
Ac
4
MAnNAz (N
3
)
Au NPs (BCN)
In cellulo imaging
201
IEDDA
Tz/TCO
mAbs (anti-HER-2, anti-EGFR and
anti-EpCAM) (TCO)
MFNPs (Tz)
QDs (Tz)
in vitro fluorescence imaging
In vitro detection of intracellular
biomarkers
202
203
Antibiotics (Vancomycin and
Daptomycin) (TCO)
Crystal violet (TCO)
MFNPs (Tz)
MFNPs (Tz)
µNMR detection of Gram-
positive bacteria/Bactericidal
treatment
Detection of Gram-positive
bacteria
204
205
Primary targeting
agent
Secondary agent
(NPs for imaging or
therapy)
Bioorthogonal reaction
(a) Pretargeting with ligands
Primary agent (NPs)
Secondary agent
(imaging agent or
therapeutic agent)
Bioorthogonal reaction
(b) Two-step targeting with NPs as
« platforms »

42
Antigen-glutathione-S-transferase
(GST) (TCO/Tz)
NPs (TCO/Tz)
SPR sensor chip (interaction,
kinetics and functionalization
studies)
206
mAbs (anti-EGFR, Anti-EpCAM,
Anti-HER2 and Anti-MUC-1) (TCO)
MFNPs (Tz)
µNMR detection of intracellular
cancer biomarkers / Profiling of
tumor cells
207
pHLIP (Tz)
HSA-NPs (TCO)
In vivo photothermal therapy
208
Trastuzumab (anti-HER2) (TCO)
SN38-Tz-NPs
In vitro/In vivo drug delivery and
anticancer effect
209
Tz/Norbornene
Anti-EGF (Tz)
QDs (Norbornene)
In vitro optical imaging
54
N
3
: azide, DBCO: dibenzocyclooctyne, BCN: bicyclononyne, Tz: tetrazine, TCO: trans-cyclooctene, MFNPs: magneto-fluorescent
nanoparticles, QDs: quantum dots, Ac
4
MAnNAz: tetraacetylated N-azidoacetyl-D-mannosamine, HSA: human serum albumin
nanoparticles, pHLIP: low pH insertion peptide, DBCO : red blood cell ghosts.
Examples of SPAAC
SPAAC approaches have recently attracted increasing attention for enhancing the targeting
ability without using biological targeting moieties such as mAbs. The metabolic
glycoengineering method has been widely studied to incorporate bioorthogonal functional
groups on cell surfaces of interest for further conjugation of NPs, fluorescent dyes or drugs.
The functionalized synthetic sugars are usually specifically delivered to the target cells by N
3
-
modified sugar molecules loaded in nanocarriers via the EPR effect or by direct intratumoral
injection of these synthetic sugars.
DBCO/N
3
cycloaddition
In 2012, Koo et al. designed SPAAC click chemistry and metabolic glycoengineering for NPs
to produce artificial glycan receptors.
195
They selected tetraacetylated N-azidoacetyl-D-
mannosamine (Ac
4
ManNAz), an unnatural sialic acid with N
3
reactive groups, to generate
targetable azido-glycans on cell surfaces specifically recognized by PEGylated liposomes
modified with DBCO (DBCO-lipo) (Figure).
Figure 17. Schematic illustration of in vivo tumor-targeting strategy for DBCO-lipo nanoparticles by bioorthogonal
copper-free click chemistry. Figure modified from ref
195
.
N
3
N
3
N
3
Outside
Inside
Target cancer cell
Copper free click chemistry
Metabolic glycoengineering
Ac
4
ManNAz
DBCO group
NIRF dye, Cy5
DBCO-liposome

43
Flow cytometry studies on 50 µM Ac
4
ManNAz-treated A549 cells showed a 13.8-fold
increase in cellular uptake of DBCO-lipo compared with PEG-lipo. These liposomes were
intravenously administered and ex vivo tumor tissue analysis highlighted the advantages of
the multivalency properties of the NPs (e.g. several reactive DBCOs per NP) and a longer
circulation time in the blood to enhance tumor targeting compared with DBCO-Cy5 (NIRF
intensity: 180% vs 6%). They also confirmed the possibility of controlling the expression of
these azide chemical precursors in a dose-dependent manner for sufficient delivery of
DBCO-lipo. They observed tumor accumulation approximately 1.8 and 1.5 times higher in
tumor-bearing mice treated with 50 mM and 5 mM Ac
4
ManNA, respectively. Moreover, this
bioorthogonal based tumor-targeting strategy was validated in three other tumor cell lines
expressing Ac
4
MAnNAz (U87MG, MCF-7 and KB).
In 2018, Meghani et al. developed clickable NPs for cancer therapy.
45
Ac
4
MAnNAz was used,
as well as N
3
-sialic acid precursors specifically recognized by prior synthesized gelatin-oleic-
DBCO nanomaterials with embedded DOX (GON-DBCO-DOX). In vitro, GON-DBCO-DOX
improved A549 and MCF-7 cell cytotoxicity compared with GON-DOX after 24 hours with 10
µg/mL of DOX (for A549: 80% vs 50%; for MCF-7: 70% vs 50%). Furthermore, confocal
images after 4 hours of incubation demonstrated 2.4-fold and 4-fold higher cellular uptake on
A549 and MCF-7 cells, respectively. A pretargeting strategy for QD-N
3
able to bind cells
metabolically modified with ManDBCO, an unnatural analog sugar of Ac
4
ManDBCO, has also
been reported.
197
The cells were first labeled with DBCO, then QD-N
3
was added as a
secondary fluorescent imaging agent. Confocal images of ManDBCO-treated 4T1 cells
showed specific and increasing cellular uptake (i.e. 30% at 2 hours vs 45% at 4 hours)
compared with no cellular uptake for cells only treated with QD-N
3
.
A pretargeted nanoradioimmunotherapy strategy was investigated by Au et al. for the specific
delivery of radiolabeled PAMAM dendrimers for a targeting and therapeutic effect on Non-
Hodgkin Lymphoma (NHL).
198
The authors expected to deposit large amounts of ionizing
radiation in the tumor site to achieve a high rate of tumor cell death while minimizing the
effects on normal tissues. Rituximab, an anti-CD20 antibody (α-CD20) commonly used in
NHL immunotherapy, was functionalized with DBCO as described in
Figure.
Figure 18. Functionalization of the primary targeting agent rituximab (α-CD20) with DBCO. (f : number of
functions). Reprinted adapted with permission from ref
198
. Copyrright
2018
American Chemical Society.

44
The pretargeting strategy consisted of injecting intravenously the DBCO-Rituximab conjugate
in the first step. After 24 hours, N
3
-Y-90 dual-modified dendrimers were administered to react
with the previously injected modified mAb. Different amounts of DBCO (2, 5, 10, 15 and 18
DBCO per mAb) were conjugated per α-CD20): the α-CD20 grafted with 10 DBCO (i.e. α-
CD20(DBCO)
10
) was found to be the most appropriate to react with the N
3
-dendrimers.
However, the dendrimer conjugated with 29 N
3
-oligoethylene glycol (i.e. PAMAM D-
(
90
Y)
8
(N)
29
) was selected for its ability to form hyper-cross-links with α-CD20(DBCO)
10
, thus
improving activation of the complement-dependent cytotoxicity (CDC). The bioorthogonal
reaction between PAMAM D-(
90
Y)
8
(N)
29
and the DBCO-labeled Raji cells was confirmed
using both confocal microscopy and flow cytometry, with MFI almost twice as high as with
the direct labeling strategy (117 vs 68). An apoptosis study also showed 10-fold higher
therapeutic efficiency with the pretargeted approach compared with direct labeling; and β
−
radiation demonstrated 3.3-fold higher inhibition of cell proliferation.
In vivo experiments performed on Raji tumor-bearing mice revealed a significantly higher rate
of DNA damage associated with a 17% increase of the survival median compared with 10%
for clinical Rituximab and α-CD20(DBCO)
10
alone. Furthermore, tumor growth decreased for
approximately 67% of the treated mice. In vivo therapeutic studies on a more aggressive
disseminated lymphoma model confirmed higher tumor growth inhibition over time compared
with the direct labeling method (46 days vs 25 days).
BCN/N
3
cycloaddition
In 2017, Lee et al. also generated artificial N
3
-glycans (Ac
4
MAnNAz) to deliver
bicyclononyne-functionalized chitosan NPs (BCN-CNPs) to human mesenchymal stem cells
(hMSCs). They hoped to enable stem cell imaging using optical imaging (BCN-CNP-Cy5.5),
MR and CT imaging techniques (BCN-CNP-IRON
and BCN-CNP-GOLD).
200
In vitro, confocal
images showed higher cellular binding for Ac
4
MAnNAz/BCN-CNP-Cy5.5 with 99% labeled vs
31.6% for BCN-CNP-Cy5.5-treated stem cells. In vivo optical imaging confirmed SPAAC
between N
3
groups on the cell surface and BCN with a NIRF intensity 15 times higher than
with BCN-CNP-Cy5.5 alone. Moreover, the imaging signal of hMSCs remained for up to 15
days after transplantation compared with 5 days for BCN-CNP-Cy5.5. Finally, MR and CT
images confirmed these observations with 5.4-fold and 2.5-fold higher signals, respectively.
Examples of IEDDA
Tetrazine/TCO cycloaddition
Among bioorthogonal reactions, the Tz/TCO reaction is well documented for the two-step NP
delivery strategy. Most publications report the use of TCO-mAbs for the first step to enable
specific cell recognition then to facilitate NP attachment for imaging, diagnostic or drug
delivery purposes.
In 2010, Weissleder and co-workers named this approach ‘bioorthogonal NP detection’
(BOND) and more precisely two-step BOND (BOND-2) (Figure).
202
In their study, they
designed Tz-magneto-fluorescent NPs (Tz-MFNPs) and tested their targeting ability on
extracellular cancer cell receptors using mAbs (anti-HER-2, anti-EGFR and anti-EpCAM)
containing TCO (TCO-mAbs). They compared this BOND-2 method, in which the cells were
first incubated with TCO-mAbs and then with MFNPs, with BOND-1, which consisted in direct
labeling of the mAbs on the NPs before cell contact. They found BOND-2 to be up to 15

45
times superior to the mAb-MFNP method, resulting in a strong fluorescence signal at the cell
membranes. This amplification can be explained by the small size and high valencies (up to
30 TCO/mAb) of the bioorthogonal functions, allowing covalent binding of several NPs with
each mAb. They applied this two-step strategy using avidin/biotin interaction and
demonstrated signals approximately twice as low as with BOND-2 on HER-2 and EpCAM
cells. Steric hindrance of avidin (66-kDa) and a lower valency than Tz-MFNPs (8 biotins vs
84 Tz, respectively) could explain these results. They successfully adapted the strategy with
the same Tz-MFNPs, in situ, to detect intracellular biomarkers on previously fixed and
permeabilized cells using TCO-anti-CK Ab (SK-OV-3, HeLa, SK-BR-3 and PANC-1) or TCO-
anti-Ki-67 Ab (S-KOV-3, SK-BR-3, A549, HT-29 and PANC-1).
203
Fluorescent images were
also obtained with Tz-QDs and TCO-anti-CK Ab or TCO-anti-Ki-67 Ab on PANC-1 cells.
Figure 19. Application of the BOND strategy for one-step (direct, BOND-1) and two-step (bioorthogonal
amplification, BOND-2) targeting of MFNPs to cells. Figure modified from
202
.
In 2014, pretargeting NPs were used to detect intracellular cancer biomarkers in human
cells. Ghazani and his team used TCO-mAbs and Tz-MFNPs to profile lung tumor cells using
µNMR technology.
207
Fine needle aspiration (FNA) and peripheral blood samples from
routine biopsies were obtained from a cohort of 35 patients. The four biomarkers previously
identified (EGFR, EpCAM, HER-2 and MUC-1) established the type of lung malignancy.
Concordance of cancer biomarkers between circulating tumor cells (CTC) and FNA was
demonstrated as well as better diagnostic accuracy compared with conventional
histopathology.
More recently, Yoo et al. transposed this strategy by using TCO-modified trastuzumab (TCO-
Trb), which can bind HER2-overexpressed NIH3T6.7 cells, and tetrazine-linked NPs (Tz-
NPs), including chemotherapeutic SN38.
209
Using fluorescence microscopy, they
demonstrated in vitro that Trb treated with 300 eq. increased Tz-NP binding and induced a
subsequent effective reduction of cell viability (up to 45% for cells treated with a
concentration of 4 µg/mL of SN38). In agreement with these in vitro results, the in vivo
biodistribution of NPs intravenously injected 12 hours after TCO-Trb administration produced
NIRF signals twice as high as those found in tumor-bearing mice that did not receive the
TCO-Trb injection.
(a) One-step BOND (BOND-1)
(b) Two-step BOND (BOND-2)
MFNP
Ab
Ab
TCO
MFNP
Tz

46
Another two-step tumor-targeting strategy based on artificial chemical receptor expression on
the tumor cell surface is also described.
208
Tz was conjugated with a low pH insertion peptide
(pHLIP) known to cross the membrane bilayer in acidic conditions. The Tz-derivated pHLIP
(pTz) was successfully anchored to HeLa cells, vascular endothelial cells (VEC) and tumor-
associated fibroblasts (TAF). TCO-conjugated human serum albumin NPs were synthesized
and modified with indocyanine green (TCO-HSA-ICG NPs = THI) enable specific pTz
binding. In vivo studies on mice bearing HeLa tumors showed 2.6-fold and 1.7-fold higher
tumor accumulation with pTz/THI compared with THI and direct active targeting HSA-ICG
NPs modified with FA, respectively. Photothermal therapeutic efficiency was higher, with a
100% survival rate after 50 days and negligible tumor relapse.
Several investigations have involved the detection of different gram-positive bacteria.
204
In
2011, vancomycin and daptomycin antibiotics were modified with TCO (i.e. vanc-TCO and
dapt-TCO) for subsequent bioorthogonal labeling with Tz-MFNPs. These antibiotics were
able to bind with the specific peptidoglycan layer of gram-positive bacteria and inhibit cell
wall synthesis. Micro-nuclear magnetic resonance system (µNMR) studies revealed up to 6-
fold higher bacterial targeting on Staphylococcus aureus compared with direct conjugates.
These results reflect the ability of the chemically modified antibiotics to preserve their
bactericidal activity. PT has also been successfully applied to the intracellular detection of
bacteria in live macrophages in combination with permeabilizing agents.
In 2012, this technique was extended to detect and classify bacteria by µNMR and optical
imaging using a crystal violet modified with TCO (CV-TCO) and Tz-MFNPs.
205
Tassa et al.
reported Tz/TCO cycloaddition to produce sensor chips using Surface Plasmon Resonance
(SPR).
206
This system is based on the specific recognition of mAbs prior to immobilization on
the gold layer of the SPR sensor surface with antigen-glutathione-S-transferase (GST)
modified with TCO or Tz. Modifications allowed the subsequent bioorthogonal reaction with
Tz derivatives or TCO-NPs, respectively. This sensor chip achieved successful interaction
and good performance in kinetic studies and also functionalized NPs for molecular imaging
using the rapid and highly specific IEDDA cycloaddition reaction.
Tz/norbornene cycloaddition
In 2010, Han et al. developed norbornene-coated QDs for live cell imaging.
54
The reaction
between Tz-Alexa 594 and norbornene-QDs incorporated approximately 16 norbornenes per
QD. This strategy was applied for the targeting of A431 human carcinoma cells after
functionalization of the epidermal growth factor (EGF) with Tz (Tz-EGF). The fluorescence
intensity observed on cells for in situ conjugation (Tz-EGF/norbornene-QD) was higher than
with direct labelling (QD-EGF).
Other reactions
PT based on non-covalent interaction between large structures has also been reported
recently. Supramolecular binding, such as the ‘host-guest’ interaction, is known to have a
faster reaction rate (i.e. k
2
= 10
9
M
-1
s
-1
) than traditional bioorthogonal covalent reactions (i.e.
k
2
= 1-10
4
M
-1
s
-1
).
210
Some mAb targeting vectors (e.g. anti-HER-2, anti-EGFR and anti-
EpCAM) functionalized with β-cyclodextrin (CD) have been used with adamantane-MFNPs
as a secondary imaging agent for profiling cancer cells. This study demonstrated, in vitro,
that this kind of labeling was 15 times superior to the direct conjugation method (mAb-
MFNPs) and twice as effective as the non-covalent avidin-biotin system. The labeling

47
protocol was finally assessed using QDs and magnetic beads demonstrating highly specific
interactions; one possible application would be the separation of HER-2-positive and -
negative cells by a magnetic sorting process.
Covalent oxime click chemistry is described in another publication for the selective and
efficient delivery of nucleic acids to cells.
211
The cell surface was first engineered with ketone
by rapid fusion of ketone-functionalized liposomes. The cells expressing ketones were then
specifically recognized by adding oxyamine/nucleic acid lipoplex, which was subsequently
internalized and released for transfection in the cells. Using flow chemistry, the in vitro study
found that the number of ketone groups expressed on the cell surface was dependent on the
keto-liposome exposure time. This system presented higher selectivity and efficiency on
fibroblast cells compared with conventional reagent transfection (i.e. 68% vs 29% and 19%
for Viafect and Lipofectamine 3000, respectively).
4.2 PT approaches with NPs as a “platform”
Recent studies on PT approaches using NPs as a “platform” are summarized in Table 6.
Table 6. General overview of PT approaches using NPs as a “platform”.
Bioorthogonal
system
Primary tumor-targeting agent
for the first step of the PT
approach
Complementary agent for the
second step of the PT
approach
Application
Refs.
SPAAC
DBCO/N
3
Ac
4
MAnNAz-liposomes (N
3
)
Zinc(II)-phtalocyanine liposomes
(DBCO)
In vitro/in vivo photothermal,
photodynamic, photoacoustic
imaging and therapy
212
MIL-100 (Fe) NPs embedding 3-
azido-D-alanine (N
3
)
Ultrasmall photosensitizer NPs
embedding TPETM molecules
(DBCO)
In vivo imaging and PDT of
bacteria
213
MSNs (DBCO)
18
F (N
3
)
In vivo PET imaging
214
MSNs-RAW (DBCO)
18
F (N
3
)
In vivo PET-CT imaging
215
Ac4MAnNAz-PEG-PLA NPs
DBCO-Ce6
In vivo photodynamic therapy
216
BCN/N
3
Ac
4
MAnNAz-chistosan NPs (N
3
)
Ce6-chitosan NPs (BCN)
In vivo photodynamic therapy
217
Ac
4
MAnNAz-(N
3
)
CNP (BCN)
In vitro/in vivo optical imaging
200,218
IEDDA
Tz/TCO
89
Zr-liposomes (TCO)
PDA@CoCrMo (Tz)
Specific removal of long-
circulating radiopharmaceuticals
219
TCO-SiO
2
NPs
11
C (Tz)
In vivo/ex vivo PET imaging
220
SMNPs (TCO)
111
In-Tz-DOTA
In vivo optical and SPECT
imaging
221
Psi (TCO)
[
18
F]FDR-tetrazine (Tz)
In vivo PET imaging
222
N
3
: azide, DBCO: dibenzocyclooctyne, BCN: bicyclononyne, Tz: tetrazine, TCO: trans-cyclooctene, Ac
4
MAnNAz: tetraacetylated N-
azidoacetyl-D-mannosamine, TPETM: 2-(1-(5-(4-(1,2,2-tris(4-methoxyphenyl)vinyl)phenyl)thiophen-2-yl)ethylidene)malononitrile),
MSNs: mesoporous silica nanoparticles, SMNPs: small molecule-based nanoparticles, PDA: polydopamine, CoCrMo: cobalt chromium
molybdenum alloy, Psi : Mesoporous Silicon.
Examples of SPAAC

48
DBCO/N
3
cycloaddition
In 2017, Du et al. also investigated a two-step tumor-targeting strategy based on metabolic
glycoengineering and SPAAC to deliver their multifunctional nanoagent to combine PTT,
PDT, PA imaging and PAT (Figure20).
212
First, Ac
4
MAnNAz was encapsulated by self-
assembly in nanomicelles (Ac
4
MAnNAz-LP) to generate the artificial chemical-receptor in
tumor cells, thanks to the EPR effect, after intravenous injection. DBCO-nanomicelles
embedded near infrared dyes zinc(II)-phthalocyanine (DBCO-ZnPc-LP) were designed for
both PTT and PA imaging. In vitro, PTT performed with a 0.4 W/cm
2
continuous laser on
A549 cells showed higher cytotoxicity for Ac
4
MAnNAz-LP/DBCO-ZnPc-LP than DBCO-ZnPc-
LP alone (80% vs 35%). Moreover, Ac
4
MAnNAz-LP/DBCO-ZnPc-LP-treated cells
demonstrated superior cytotoxicity with a combination of PTT/PAT compared with PTT and
PAT alone with 63%, 22% and 20% cytotoxicity, respectively. PA imaging on in vivo
experiments revealed 3-fold higher tumor uptake after 24 hours for Ac
4
MAnNAz-LP/DBCO-
ZnPc-LP compared with DBCO-ZnPC-LP. ICP-MS analyses showed that mice pre-treated
with Ac
4
MAnNAz-LP for 3 days had a higher tumor uptake 6 hours after injection than no
those with no pre-treatment (6.30% IA/g vs 1.85% IA/g), and tumor growth inhibition over
time (18 days) with synergistic PTT/PAT.
Figure 20. Schematic illustration of PA Imaging-Guided Synergistic PTT/PAT with the Bioorthogonal Metabolic
Glycoengineering-Activated Tumor Targeting Nanoagent. Reprinted adapted with permission from ref
212
.
Copyright 2017 American Chemical Society.
In 2018, Mao et al. performed specific in vivo imaging of bacteria and antibacterial treatment
using clickable NPs.
213
Two different NPs were administered for subsequent bioorthogonal
conjugation. Firstly, MIL-100 (Fe) NPs encapsulating the 3-azido-D-alanine metabolic
precursor (D-AzAla@MIL-100 (Fe)) were injected and accumulated in the infected regions of
mice bearing methicillin-resistant Staphylococcus aureus (MRSA). These NPs decomposed
in the presence of H
2
O
2
secreted by the immune cells and released unnatural azido groups
which were then metabolically expressed on MRSA bacterial walls. In the second step,
ultrasmall DBCO-modified photosensitizer NPs integrating TPETM molecules (US-TPETM
NPs) were administered for both imaging and photodynamic therapy (PDT). In vivo azido

49
incorporation efficiency was evaluated using a DBCO-Cy5 fluorophore and demonstrated
3.2-fold higher fluorescence thanks to H
2
O
2
responsiveness compared with normal tissues.
In vivo, a stronger fluorescent signal was obtained after 24 hours in bacteria-bearing mice
pre-treated with D-AzAla@MIL-100 (Fe) compared with mice only treated with US-TPETM
NPs. The antibacterial effect was also significantly enhanced after white light irradiation for
10 min (p < 0.05).
In 2013, Lee et al. described the first preclinical pretargeting application of SPAAC in PET
imaging.
214
In situ formation of
18
F-DBCOT-PEG-MSNs thanks to the two-step strategy was
reported for in vivo real-time tracking imaging (pharmacokinetic studies). DBCO-PEG-MSNs
were first injected intravenously into U87MG tumor-bearing mice to encourage accumulation
thanks to the EPR effect. 24 hours later, [
18
F]fluoropentaethylene glycolic N
3
([
18
F]2) was
administered and clicked specifically to the NPs within 2 hours. Biodistribution studies
showed a significantly higher signal for DBCO-PEG-MSNs-pre-treated tumors (250 µg, 30
nmol DBCO) compared with [
18
F]2 alone, at 2.6 MBq (1.4% IA/g vs 0.6% IA/g).
A pretargeting PET imaging strategy was also assessed in 2019 for macrophage cell tracking
in tumors and atherosclerosis plaques using
18
F labeling to investigate the ability of the
macrophages to accumulate in the affected areas.
215
DBCO-MSN cells were thus incubated
with RAW 264.7 macrophage cells for 2 hours at 37 °C to obtain the desired DBCO-MSN-
RAW cells. These DBCO-MSN-RAW cells were then intravenously injected into U87MG
tumor-bearing mice. Between one and eight days later, the mice were injected with 11.1 MBq
of
18
F-N
3
. PET-CT images demonstrated a larger accumulation of radioactivity at the tumor-
site compared with
18
F-N
3
alone (3.8% IA/g vs 2.5% IA/g). Finally, this specific binding and
macrophage tracking method was also assessed on mice with atherosclerosis and showed
similar efficient accumulation in the atherosclerotic aorta area.
BCN/N
3
cycloaddition
One study reports that the tumor accumulation of glycol chitosan NPs (CNPs) can be
increased by metabolic glycoengineering.
217
CNPs functionalized with Ac
4
MAnNAz
(Ac
4
MAnNAz-CNP) by hydrophobic interaction were intravenously injected into mice bearing
A549 tumors for accumulation thanks to the EPR effect. Other CNPs functionalized with BCN
and chlorin e6 photosensitizers (BCN-Ce6-CNPs) were subsequently injected as secondary
agents. In vitro, the pre-targeting strategy showed significantly higher cytotoxicity on A549
cells compared with the non-pre-treated BCN-Ce6-CNPs after laser irradiation (90% vs
40%). In vivo, the biodistribution study revealed a significant 2-fold higher tumor uptake for
Ac
4
MAnNAz-CNP/BCN-Ce6-CNPs compared with BCN-Ce6-CNPs. Moreover, efficient
photodynamic therapy was observed 21 days after laser irradiation with no tumor relapse
compared with BCN-Ce6-CNPs (p<0.01), for which growth restarted after 4 days.
Examples of IEDDA
Tetrazine/TCO cycloaddition
In 2016, Denk et al. performed in vivo click experiments using TCO-MSNs and low-molecular
weight
11
C-tetrazine for PET imaging (Figure 21).
220
They exploited the ability of these NPs
to achieve rapid and exclusive accumulation in the lungs for the investigation of pretargeted
PET imaging using bioorthogonal chemistry. TCO-MSNs were first administered for effective
accumulation in lungs, before the
11
C-Tz was injected five minutes later. Two-step protocol

50
PET imaging demonstrated a 3-fold increase in activity concentration, and therefore in the
gamma counter signal, compared with
11
C-Tz. In recent years, pretargeting NPs have been
introduced as a suitable tool for nuclear imaging and radiotherapy.
64
Figure 21. (A) In vivo click experiment using TCO-and s-TCO-modified silica nanoparticles (chemical structure
shown for TCO). (B) Activity concentration measured in vivoby PET imaging (50-60 min) and ex vivo(after 60 min,
gamma counter) showing increased lung uptake in female BALC/c mice when using s-TCO-MSNs (n=5) and
TCO-MSNs (n=2). (C) Pretargeted PET image (sagittal view, 40-60 min): TCO-MSNs + [11C]-1(scale bar unit:
SUV; b = brain, bl = bladder, k = kidney, lu = lung. Reprinted adapted with permission from ref
220
. Copyright 2016
American Chemical Society.
In 2019, van Onzen et al. used IEDDA cycloaddition to radiolabel π-conjugated small
molecule-based NPs (SMNPs) for both optical and SPECT imaging.
221
The covalent
conjugation between the
111
In-radiolabeled Tz-DOTA and TCO-NPs was confirmed
intrinsically. SMNPs were used as dual-imaging agents for biodistribution studies and
accumulated in the liver and spleen (80.4% IA/g and 34.8% IA/g, respectively) 4 hours after
injection but were efficiently cleared from the blood after 10 min (0.9% IA/g). However, this
imaging strategy is limited for long-life radionuclides (e.g.
64
Cu and
89
Zr) which could deposit
large amounts of radiation in non-targeted organs (e.g. liver and spleen).
To circumvent this problem, Brand et al. recently designed an IEDDA-based platform for the
specific removal of long-circulating radiopharmaceuticals from the systemic circuit.
219
Tz-
polydopamine-coated metal disks (Tz-PDA@CoCrMo) were developed to remove the TCO-
89
Zr-radiolabeled liposomes (
89
Zr-TCO-LNP) that remained in the blood after EPR
accumulation in tumor cells. Phosphor autoradiography assays demonstrated 2-fold higher
radioactivity on the disk for
89
Zr-TCO-LNP compared with PDA@CoCrMo.
5. Click chemistry for multifunctionalized NPs
In the previous paragraphs of this manuscript, we have attempted to identify the potential
roles of click chemistry in the field of nanoparticles. This early 20
th
century chemistry offers a
solution for encapsulating therapeutic agents, functionalizing imaging agents or therapeutic
agents on the surface of NPs or grafting targeting agents to address NPs specifically
according to the expression or overexpression of tumor receptors in order to amplify tumor
uptake. Bioorthogonal chemistry (SPAAC or IEDDA) also provides a targeting approach
based more specifically on monoclonal antibodies, probably due to the rapid development of
anti-cancer immunotherapy over the last ten years. In this section, we present the
opportunities offered by click chemistry for the development of multimodal NPs (nano-objects
combining several imaging modalities: PET, SPECT, NIRF, MRI) or theranostic NPs (nano-
objects combining imaging and therapeutic agents). Among all the articles reviewed to
establish this bibliographic analysis, we focused particularly on articles published since 2016.

52
Table 7. General overview of multimodal or/and theranostic NP.
Multimodal
or/and
Theranostic NP
Type
of NP
Type of
click chemistry
Type of imaging
Therapeutic
approach
Passive or Active targeting
In vitro/In vivo
studies
Refs.
Multimodal
Glycol chitosan NP
N
3
/DBCO
FITC/PET
Passive
In vivo
108
Liposome
N
3
/DBCO
FITC/PET
Passive
In vivo
109
Iron nanoparticle
N
3
/alkyne
MRI/HIR
Active
In vitro
132
Porous silica NP
N
3
/DBCO
FITC/PET
Active
In vitro/in vivo
134
Glycol chitosan NP
N
3
/BCN
FITC/MRI
Active
In vitro/in vivo
217
Theranostic
Liposome
N
3
/DBCO
FITC/PA
PTT
Active
In vivo
212
Glycol chitosan NP
N
3
/BCN
FITC
PDT
Active
In vitro/in vivo
217
HSA-NP (albumine)
Tz/TCO
FITC
PTT
Active
In vitro/in vivo
208
Liposome
N
3
/BCN
FITC
Drug carrier
Passive
In vitro/in vivo
229
Liposome
Tz/vinyl ether
FITC
PTT/Drug carrier
Passive
In vitro/in vivo
231
LQ /DOX-ZnPc nanocomposite
N
3
/DBCO
PTT/Drug carrier
Active
In vitro/in vivo
223
Multimodal/Theranostic
Porous silica NP
N
3
/DBCO
FITC/SPECT
Drug carrier
Active
In vitro/in vivo
224
Ac4MAnNAz-PEG-PLA NPs
+ DBCO-PEG-PAEAM NPs
MRI
PDT
Active
In vitro/in vivo
225
Liposome
NIRF
PTT
Active
226
53
N
3
: azide, DBCO: dibenzocyclooctyne, BCN: bicyclononyne, FITC : Fluoresceine-5-isothiocyanate , PET : positron-emission tomography, SPECT: Single-photon emission computed tomography, PDT : photodynamic therapy, PTT :
photothermal therapy, LQ : (LMWH)-quercetin (Qu) conjugate, DOX : doxorubicin, ZnPc : zinc phthalocyanine, PAEAM : poly(2-azepane ethyl methacrylate), NIRF : Near infrared fluorescence imaging.

54
5.1. NPs using click chemistry for multimodal imaging
As indicated by Lee et al.,
108
imaging techniques, such as computed tomography, magnetic
resonance imaging, and positron emission tomography (PET), off er many potential benefits
for the diagnosis and treatment of cancers. Each method has its own strengths and
weaknesses. Multimodal imaging techniques have thus been pinpointed as an alternative
method to overcome the limitations of each individual imaging technique. The authors also
developed a dual optical/PET imaging CNP (glycol chitosan nanoparticle). The azido group
available on the surface of the NP allows grafting of the 64Cu-radiolabeled DOTA complex
and the activatable MMP-probe via copper-free click chemistry (Figure ). To validate the use
of dual PET/activatable NIRF probe-labeled CNPs for tracking nanoparticles and imaging the
tumor in vivo, whole-body NIRF and PET images were obtained from A549 tumor-bearing
mice after intravenous injection of AMP-CNP-DOTA-
64
Cu. The PET images revealed that the
accumulation of AMP-CNP-DOTA-
64
Cu in the tumor increased gradually with time, reaching
a plateau 24 hours after injection (5% ID/g). To confirm these in vivo multimodal imaging
observations, the NIRF signals of the tumor and other major organs were quantified and the
highest NIRF signal intensity was found in the tumor region.
Figure 22. Chemical structure of azide-functionalized glycol chitosan-5β-cholanic acid conjugate (CNP-N
3
) and
schematic illustration for the labelling of DOTA-Lys-PEG
4
-DBCO with
64
CU and AMP-DBCO (AMP-DBCO is
composed of MMP-specific peptide, NIRF dye (Cy5.5), dark quencher (BHQ-3), and DBCO) onto azide-
functionalized CNP via bio-orthogonal click chemistry. Reprinted with permission from ref
108
. Copyright 2014
American Chemical Society.
In a similar approach, Perez-Medina et al. developed a dual-modality liposomal nanoparticle
combining a PET label and a near-infrared (NIR) fluorophore, bringing together the best of
both worlds, i.e. the high sensitivity and tissue penetration of PET and the cellular resolution
of optical imaging.
227
In this study, copper-free click chemistry was used to graft the labelling
55
89
Zr probe. The fluorescent dyes were incorporated into the lipid bilayer of the liposomal NPs.
In vivo biodistribution of the
89
Zr-CLL was compared with that of
89
Zr-SCL in mice bearing
4T1 breast cancer xenografts (for the latter, no click chemistry was used and the radionuclide
was chelated by DFO grafted onto the surface of the NPs). However, no
89
Zr-CLL signal was
detected in the tumor, unlike the
89
Zr-SCL (up to 24% ID/g in the tumor).
Yuan et al. used the powerful click chemistry (SPAAC) method to generate multifunctional
nanomaterials to allow MRI detection of NPs (superparamagnetism of iron NPs),
fluorescence (click attachment of fluorochromes), and radioactivity.
132
The click chemistry
group available on the surface of the NP thus allows the grafting of different fluorescent
probes as well as the association of targeting agents (folate, RGD or protamine). This
multimodal approach to nanoparticles, which can be surface functionalized by different
targeting agents using click chemistry, could enable the design of a "universal" nano-object,
suitable for a range of cancer pathologies. This nanoparticle has only been evaluated in vitro
for some targeting agents.
Wang et al. developed azido-modified silica nanoconjugates (azido-/Cy5-NCs) that not only
enabled dual-modal PET/CT and fluorescence imaging but could also target the cell-surface
DBCO groups via SPAAC click chemistry.
134
Azido and DOTA (for radiolabeling with
64
Cu)
groups were grafted onto the surface of NCs and fluorescent dyes were encapsulated inside.
For this in vivo study, LS174T tumors were developed in athymic nude mice by
subcutaneous injection of LS174T cells into both flanks, then Ac
4
ManDBCO moieties (5
mg/kg) were injected intratumorally (i.t.). Ac
4
ManDBCO can be incorporated into the cell
membrane surface, thus creating "artificial tumor receptors". The combination of the
Ac
4
ManDBCO system and click chemistry demonstrated advantages in terms of high
targeting efficiency, absence of immunogenicity, and easy manufacture.
64
Cu-/azido-/Cy5-
NCs or
64
Cu-/Cy5-NCs were injected intravenously and biodistribution was monitored by
PET/CT imaging. Measurement of the radioactivity of the tissues harvested 24 hours after
injection showed a 1.7-fold accumulation of
64
Cu-/azido-/Cy5-NCs in the tumors on the left
pretreated with Ac4ManDBCO compared with the tumors on the right that were not
pretreated with glycoengineering sugar. However, the difference between the left- and
righthand tumors was negligible in terms of accumulation of
64
Cu-/Cy5-NCs without azido
modification. Moreover, confocal imaging of tissue sections of Ac
4
ManDBCO-treated tumors
showed a noticeable amount of azido-/Cy5-NCs accumulated in tumors 6 hours after
injection, and a clear Cy5 fluorescence contrast was observed between the tumors on the left
pretreated with Ac
4
ManDBCO and the tumors on the right pretreated with PBS.
In 2017, Lee et al. also investigated this glycoengineering system combined with modified
glycol chitosan nanoparticles (BCN-CNPs) to deliver different imaging agents: Cy5.5, iron
oxide NPs and gold NPs were conjugated with or encapsulated into BCN-CNPs for optical,
MRI and CT imaging.
200
They started by testing three different glycoengineering systems
(Ac
4
ManN
3
, Ac
4
GalN
3
and Ac
4
GlcN
3
). Importantly, Ac
4
ManN
3
-treated hMSCs produced a
significantly higher signal in membrane extract compared with other hMSCs treated with
Ac
4
GalN
3
or Ac
4
GlcN
3
. To evaluate this imaging method to target chemical receptors, they
then tested the imaging efficiency (MR and CT) of BCN-CNPs containing iron oxide
nanoparticles (BCN-CNP-IRON) or gold nanoparticles (BCN-CNP-GOLD) to track stem cells
in live animals. Approximately 100% of the cells were labeled with each nanoparticle,
showing positive signals for both imaging techniques.
56
5.2. NPs using click chemistry for a theranostic approach
The two-step tumor-targeting strategy based on metabolic glycoengineering and click
chemistry, an emerging tumor-targeting technique that has already demonstrated high tumor-
specificity with a remarkable advantage over biological receptors, has also been used to
develop theranostic NPs. The artificial chemical-receptor can be expressed on the cell
surface in large quantities regardless of the type or subpopulation of the tumor cells. Du et al.
prepared a nanoagent (DBCO-ZnPc-LP) by self-assembling a single lipophilic near-infrared
(NIR) dye, zinc(II)-phthalocyanine (ZnPc), with a lipid-poly(ethylene glycol) (LP), and then
modified it further with dibenzyl cyclootyne (DBCO) to introduce the two-step chemical tumor-
targeting strategy based on metabolic glycoengineering and click chemistry.
228
To improve
the efficiency of Ac4ManN
3
delivery to the tumor site, the Ac4ManN
3
was encapsulated by
DSPS-PEG2000-NH
2
to obtain the nanomicelle Ac
4
ManN
3
-LP, which successfully generated
artificial receptors after intravenous injection both in vitro and in vivo. DBCO-ZnPc-LP is a
multifunctional phototheranostic NP that combines photothermal therapy (PTT) and
photoacoustic therapy (PAT). After intravenous injection of DBCO-ZnPc-LP into A549 tumor-
bearing mice pretreated with either saline or Ac
4
ManN
3
-LP, the PA signal in the tumor sites
was substantially higher in the group pretreated with Ac4ManN
3
-LP; this may be attributed to
the dual targeting eff ect of EPR and the subsequent binding reaction with the “receptor-like”
azides on the tumors. With the significant tumor-targeting strategy, PA imaging, and the
synergistic eff ect of PTT and PAT, the positions of the solid tumors are identified precisely
and eradicated completely with few side eff ects in vivo, compared with PAT or PTT
approaches. These targeting NPs can not only convert NIR light into heat for eff ective
thermal ablation but also induce a thermal-enhanced ultrasound shockwave boost to trigger
highly localized mechanical damage, achieving a synergistic anti-tumor eff ect.
Lee et al. also developed NPs based on metabolic glycoengineering and click chemistry
associating NIRF and PDT approaches (Figure ).
229
Like Du et al., they intravenously
injected the Ac
4
ManN
3
after loading it into CNPs to generate artificial receptors. They then
developed a second BCN-Ce6-CNP functionalized on the surface by 39 molecules of Chlorin
e6 (photosensitizer) and 37 molecules of BCN (bioorthogonal agent), which were
intravenously injected into tumor-bearing mice pretreated with saline, free Ac
4
ManN
3
, or
Ac
4
ManN
3
-CNP. Signal intensity in the tumor tissue was approximately 1.8 times higher in
the mice pretreated with Ac
4
ManN
3
-CNP than in the groups pretreated with saline or free
Ac4ManNAz, and more than 10 times higher than in those pretreated with free Ce6. After
laser irradiation, significant bleeding and black scab generation were observed in the mice
treated with Ac
4
ManN
3
-CNP and BCN-Ce6-CNP. This is direct proof of tumor tissue
destruction by excessive local generation of cytotoxic singlet oxygen.

57
Figure 23. Schemes of the tumor-targeting strategy using nano-sized metabolic precursors (Nano-MPs) and
bioorthogonal click chemistry. (a) The synthesis of Nano-MPs containing azide groups (-N3). (b) Illustration for the
tumor tissue-specific generation of azide groups by Nano-MPs and metabolic glycoengineering. Then, the
generated azide groups on tumor cells can be specifically targeted with bioorthogonal chemical group-conjugated
Cy5.5 or liposomes via in vivo bioorthogonal click chemistry. Reprinted adapted with permission from ref
229
.
Copyright 2017 Elsevier.
Lu et al. also used the glycoengineering system with IEDDA biorthogonal chemistry to create
tetrazine artificial receptors on tumors after intravenous injection of pHLIP-Tz (Figure).
208
Indocyanine green (ICG)-loaded and trans-cyclooctene (TCO)-conjugated human serum
albumin (HSA) nanoparticles (TCO-HSA-ICG NPs, denoted THI) were fabricated as
representative carriers and administrated in a second time for both NIRF imaging and PTT.
Tumor accumulation in the pTz/THI group was about 2.6 times higher than that of the THI
groups. The tumors in the pTz/THI group were completely ablated after one session
photothermal therapy, with negligible tumor growth or regrowth and a survival rate of 100%
at day 50.

58
Figure 24. Schematic illustration of the iEDDA‐based two‐step tumor‐targeting strategy. a) Preparation of
TCO‐HSA‐ICG NPs (THI). b) Tz‐modified pHLIP (pTz) transferred to tumor in a pH‐dependent manner and
generated chemical receptors on the membrane of various cells, which were subsequently used as the baits for
THI binding followed with photothermal therapy. Reprinted adapted with permission from ref
208
. Copyright 2018
Wiley-VCH.
Qiao and co-workers developed a copper-free click-targeting LMWH-quercetin
nanocomposite system (DLQ/DZ) that improved the specific co-delivery of doxorubicin and
the photosensitizer zinc phthalocyanine (PTT application) against breast cancer cells by
glycoengineering the tumor cell surface with an azide-modified sugar (Ac4ManN
3
) (Figure
25).
223
This co-treatment combination (chemotherapeutic agent and PTT) was studied
because it is known that PTT enhances tumor sensitivity to chemotherapeutic agents. The
use of artificial receptors and the glycoengineering system necessitated this synergic
treatment (with intratumoral rather than intravenous injection of Ac4ManN
3
). With the
DLQ/DZ system, the esterase or acidic tumor environment hydrolyzed the ester bond of the
DLQ to release both ZnPc and DOX. ZnPc was then irradiated by NIR laser to increase
temperature inside the tumor cells to cause irreversible apoptosis via tumor cell protein
denaturation and coagulative necrosis. The authors observed that Ac4ManNAz+DLQ/DZ
treatment augmented the anti-cancer effect of combined chemotherapy and PTT (96.1%
inhibition rate), nearly eliminating the tumor, thus demonstrating the mutually synergistic
performance of this therapeutic approach.

59
Figure 25. Schematic engineered of the click-targeting nanocomposites for chemo-photothermal synergistic
therapy. Stage I: Production of azide groups on the surface of tumor cells by metabolism glycoengineering. Stage
II: (A) Nanocomposites accumulated in tumor tissue through EPR effect. (B) Nanocomposites bound to tumor cell
via bio-orthogonal copper-free click chemistry. (C) Chemotherapy combined with photothermal therapy to
synergize anti-tumor by DNA damage of doxorubicin (DOX) and thermal ablation of zinc phthalocyanine (ZnPc)
upon laser irradiation. Reprinted adapted with permission from ref
223
. Copyright 2020 Wiley-VCH.
Xie et al. conducted two very interesting studies on prodrugs that can be cleaved in vivo by a
copper-free biorthogonal approach (Figure).
230
First, they studied a nanosystem for NIRF
and prodrug activation. Then, they developed a tetrazine derivative linked with a near-
infrared dye (Tz-NR) to be used as the trigger, as well as a fluorescence probe and the
camptothecin vinyl ether prodrug (vinyl ether-masked CPT). The trigger, Tz-NR, mediates
cleavage of the vinyl ether group and induces the release of CPT. The molecular prodrug
and Tz-NR can be encapsulated separately into liposomes, forming a two-component
liposomal bioorthogonal system for imaging and tumor inhibition. As long as the tetrazine is
connected to the NR probe, there is no fluorescence (TBET-based system). Fluorescence
imaging is only visible once the iEDDA chemical reaction has taken place because the TBET
phenomenon is then no longer effective. The presence of fluorescence proves that the CPT
has been released. In vivo fluorescence imaging was performed by intravenously injecting
both the liposomal NIR fluorescent dye LIP-NR-Tz and the LIP-prodrug into tumor-bearing
mice and observing fluorescence images at predetermined times (0, 1, 4, 12, 24, and 48
hours) after injection. In the tumor region, fluorescence emerged 4 hours after injection,
reaching a maximum after 12 hours and then gradually decreasing. The tumor inhibition
capability of the liposomal system was investigated in an HeLa xenograft tumor model and
proved very effective. High accumulation and retention, associated with the efficiency of the
bioorthogonal reaction of the liposomal bioorthogonal system, may contribute to this high
anti-tumor efficacy. Based on this success, the authors then developed a similar tetrazine-
mediated bioorthogonal system for pro-drug activation, photothermal therapy and
optoacoustic imaging. This time, the system’s trigger was fabricated by immobilizing
PEGylated tetrazine on the gold nanorods (AuNR-PEG-Tz), and the bioorthogonal prodrug

60
was synthesized by caging the drug camptothecin with vinyl ether, and encapsulating it with
phospholipid liposomes (LIP-VE-CPT). The combined therapy group (AuNR-PEG-Tz + LIP-
VE-CPT + laser) displayed the highest tumor inhibition efficacy and the lowest relative tumor
volume during this investigation.
Figure 26. Schematic overview of the two-component Bioorthogonal Nanosystem for Imaging and Tumor
inhibition. Reprinted adapted with permission from ref
230
. Copyright 2019 American Chemical Society.
5.3. NPs using click chemistry for imaging modality and a theranostic
approach
Wang et al. developed a dual-labeled iRGD-modified multifunctional porous silicon
nanoparticle (Psi NP) modified by DBCO and amino groups on its surface and able to
encapsulate a chemotherapeutic agent (sorafenib).
134
The Alexa Fluor 488 and DBCO were
conjugated with the Psi NPs via amide linkage. The DBCO groups were then used to graft
both peptide iRGD and macrocycle DOTA for radiolabeling via SPAAC. Mice bearing
prostate cancer xenografts were studied by SPECT imaging, and in vivo biodistribution of
111
In-radiolabeled multifunctional Psi and Psi-iRGD NPs was determined after intravenous
administration. The intravenously administered iRGD-modified NPs achieved higher tumor-
specific accumulation compared with the PSi NPs, based on the 4.4 vs. 2.7 tumor-to-muscle
ratios of the injected dose per gram (ID%/g), for Psi-iRGD and Psi, respectively. iRGD
peptide surface modification of the nanocarriers increased the tumor accumulation of the
latter. The antitumor effect of the multifunctional NPs loaded with Sorafenib (SF) was then
assessed in vivo. After two cycles of intravenous administration 24 hours apart, both SF-
loaded NPs (Psi-SF and PSi-iRGD-SF corresponding to 3 mg/kg of SF) affected tumor
growth in a manner similar to that of the free SF. The authors indicated that this

61
disappointing result was probably due to the fast release of the SF from the Psi matrix into
the blood after intravenous administration. Nevertheless, the authors demonstrated the
development and efficacy of a multifunctional Psi nanocarrier, making the multimodal
nanosystem presented a promising nanotheranostic PSi system for cancer diagnosis and
treatment.
6. Nanoparticles, click chemistry and protein corona
Through the different examples from the literature cited in the previous paragraphs of this
review, we can ascertain the rightful place of click chemistry in the field of nanomedicine.
Although the literature proposes few comparisons of traditional functionalization of NPs and
click chemistry, it seems very clear that this chemistry constitutes an asset for grafting
molecules, radiosensitizing elements, optical imaging or ligands, regardless of the type of
NP. Compared with traditional coupling reactions, which require organic solvents, high
temperature/pressure or toxic catalysts, click chemistry avoids these constraints and
represents an opportunity for an effective, direct reaction in the aqueous phase in biological
matrices (Table 6).
Table 8. Main advantages and disadvantages of click chemistry reactions currently used in nanomedicine
Click
chemistry
Advantages
Disadvantages
CuAAC
- These click groups are small (no steric
hindrance)
- Many azide and alkyne groups are
commercially available
- Ease of adding azide and/or alkyne groups
on NP
- Possibility of reaction in both organic and
aqueous media
- High second order reaction rate constant
- Requires copper catalysis and risk of copper
contamination on the surface of NP (problem for
human application)
- Risk of interaction between azide groups on the
surface of NP due to the zwitterionic form
Thiol-ene
- Ease of adding alkene groups on NP
- Click groups are small (no steric hindrance)
- Possibility of reaction in both organic and
aqueous media
- Soft conditions (pH, temperature) for the
reaction
- No catalyst
- Difficulties working with thiol groups
- Risk of polymerization between different alkene
groups on the surface of NP
- Risk of formation of intra or inter disulfide bridge
- Risk of competition with other thiols of the
environment
SPAAC
- Bioorthogonal entities are commercially
available
- Moderate reaction kinetics
- Reaction in both organic and aqueous
media
- Soft conditions (pH, temperature) for the
reaction
- One small and one large structure
- No catalyst
- Risk of p-stacking with strained-alkyne group if
their density is too high on the surface of NP
- Moderate second order reaction rate constant
IEDDA
- Biorthogonal entities are commercially
available
- Very high second order reaction rate
constant
- -No catalyst
- Tetrazine and TCO do not react with azide
groups: possibilities to have tetrazine (or
TCO) and azide groups on the surface of a
same NP
- Reaction in both organic and aqueous
media
- Isomerization of TCO
- Risk of p-stacking with tetrazine groups if their
density is too high on the surface of NP
- Instability of the reactive groups under too acidic or
basic conditions
- Two large structures
The thiol-ene approach is much less used than the other three click reactions. However, it
could prove to be relevant with regard to the biomolecules that can be grafted onto the NPs.
No azide or alkyne functional groups are found among native biomolecules, thus imposing
the specific introduction of these groups into proteins or DNA. Compared with the azide–
62
alkyne reaction, the thiol functional group of cysteine-containing proteins makes
bioconjugation more readily achievable through a thiol–ene click reaction.
62,232
One of the main advantages of click chemistry is that it appears to allow the modification of
molecules or ligands on the surface of NPs, regardless of the design of the nano-object itself.
Once the click entities are available on the surface of the nanoparticle, they can be used to
graft different groups depending on the desired application. This is particularly important,
since many molecules functionalized with bioorthogonal entities are now commercially
available. The bioorthogonality of these different reactions thus makes it possible to envisage
incorporating different functionalities into the same NP (for example, a fluorescent probe, a
drug, a peptide, a monoclonal antibody, etc.). Concerning the PT approach, we think that a
single nano-object, functionalized on the surface with bioorthogonal entities, could be used
for different cancer pathologies. The complementary bioorthogonal entities simply have to be
grafted onto monoclonal antibodies with a high affinity for the receptors (over)expressed in
the tumor. However, click chemistry is not yet a paradigm in nanomedicine. When NPs are
exposed to biological fluids and/or a tumoral microenvironment, their surface becomes
covered with biomolecules and/or proteins. The "protein corona" that forms around the NPs
is described as "hard" or "soft", depending on the binding of the proteins adsorbed onto the
surfaces of the NPs. The energetically favorable process of protein corona formation is very
complex and has a significant impact on the original physicochemical properties of the NPs,
possibly altering their biological identity between in vitro and in vivo evaluations.
233
The
presence of proteins is all the more important in the PT strategy. Aside from the possible
steric hindrance of the covalent reaction between the two-bioorthogonal entities present
respectively on the antibody and the NP, the PT strategy cannot be implemented if the
protein corona prevents the click reaction at the tumor site. Moreover, in the case of the PT
strategy, can we still use the term active targeting? For nanoparticles, the notion of anchoring
is likely to be more relevant than the concept of active targeting. For the PT strategy, the NP,
which is injected in a second step, is distributed in vivo by the EPR effect, and then
“attached” to the mAb when click chemistry takes place at the tumor site.
In any case, perfect engineering of the surface ligands is essential for the future of PT
approaches with NPs. Recent works have discussed the importance of using non-fouling
moieties to prevent protein corona formation as this protein layer aff ects the properties of
nanomaterials, altering their behavior and masking engineered functionalities.
234
Jiang et al.
recently published a complete review of antifouling strategies for selective in vitro and in vivo
sensing.
235
A wide range of molecular systems, such as polyethyleneglycol (linear or
branched PEG, >10 EG units), zwitterionic species, peptides with alternating or random
mixed-charge and other polymers like polysaccharides, polyoxazolines, poly(hydroxy
acrylates), and hyperbranched polyglycerol (HPG), have been shown to possess significant
antifouling properties, mainly resulting from their high hydration. Moyano et al. first described
the antifouling effect of sulfobetaine zwiterrionic head groups for NPs. AuNPs coated with
PEG3-sulfobeteaine ligands did not adsorb proteins at moderate serum protein
concentrations (10 to 55% plasma).
236
Another team found that sulfobetaine arms provided a
corona-free property, even in the presence of other functions.
237
The dual functionalization of
NPs is a promising strategy for targeted delivery purposes, because careful balance of a
zwitterionic moiety and a proper targeting function on the NP surface should control the bio-
nano interaction while maintaining targeting capabilities. However, the choice of the best
surface ligands will depend on the in vivo route chosen and the therapeutic strategy. The
63
dilemma might be the choice of an appropriate NP design. Surface engineering will differ
depending on whether or not we want to activate the cell’s uptake machinery in a process
known as endocytosis. Zhang and his colleagues referred to a soft reversible adsorption of
the protein corona on 2nm core AuNPs coated with a PEG3-quaternary ammonium ligand.
238
In this study, no adsorption was observed with the PEG3-sulfobetaine ligand. Ultimately, the
antifouling effect does not allow cell uptake of NPs by endocytosis. In this case, the best
functionalization strategy involved the PEG3-ammonium ligand, allowing soft and reversible
adsorption of the proteins associated with cell penetrating by endocytosis. This
functionalization method will probably not be preferred for in vivo pre-targeting. Very densely
charged surfaces with zwitterionic ligands will be preferred to avoid any protein adsorption
and thus allow the in vivo click reaction. It is therefore important to conduct studies on the
formation of this protein corona depending on the nature of the bioorthogonal entities grafted
onto the surface of the NPs, their number, and the overall charge of the nano-object before
assuming that the PT approach can be applied to any type of nanoparticle.
7. CONCLUSIONS AND OUTLOOK
Theranostic NPs have the potential to revolutionize future disease management. In
particular, their development has emerged as an encouraging strategy for personalized
medicine in the fields of cancer diagnosis and therapy. However, their decoration still
encounters various engineering difficulties, including achieving good reproducibility of
nanoparticle batches, making them biocompatible, and improving their ability to target the
TME specifically. Even a decade ago, most NPs were surface functionalized by carbodiimide
and maleimide reactions.
Advances in organic chemistry have apparently opened up a new field of NP engineering.
The click chemistry discovered by Huisgen in the 1960s represents a practical organic
reaction for the quantitative synthesis of progressively growing polymers. However,
Sharpless coined the term "click chemistry" to define highly efficient synthetic reactions which
tolerate various functional groups and occur under mild synthetic conditions. Numerous
published studies highlight the advantages of click chemistry as a ligation tool for potential
applications as non-targeting nanocarriers or theranostic vehicles for cancers. High yields
(often higher than 95%), specificity, diverse reaction conditions and scale-expansion are
assets for click chemistry, offering the possibility of creating complex, stable NPs. This is
attracting growing interest for the surface incorporation of non-targeted molecules (e.g.
fluorophores and contrast agents for multimodal imaging) and for the specific and efficient
encapsulation of insoluble chemotherapeutic agents to improve the therapeutic index.
Micelles, liposomes, dendrons, dendrimers and polymeric NPs thus continue to be the
nanocarriers most employed in this area. They show efficient cell internalization and
antiproliferative activity, even if, for most of them, efficacy remains to be evaluated either in
vivo or in clinics.
Click chemistry also allows the grafting of ligands onto NPs for active targeting and
accumulation in specific cancer cells. Regarding the comparison of clicked targeted NPs and
non-targeted NPs, active targeting seems to be especially effective thanks to the favorable
and selective interaction of NPs with their biological targets. Targeting ligands allow
restricted access only to cancer cells for the intracellular delivery of genes (i.e., for gene
knockdown), therapeutic agents (i.e., for an anticancer effect) or imaging agents. They
substantially improve cellular uptake and internalization, generally allowing the promotion of
64
cancer cell cytotoxicity, apoptosis and transfection efficiency through the well-exploited
receptor-mediated endocytosis pathway. However, CuAAC is a cooper-catalyst reaction and,
despite engineering developments to purify these nanosystems, residual traces of copper
may be present on the nano-object, thus posing a problem for clinical transfer.
The concept of bioorthogonal chemistry (copper-free click chemistry) seems capable of
solving this bioaccounting problem and suggests the advent of a new era for therapeutic and
theranostic NPs. The notion of bioorthogonality was developed by Bertozzi to define a
chemistry that required that the functional groups be inert towards biological
macromolecules. Bioorthogonality ensures a reaction capable of taking place under
physiological conditions (pH and temperature) while avoiding toxicity in living systems.
SPAAC and IEDDA systems certainly seem to characterize the future of nanomedicine for
several reasons:
(1) These two systems appear to have constituted a “classical” functionalization
chemistry in the field of NPs
(2) No catalyst is needed to conjugate the two-bioorthogonal entities and they are
applicable regardless of the type of NP: organic or inorganic.
The PT approach appears very promising for nanomedicine. According to this strategy, the
NPs injected in a second step distribute themselves due to the EPR effect. It is also true that,
according to Wilhem's meta-analysis, less than 1% of the injected NPs reach the tumor
volume. However, a first injection of the modified antibody appears to allow better tumor
concentration of the NPs by click reaction, as demonstrated by Haun et al., who compared
BOND-1 (one step targeting) and BOND-2 (two-step targeting) approaches.
202
As the antibody is dissociated from the NP, several perspectives can be envisaged:
- This approach would make it possible to envisage using the same NPs for the same
type of cancer but with different mAbs targeting different biomarkers of interest. For
example, a single NP could be used with either anti-EGFR or anti-HER2 mAb for
breast cancer. If this in vivo ligation is effective with an anticancer mAb, we can
assume that it would also be effective with mAbs used for other pathologies, such as
inflammation for example.
However, some significant questions and problems still remain to be resolved:
The PT approach is only possible if the antibodies modified with the first bioorthogonal
entity do not internalize before administration of the NPs. But do the bioorthogonal entities
promote internalization of the modified antibody? If this difficulty is confirmed, it will probably
be necessary to develop engineered antibodies to limit their internalization over the first 24 or
48 hours in order to allow grafting of the NP onto the tumor. Will different approaches be
required for chimeric, humanized and human antibodies?
As the mAb is not directly grafted onto the NP, the size and surface of the NP must be
controlled to avoid both renal clearance and uptake by the mononuclear phagocyte system
after formation of a protein corona that contains ions, opsonins and other serum proteins,
thus maximizing blood circulation time and allowing the NPs to reach the tumor site to enable
the click reaction.

65
The NPs must be functionalized on the surface to avoid the formation of the protein
corona which would be partly responsible for non-ligation in vivo.
Rather than using antibodies, should we not develop the notion of glycoengineering to
modify the surface of the tumors and allow the attachment of NPs in a second phase?
Regardless of the approach used (passive or active targeting in two stages (PT approach or
glycoengineering), it is clear that biodistribution of the NP will take place due to the EPR
effect. One solution for developing multimodal or theranostic NPs may therefore be an
approach where the NP acts as a “tumor platform”, with bioorthogonal entities on the surface
capable of grafting theranostic agents, PTT or radiosensitive agents, or even prodrugs in a
second phase.
AUTHOR INFORMATIONS
Corresponding Authors
Emmanuel Moreau – Université Clermont Auvergne, Imagerie Moléculaire et Stratégies
Théranostiques, BP 184, F-63005 Clermont-Ferrand, France. Inserm, U 1240, F-63000
Clermont-Ferrand, France. Centre Jean Perrin, F-63011 Clermont-Ferrand, France.;
orcid.org/0000-0002-8004-5019; Email: emmanuel.moreau@uca.fr.
Carole Chaix – Interfaces and Biosensors, UMR 5280, CNRS, Villeurbanne, France ;
Authors
Ludivine Taiariol - Université Clermont Auvergne, Imagerie Moléculaire et Stratégies
Théranostiques, BP 184, F-63005 Clermont-Ferrand, France. Inserm, U 1240, F-63000
Clermont-Ferrand, France. Centre Jean Perrin, F-63011 Clermont-Ferrand, France.; 0000-
Carole Farre – Université de Lyon, CNRS, Université Claude Bernard Lyon 1, Institut des
Sciences Analytiques, UMR 5280, 5 rue de la Doua, F-69100 Villeurbanne, France;
orcid.org/0000-0003-0783-289X; Email : carole.farre@univ-lyon1.fr.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given
approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest
66
Biographies
Ludivine Taiariol obtained her MSc in Drug Sciences from the University of Clermont-
Ferrand (France) in 2016. She then joined UMR 1240 INSERM/IMoST research laboratory
for her Ph.D. under the supervision of Doctor Emmanuel Moreau and Doctor Carole Chaix.
She received her Ph.D. in Chemistry of Materials, Nanomaterials and Processes from the
University of Clermont-Ferrand (France) in 2019. Her thesis subject focused on the
development of novel silica nanoparticles for the application in external radiotherapy. Her
skills are at the interface between organic chemistry/chemistry of nanomaterials and
biochemistry.
Carole Chaix received her Ph.D. in Biological and Medical Engineering from the University
of Grenoble (France) in 1990. She is presently Research Director at the Institute of Analytical
Sciences of Lyon and head of the Interfaces and Biosensors group. She has extensive
experience in the functionalization of biomolecules and the surface chemistry of materials.
Her research focus on the development of bio-functionalized nanomaterials and bioanalytical
systems such as biosensors or biochips for applications in nanomedicine and biological
analysis. She is the author or co-author of more than 80 articles and book chapters.
Carole Farre obtained her MSc in Biomolecular Chemistry from the University of Montpellier
(France) in 2006. She is currently working as an Engineer at the Institute of Analytical
Sciences of Lyon. Her research focuses on nanoparticle functionalization with biomolecules,
in particular DNA. She is also working on new molecules for DNA labelling and their
integration into bioanalytical systems.
Emmanuel Moreau received his Ph.D. in pharmaceutical sciences from the University of
Clermont-Ferrand (France) in 2001. He did post-doctoral studies at the Saint-François
d'Assises research centre in Quebec City (Canada) from 2002 to 2004. He is presently
assistant professor at the Faculty of Pharmacy in Clermont-Ferrand (France). Since 2005, he
has been conducting his research in an INSERM unit in Clermont-Ferrand (France). He is
the leader of the "Targets and tools for imaging and therapy" team combining the
complementary expertise of chemists, radiochemists, biologists and clinicians. This research
focuses on the development of new radiopharmaceuticals for melanoma, colorectal cancer
and breast cancer. He is author and co-author of about fifty publications and has supervised
about ten Ph.D students.
ACKNOWLEDGEMENTS
This project is based upon work supported in part by the “Cancéropôle Rhône-Alpes
Auvergne CLARA Oncostarter” (Grant support CVPPRCAN000184) (E.M), the “Ligue
Régionale contre le Cancer” (Grant support R21JPRARPDD) (E.M), Ludivine Tairaiol's
doctoral work was supported by funding from the French Ministry of Research and the
doctoral school of basic sciences in Clermont-Ferrand (2016-2019).
ABBREVIATIONS
Ac
4
MAnNAz = tetraacetylated N-azidoacetyl-D-mannosamine
AMP = activatable MMP-specific peptide probe
AuNP = gold nanoparticle
67
AuNC = gold nanocluster
AuNR: gold nanorod
AZT = acetazolamide
BBB = Blood-brain barrier
BCN = bicyclo[6.1.0]nonyne
BHQ-3 = non-fluorescent Black Hole Quencher-3
BOND = bioorthogonal NP detection
CA = carbonic anhydrase
CD20: β-lymphocyte antigen
Ce6 = Chlorin e6
CF7N = Cy7-modified chitosan nanoparticle
CPP = cell penetrating peptide
CPT = camptothecin
CT = computed tomography
CuAAC = copper(I)-catalyzed Azide-Alkyne [3+2] Cycloaddition
CDDP= cisplatin
CNP = chitosan nanoparticle
CPP = cell penetrating peptide
CPT = camptothecin
CSPT = chain-shattering polymeric therapeutics
Cy = cyanine
β-CD = β-cyclodextrin
DACH-Pt= dichloro(1,2-diaminocyclohexane)platinum(II)
DBCO = dibenzocyclooctyne
DLQ = DBCO-LQ
D-mino = hydroxyl-G6 PAMAM dendrimer–9-amino-minocycline conjugate
DOTA = 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid
DOX = doxorubicin
DSPS = 1,2-Distearoyl-sn-glycero-3-phospho-L-serine
DZ = DOX-zinc phthalocyanine conjugate
EDC = 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
EGFR = epidermal growth factor receptor
68
EPR = enhanced permeability and retention
FA = folic acid
FCPZnO = FA-conjugated hollow ZnO nanoparticle
FITC= fluorescein isothiocyanate
FNC = folate-decorated nanoceria
FND = fluorescent nanodiamond
FR = folate receptor
GEM = gemcitabine
GFP = green fluorescent protein
GLA = glutamate urea
GON = gelatin-oleic acid nanoparticle
GSH = glutathione
GT = ganetespib
HA = hyaluronic acid
HAase = hyaluronidase
HDAC = protein histone deacetylase
HDACi = histone deacetylase inhibitor
HER2 = human epidermal growth factor receptor 2
HNE = epithelial tumor cell line
ICP = infinite‐coordination‐polymer
IC50 = half-maximal inhibitory concentration
IEDDA = inverse-electron-demand Diels-Alder [4+2]
LIP = liposome
LHRH = luteinizing hormone releasing hormone
LMWH = low molecular weight heparin
LQ = (LMWH)-quercetin (Qu) conjugate
mAb = monoclonal antibody
MBG = mesoporous bioactive glass
MCF-7 = human breast adenocarcinoma cell line
MDA-MB = epithelial, human breast cancer cell line
MFI = mean fluorescence intensity
MFNP = magneto-fluorescent nanoparticle
69
MMP = matrix metalloproteinase
MPLA = monophosphoryl lipid A
MR = magnetic resonance
MRI: magnetic resonance imaging
MRSA = methicillin-resistant Staphylococcus aerus
MSN = mesoporous silica nanoparticle
MTX = methotrexate
NC = nanoconjugate
ND= nanodiamond
NHS = N-hydroxysuccinimide
NIR = near infrared
NIRF: near infrared fluorescence
NMOFs = metal-organic framework nanoparticles.
NP = nanoparticle
PAMAM = polyamidoamine
PAT= photoacoustic therapy
PDA = polydopamine
PD-L1 = programmed death-ligand 1
PDT = photodynamic therapy
PEG = polyethylene glycol
PET = positron-emission tomography
pHLIP = pH (low) insertion peptide
pHLIP-Tz = pH (low) insertion peptide conjugated to tetrazine
PLGA = poly(lactic-co-glycolic acid)
PT = pretargeting
PTT = photothermal therapy
PTX = paclitaxel
PSMA = prostate-specific membrane antigen
Psi = porous silicon
QD = quantum dot
RAW = monocyte/macrophage-like cell line
RGD = Arginine-Glycine-Aspartic acid
70
RGE = neuropilin-1-targeted peptide (RGERPPR)
RTX = rituximab
ROMP = ring-opening metathesis copolymerization
RSC = lipid-based complexes encapsulating siRNA
RTV = relative tumor volume
SERS = Surface-Enhanced Raman Scattering
siRNA = small interfering RNA
SKOV-3 = ovarian cancer cell line
SMNPs = small molecule-based nanoparticles
SN-38 = 7-ethyl-10-hydroxycamptothecin
SPAAC = Strain-Promoted Alkyne-Azide Cycloaddition
SPECT = single-photon emission computed tomography
SPION = SuperParamagnetic Iron Oxide nanoparticle
SPR = surface plasmon resonance
TAF = tumor-associated fibroblasts
Tat = Trans-activating transcriptional activator
TBET = through-bond energy transfer
TCO = trans-cyclooctene
TME = tumor microenvironment
TNF-α = tumor necrosis factor α
TPETM =
2‐(1‐(5‐(4‐(1,2,2‐tris(4‐methoxyphenyl)vinyl)phenyl)thiophen‐2‐yl)ethylidene)malononit
rile
Tz = tetrazine
UCNP: upconversion nanoparticle
USPION = Ultra Small SuperParamagnetic Iron Oxide nanoparticle
VEC = vascular endothelial cells
ZnPc = zinc phthalocyanine
REFERENCES
(1) Dr. Niranjani Chaurasia. Nanotechnology and Nanomaterials in Everyday Life. Int. J.
Sci. Res. IJSR 2017, 6, 1560–1562.
71
(2) Weltring, K.-M.; Gouze, N.; Martin, N.; Pereira, N.; Baanante, I.; Gramatica, F.
Nanomedicine Strategic Research and Innovation Agenda. Nanomedicine European
Technology Platform January 2016.
(3) Nakhlband, A.; Eskandani, M.; Omidi, Y.; Saeedi, N.; Ghaffari, S.; Barar, J.; Garjani,
A. Combating Atherosclerosis with Targeted Nanomedicines: Recent Advances and
Future Prospective. BioImpacts 2018, 8, 59–75.
(4) Jiang, W.; Rutherford, D.; Vuong, T.; Liu, H. Nanomaterials for Treating
Cardiovascular Diseases: A Review. Bioact. Mater. 2017, 2, 185–198.
(5) Kanwar, J. R.; Sun, X.; Punj, V.; Sriramoju, B.; Mohan, R. R.; Zhou, S.-F.; Chauhan,
A.; Kanwar, R. K. Nanoparticles in the Treatment and Diagnosis of Neurological
Disorders: Untamed Dragon with Fire Power to Heal. Nanomedicine Nanotechnol.
Biol. Med. 2012, 8, 399–414.
(6) Zazo, H.; Colino, C. I.; Lanao, J. M. Current Applications of Nanoparticles in
Infectious Diseases. J. Controlled Release 2016, 224, 86–102.
(7) DiSanto, R. M.; Subramanian, V.; Gu, Z. Recent Advances in Nanotechnology for
Diabetes Treatment: Nanotechnology for Diabetes Treatment. Wiley Interdiscip. Rev.
Nanomed. Nanobiotechnol. 2015, 7, 548–564.
(8) Iavicoli, I.; Fontana, L.; Leso, V.; Bergamaschi, A. The Effects of Nanomaterials as
Endocrine Disruptors. Int. J. Mol. Sci. 2013, 14, 16732–16801.
(9) Oliveira, I. M.; Gonçalves, C.; Reis, R. L.; Oliveira, J. M. Engineering Nanoparticles
for Targeting Rheumatoid Arthritis: Past, Present, and Future Trends. Nano Res.
2018, 11, 4489–4506.
(10) Riffault, M.; Six, J.-L.; Netter, P.; Gillet, P.; Grossin, L. PLGA-Based Nanoparticles: A
Safe and Suitable Delivery Platform for Osteoarticular Pathologies. Pharm. Res.
2015, 32, 3886–3898.
(11) Awasthi, R.; Roseblade, A.; Hansbro, P. M.; Rathbone, M. J.; Dua, K.; Bebawy, M.
Nanoparticles in Cancer Treatment: Opportunities and Obstacles. Curr. Drug Targets
2018, 19, 1696–1709.
(12) Riehemann, K.; Schneider, S. W.; Luger, T. A.; Godin, B.; Ferrari, M.; Fuchs, H.
Nanomedicine-Challenge and Perspectives. Angew. Chem. Int. Ed. 2009, 48, 872–
897.
(13) Prabhu, P.; Patravale, V. The Upcoming Field of Theranostic Nanomedicine: An
Overview. J. Biomed. Nanotechnol. 2012, 8, 859–882.
(14) Baetke, S. C.; Lammers, T.; Kiessling, F. Applications of Nanoparticles for Diagnosis
and Therapy of Cancer. Br. J. Radiol. 2015, 88, 20150207.
(15) Greish, K.; Mathur, A.; Bakhiet, M.; Taurin, S. Nanomedicine: Is It Lost in
Translation? Ther. Deliv. 2018, 9, 269–285.
(16) Wilhelm, S.; Tavares, A. J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H. F.; Chan, W. C.
W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014.
(17) Meldal, M.; Tornøe, C. W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev.
2008, 108, 2952–3015.
(18) Wang, Q.; Chan, T. R.; Hilgraf, R.; Fokin, V. V.; Sharpless, K. B.; Finn, M. G.
Bioconjugation by Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition. J. Am.
Chem. Soc. 2003, 125, 3192–3193.
(19) Verma, S. A Novel Loom of Click Chemistry in Drug Discovery. Int. J. Drug Dev. Res.
2015, 18–21.
(20) Bertozzi, C. R. Chemical Glycobiology. Science 2001, 291, 2357–2364.
(21) Kim, E.; Koo, H. Biomedical Applications of Copper-Free Click Chemistry: In vitro , in
vivo , and Ex Vivo. Chem. Sci. 2019, 10, 7835–7851.
(22) Ladd, E.; Sheikhi, A.; Li, N.; van de Ven, T. G. M.; Kakkar, A. Design and Synthesis
of Dendrimers with Facile Surface Group Functionalization, and an Evaluation of
Their Bactericidal Efficacy. Molecules 2017, 22, 868–896.
(23) Anandkumar, D.; Rajakumar, P. Synthesis and Anticancer Activity of Bile Acid
Dendrimers with Triazole as Bridging Unit through Click Chemistry. Steroids 2017,
125, 37–46.
72
(24) Sadowski, L. P.; Edem, P. E.; Valliant, J. F.; Adronov, A. Synthesis of Polyester
Dendritic Scaffolds for Biomedical Applications. Macromol. Biosci. 2016, 16, 1475–
1484.
(25) Fischer, G.; Wängler, B.; Wängler, C. Optimized Solid Phase-Assisted Synthesis of
Dendrons Applicable as Scaffolds for Radiolabeled Bioactive Multivalent Compounds
Intended for Molecular Imaging. Mol. Basel Switz. 2014, 19, 6952–6974.
(26) Singh, P.; Pandit, S.; Mokkapati, V. R. S. S.; Garg, A.; Ravikumar, V.; Mijakovic, I.
Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol.
Sci. 2018, 19, 1979–1995.
(27) Mao, W.; Kim, H. S.; Son, Y. J.; Kim, S. R.; Yoo, H. S. Doxorubicin Encapsulated
Clicked Gold Nanoparticle Clusters Exhibiting Tumor-Specific Disassembly for
Enhanced Tumor Localization and Computerized Tomographic Imaging. J. Control.
Release 2018, 269, 52–62.
(28) Tural, S.; Ece, M. Ş.; Tural, B. Synthesis of Novel Magnetic Nano-Sorbent
Functionalized with N-Methyl-D-Glucamine by Click Chemistry and Removal of Boron
with Magnetic Separation Method. Ecotoxicol. Environ. Saf. 2018, 162, 245–252.
(29) Shoghi, E.; Mirahmadi-Zare, S. Z.; Ghasemi, R.; Asghari, M.; Poorebrahim, M.; Nasr-
Esfahani, M.-H. Nanosized Aptameric Cavities Imprinted on the Surface of Magnetic
Nanoparticles for High-Throughput Protein Recognition. Mikrochim. Acta 2018, 185,
241.
(30) Finetti, C.; Sola, L.; Pezzullo, M.; Prosperi, D.; Colombo, M.; Riva, B.; Avvakumova,
S.; Morasso, C.; Picciolini, S.; Chiari, M. Click Chemistry Immobilization of Antibodies
on Polymer Coated Gold Nanoparticles. Langmuir 2016, 32, 7435–7441.
(31) Abadeer, N. S.; Brennan, M. R.; Wilson, W. L.; Murphy, C. J. Distance and Plasmon
Wavelength Dependent Fluorescence of Molecules Bound to Silica-Coated Gold
Nanorods. ACS Nano 2014, 8, 8392–8406.
(32) Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis,
Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534.
(33) Lu, J.; Liong, M.; Zink, J. I.; Tamanoi, F. Mesoporous Silica Nanoparticles as a
Delivery System for Hydrophobic Anticancer Drugs. Small 2007, 3, 1341–1346.
(34) De Crozals, G.; Bonnet, R.; Farre, C.; Chaix, C. Nanoparticles with Multiple
Properties for Biomedical Applications: A Strategic Guide. Nano Today 2016, 11,
435–463.
(35) Slowing, I.; Viveroescoto, J.; Wu, C.; Lin, V. Mesoporous Silica Nanoparticles as
Controlled Release Drug Delivery and Gene Transfection Carriers. Adv. Drug Deliv.
Rev. 2008, 60, 1278–1288.
(36) Chen, Z.; Sun, M.; Luo, F.; Xu, K.; Lin, Z.; Zhang, L. Stimulus-Response Click
Chemistry Based Aptamer-Functionalized Mesoporous Silica Nanoparticles for
Fluorescence Detection of Thrombin. Talanta 2018, 178, 563–568.
(37) Datz, S.; Argyo, C.; Gattner, M.; Weiss, V.; Brunner, K.; Bretzler, J.; von Schirnding,
C.; Torrano, A. A.; Spada, F.; Vrabel, M.; et al. Genetically Designed Biomolecular
Capping System for Mesoporous Silica Nanoparticles Enables Receptor-Mediated
Cell Uptake and Controlled Drug Release. Nanoscale 2016, 8, 8101–8110.
(38) Noureddine, A.; Gary-Bobo, M.; Lichon, L.; Garcia, M.; Zink, J. I.; Wong Chi Man, M.;
Cattoën, X. Bis-Clickable Mesoporous Silica Nanoparticles: Straightforward
Preparation of Light-Actuated Nanomachines for Controlled Drug Delivery with Active
Targeting. Chem. Weinh. Bergstr. Ger. 2016, 22, 9624–9630.
(39) Chertok, B.; Moffat, B. A.; David, A. E.; Yu, F.; Bergemann, C.; Ross, B. D.; Yang, V.
C. Iron Oxide Nanoparticles as a Drug Delivery Vehicle for MRI Monitored Magnetic
Targeting of Brain Tumors. Biomaterials 2008, 29, 487–496.
(40) Lee, H.-Y.; Li, Z.; Chen, K.; Hsu, A. R.; Xu, C.; Xie, J.; Sun, S.; Chen, X. PET/MRI
Dual-Modality Tumor Imaging Using Arginine-Glycine-Aspartic (RGD)-Conjugated
Radiolabeled Iron Oxide Nanoparticles. J. Nucl. Med. 2008, 49, 1371–1379.
(41) Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X.
PET/NIRF/MRI Triple Functional Iron Oxide Nanoparticles. Biomaterials 2010, 31,
3016–3022.
73
(42) Massaad-Massade, L.; Boutary, S.; Caillaud, M.; Gracia, C.; Parola, B.; Gnaouiya, S.
B.; Stella, B.; Arpicco, S.; Buchy, E.; Desmaële, D.; et al. New Formulation for the
Delivery of Oligonucleotides Using “Clickable” SiRNA-Polyisoprenoid-Conjugated
Nanoparticles: Application to Cancers Harboring Fusion Oncogenes. Bioconjug.
Chem. 2018, 29, 1961–1972.
(43) Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic Iron
Oxide Nanoparticles (SPIONs): Development, Surface Modification and Applications
in Chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46.
(44) Arriortua, O. K.; Insausti, M.; Lezama, L.; Gil de Muro, I.; Garaio, E.; de la Fuente, J.
M.; Fratila, R. M.; Morales, M. P.; Costa, R.; Eceiza, M.; et al. RGD-Functionalized
Fe3O4nanoparticles for Magnetic Hyperthermia. Colloids Surf. B Biointerfaces 2018,
165, 315–324.
(45) Meghani, N. M.; Amin, H. H.; Park, C.; Park, J.-B.; Cui, J.-H.; Cao, Q.-R.; Lee, B.-J.
Design and Evaluation of Clickable Gelatin-Oleic Nanoparticles Using Fattigation-
Platform for Cancer Therapy. Int. J. Pharm. 2018, 545, 101–112.
(46) Liu, Y.; Hou, W.; Sun, H.; Cui, C.; Zhang, L.; Jiang, Y.; Wu, Y.; Wang, Y.; Li, J.;
Sumerlin, B. S.; et al. Thiol-Ene Click Chemistry: A Biocompatible Way for
Orthogonal Bioconjugation of Colloidal Nanoparticles. Chem. Sci. 2017, 8, 6182–
6187.
(47) Neouze, M.-A.; Schubert, U. Surface Modification and Functionalization of Metal and
Metal Oxide Nanoparticles by Organic Ligands. Monatshefte Für Chem. - Chem.
Mon. 2008, 139, 183–195.
(48) Subbiah, R.; Veerapandian, M.; S. Yun, K. Nanoparticles: Functionalization and
Multifunctional Applications in Biomedical Sciences. Curr. Med. Chem. 2010, 17,
4559–4577.
(49) G.T. Hermanson. Bioconjugate Techniques. Acad. Press 2013.
(50) Algar, W. R.; Prasuhn, D. E.; Stewart, M. H.; Jennings, T. L.; Blanco-Canosa, J. B.;
Dawson, P. E.; Medintz, I. L. The Controlled Display of Biomolecules on
Nanoparticles: A Challenge Suited to Bioorthogonal Chemistry. Bioconjug. Chem.
2011, 22, 825–858.
(51) Nakajima, N.; Ikada, Y. Mechanism of Amide Formation by Carbodiimide for
Bioconjugation in Aqueous Media. Bioconjug. Chem. 1995, 6, 123–130.
(52) Rahim, M. K.; Kota, R.; Lee, S.; Haun, J. B. Bioorthogonal Chemistries for
Nanomaterial Conjugation and Targeting. Nanotechnol. Rev. 2013, 2, 215–227.
(53) Such, G. K.; Johnston, A. P. R.; Liang, K.; Caruso, F. Synthesis and Functionalization
of Nanoengineered Materials Using Click Chemistry. Prog. Polym. Sci. 2012, 37,
985–1003.
(54) Han, H.-S.; Devaraj, N. K.; Lee, J.; Hilderbrand, S. A.; Weissleder, R.; Bawendi, M.
G. Development of a Bioorthogonal and Highly Efficient Conjugation Method for
Quantum Dots Using Tetrazine−Norbornene Cycloaddition. J. Am. Chem. Soc. 2010,
132, 7838–7839.
(55) Kotagiri, N.; Li, Z.; Xu, X.; Mondal, S.; Nehorai, A.; Achilefu, S. Antibody Quantum
Dot Conjugates Developed via Copper-Free Click Chemistry for Rapid Analysis of
Biological Samples Using a Microfluidic Microsphere Array System. Bioconjug.
Chem. 2014, 25, 1272–1281.
(56) Kaplun, V.; Stepensky, D. Efficient Decoration of Nanoparticles Intended for
Intracellular Drug Targeting with Targeting Residues, as Revealed by a New Indirect
Analytical Approach. Mol. Pharm. 2014, 11, 2906–2914.
(57) Williams, S.; Neumann, A.; Bremer, I.; Su, Y.; Dräger, G.; Kasper, C.; Behrens, P.
Nanoporous Silica Nanoparticles as Biomaterials: Evaluation of Different Strategies
for the Functionalization with Polysialic Acid by Step-by-Step Cytocompatibility
Testing. J. Mater. Sci. Mater. Med. 2015, 26, 125–131.
(58) Jewett, J. C.; Bertozzi, C. R. Cu-Free Click Cycloaddition Reactions in Chemical
Biology. Chem. Soc. Rev. 2010, 39, 1272–1279.
(59) Dommerholt, J.; van Rooijen, O.; Borrmann, A.; Guerra, C. F.; Bickelhaupt, F. M.; van
Delft, F. L. Highly Accelerated Inverse Electron-Demand Cycloaddition of Electron-
Deficient Azides with Aliphatic Cyclooctynes. Nat. Commun. 2014, 5, 5378–5384.
74
(60) Korthals, B.; Morant-Miñana, M. C.; Schmid, M.; Mecking, S. Functionalization of
Polymer Nanoparticles by Thiol−Ene Addition. Macromolecules 2010, 43, 8071–
8078.
(61) Ruizendaal, L.; Pujari, S. P.; Gevaerts, V.; Paulusse, J. M. J.; Zuilhof, H.
Biofunctional Silicon Nanoparticles by Means of Thiol-Ene Click Chemistry. Chem. -
Asian J. 2011, 6, 2776–2786.
(62) Hoyle, C. E.; Bowman, C. N. Thiol-Ene Click Chemistry. Angew. Chem. Int. Ed. 2010,
49, 1540–1573.
(63) Hoyle, C. E.; Lowe, A. B.; Bowman, C. N. Thiol-Click Chemistry: A Multifaceted
Toolbox for Small Molecule and Polymer Synthesis. Chem. Soc. Rev. 2010, 39,
1355–1387.
(64) Stéen, E. J. L.; Edem, P. E.; Nørregaard, K.; Jørgensen, J. T.; Shalgunov, V.; Kjaer,
A.; Herth, M. M. Pretargeting in Nuclear Imaging and Radionuclide Therapy:
Improving Efficacy of Theranostics and Nanomedicines. Biomaterials 2018, 179,
209–245.
(65) Devaraj, N. K.; Weissleder, R. Biomedical Applications of Tetrazine Cycloadditions.
Acc. Chem. Res. 2011, 44, 816–827.
(66) Gregoritza, M.; Brandl, F. P. The Diels-Alder Reaction: A Powerful Tool for the
Design of Drug Delivery Systems and Biomaterials. Eur. J. Pharm. Biopharm. Off. J.
Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2015, 97, 438–453.
(67) Yi, G.; Son, J.; Yoo, J.; Park, C.; Koo, H. Application of Click Chemistry in
Nanoparticle Modification and Its Targeted Delivery. Biomater. Res. 2018, 22–29.
(68) Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in
Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the
Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392.
(69) Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle Design Strategies for Enhanced
Anticancer Therapy by Exploiting the Tumour Microenvironment. Chem Soc Rev
2017, 3830–3852.
(70) Kobayashi, H.; Watanabe, R.; Choyke, P. L. Improving Conventional Enhanced
Permeability and Retention (EPR) Effects; What Is the Appropriate Target?
Theranostics 2014, 4, 81–89.
(71) Barenholz, Y. (Chezy). Doxil® — The First FDA-Approved Nano-Drug: Lessons
Learned. J. Controlled Release 2012, 160, 117–134.
(72) Miele, E.; Spinelli, G. P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-Bound
Formulation of Paclitaxel (Abraxane ABI-007) in the Treatment of Breast Cancer. Int.
J. Nanomedicine 2009, 4, 99–105.
(73) Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H.
PEG–PLGA Copolymers: Their Structure and Structure-Influenced Drug Delivery
Applications. J. Controlled Release 2014, 183, 77–86.
(74) Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J. M.; Peer, D. Progress and Challenges
towards Targeted Delivery of Cancer Therapeutics. Nat. Commun. 2018, 9, 1410.
(75) Mei, L.; Rao, J.; Liu, Y.; Li, M.; Zhang, Z.; He, Q. Effective Treatment of the Primary
Tumor and Lymph Node Metastasis by Polymeric Micelles with Variable Particle
Sizes. J. Control. Release Off. J. Control. Release Soc. 2018, 292, 67–77.
(76) Mei, L.; Liu, Y.; Rao, J.; Tang, X.; Li, M.; Zhang, Z.; He, Q. Enhanced Tumor
Retention Effect by Click Chemistry for Improved Cancer Immunochemotherapy.
ACS Appl. Mater. Interfaces 2018, 10, 17582–17593.
(77) Toomari, Y.; Namazi, H.; Akbar, E. A. Synthesis of the Dendritic Type β-Cyclodextrin
on Primary Face via Click Reaction Applicable as Drug Nanocarrier. Carbohydr.
Polym. 2015, 132, 205–213.
(78) Denis, I.; El Bahhaj, F.; Collette, F.; Delatouche, R.; Gueugnon, F.; Pouliquen, D.;
Pichavant, L.; Héroguez, V.; Grégoire, M.; Bertrand, P.; et al. Vorinostat-Polymer
Conjugate Nanoparticles for Acid-Responsive Delivery and Passive Tumor Targeting.
Biomacromolecules 2014, 15, 4534–4543.
(79) Yu, Y.; Chen, C.-K.; Law, W.-C.; Weinheimer, E.; Sengupta, S.; Prasad, P. N.;
Cheng, C. Polylactide-Graft-Doxorubicin Nanoparticles with Precisely Controlled Drug
Loading for PH-Triggered Drug Delivery. Biomacromolecules 2014, 15, 524–532.
75
(80) Zhu, C.; Xiao, J.; Tang, M.; Feng, H.; Chen, W.; Du, M. Platinum Covalent Shell
Cross-Linked Micelles Designed to Deliver Doxorubicin for Synergistic Combination
Cancer Therapy. Int. J. Nanomedicine 2017, 12, 3697–3710.
(81) Noureddine, A.; Lichon, L.; Maynadier, M.; Garcia, M.; Gary-Bobo, M.; Zink, J. I.;
Cattoën, X.; Wong Chi Man, M. Controlled Multiple Functionalization of Mesoporous
Silica Nanoparticles: Homogeneous Implementation of Pairs of Functionalities
Communicating through Energy or Proton Transfers. Nanoscale 2015, 7, 11444–
11452.
(82) Cai, K.; Yen, J.; Yin, Q.; Liu, Y.; Song, Z.; Lezmi, S.; Zhang, Y.; Yang, X.; Helferich,
W. G.; Cheng, J. Redox-Responsive Self-Assembled Chain-Shattering Polymeric
Therapeutics. Biomater. Sci. 2015, 3, 1061–1065.
(83) Abánades Lázaro, I.; Haddad, S.; Sacca, S.; Orellana-Tavra, C.; Fairen-Jimenez, D.;
Forgan, R. S. Selective Surface PEGylation of UiO-66 Nanoparticles for Enhanced
Stability, Cell Uptake, and PH-Responsive Drug Delivery. Chem 2017, 2, 561–578.
(84) Mai, K.; Lin, J.; Zhuang, B.; Li, X.; Zhang, L.-M. Cationic Dendronization of Amylose
via Click Chemistry for Complexation and Transfection of Plasmid DNA. Int. J. Biol.
Macromol. 2015, 79, 209–216.
(85) Mai, K.; Zhang, S.; Liang, B.; Gao, C.; Du, W.; Zhang, L.-M. Water Soluble Cationic
Dextran Derivatives Containing Poly(Amidoamine) Dendrons for Efficient Gene
Delivery. Carbohydr. Polym. 2015, 123, 237–245.
(86) Chen, R.; Zhunang, B.; Li, Z.; Li, W.; Huang, P.; Pang, J.; Zhou, Y.; Lin, Q.; Zhou, Q.;
Ye, X.; et al. Nanocomplexation of Thrombin with Cationic Amylose Derivative for
Improved Stability And Hemostatic Efficacy. Int. J. Nanomedicine 2015, 939–947.
(87) Yan, Y.; Fu, J.; Wang, T.; Lu, X. Controlled Release of Silyl Ether Camptothecin from
Thiol-Ene Click Chemistry-Functionalized Mesoporous Silica Nanoparticles. Acta
Biomater. 2017, 51, 471–478.
(88) Cai, L.; Xu, G.; Shi, C.; Guo, D.; Wang, X.; Luo, J. Telodendrimer Nanocarrier for Co-
Delivery of Paclitaxel and Cisplatin: A Synergistic Combination Nanotherapy for
Ovarian Cancer Treatment. Biomaterials 2015, 37, 456–468.
(89) He, H.; Xiao, H.; Kuang, H.; Xie, Z.; Chen, X.; Jing, X.; Huang, Y. Synthesis of
Mesoporous Silica Nanoparticle-Oxaliplatin Conjugates for Improved Anticancer Drug
Delivery. Colloids Surf. B Biointerfaces 2014, 117, 75–81.
(90) Eroglu, S., M.; Toksoy Oner, E.; Cansever Mutlu, E.; Sennaroglu Bostan, M. Sugar
Based Biopolymers in Nanomedicine; New Emerging Era for Cancer Imaging and
Therapy. Curr. Top. Med. Chem. 2017, 17, 1507–1520.
(91) Bacinello, D.; Garanger, E.; Taton, D.; Tam, K. C.; Lecommandoux, S. Enzyme-
Degradable Self-Assembled Nanostructures from Polymer-Peptide Hybrids.
Biomacromolecules 2014, 15, 1882–1888.
(92) Kim, H. S.; Yoon, S.; Son, Y. J.; Park, Y.; Jung, Y. M.; Yoo, H. S. High-Yield Clicking
and Dissociation of Doxorubicin Nanoclusters Exhibiting Differential Cellular Uptakes
and Imaging. J. Control. Release Off. J. Control. Release Soc. 2015, 217, 64–73.
(93) Zhang, C.; Pan, D.; Li, J.; Hu, J.; Bains, A.; Guys, N.; Zhu, H.; Li, X.; Luo, K.; Gong,
et al. Enzyme-Responsive Peptide Dendrimer-Gemcitabine Conjugate as a
Controlled-Release Drug Delivery Vehicle with Enhanced Antitumor Efficacy. Acta
Biomater. 2017, 55, 153–162.
(94) Li, N.; Li, N.; Yi, Q.; Luo, K.; Guo, C.; Pan, D.; Gu, Z. Amphiphilic Peptide Dendritic
Copolymer-Doxorubicin Nanoscale Conjugate Self-Assembled to Enzyme-
Responsive Anti-Cancer Agent. Biomaterials 2014, 35, 9529–9545.
(95) Sharma, R.; Kim, S.-Y.; Sharma, A.; Zhang, Z.; Kambhampati, S. P.; Kannan, S.;
Kannan, R. M. Activated Microglia Targeting Dendrimer-Minocycline Conjugate as
Therapeutics for Neuroinflammation. Bioconjug. Chem. 2017, 28, 2874–2886.
(96) Jiang, P.; Li, S.; Lai, J.; Zheng, H.; Lin, C.; Shi, P.; Wang, Y. Nanoparticle-
Programmed Surface for Drug Release and Cell Regulation via Reversible
Hybridization Reaction. ACS Appl. Mater. Interfaces 2017, 9, 4467–4474.
(97) Denis, I.; el Bahhaj, F.; Collette, F.; Delatouche, R.; Gueugnon, F.; Pouliquen, D.;
Pichavant, L.; Héroguez, V.; Grégoire, M.; Bertrand, P.; et al. Histone Deacetylase
76
Inhibitor-Polymer Conjugate Nanoparticles for Acid-Responsive Drug Delivery. Eur. J.
Med. Chem. 2015, 95, 369–376.
(98) El Bahhaj, F.; Denis, I.; Pichavant, L.; Delatouche, R.; Collette, F.; Linot, C.;
Pouliquen, D.; Grégoire, M.; Héroguez, V.; Blanquart, C.; et al. Histone Deacetylase
Inhibitors Delivery Using Nanoparticles with Intrinsic Passive Tumor Targeting
Properties for Tumor Therapy. Theranostics 2016, 6, 795–807.
(99) Xu, L.; Zolotarskaya, O. Y.; Yeudall, W. A.; Yang, H. Click Hybridization of Immune
Cells and Polyamidoamine Dendrimers. Adv. Healthc. Mater. 2014, 3, 1430–1438.
(100) Gondi, C. S.; Rao, J. S. Cathepsin B as a Cancer Target. Expert Opin. Ther. Targets
2013, 17, 281–291.
(101) Lyu, Y.; Zhen, X.; Miao, Y.; Pu, K. Reaction-Based Semiconducting Polymer
Nanoprobes for Photoacoustic Imaging of Protein Sulfenic Acids. ACS Nano 2017,
11, 358–367.
(102) Xing, Y.; Zhu, J.; Zhao, L.; Xiong, Z.; Li, Y.; Wu, S.; Chand, G.; Shi, X.; Zhao, J.
SPECT/CT Imaging of Chemotherapy-Induced Tumor Apoptosis Using 99mTc-
Labeled Dendrimer-Entrapped Gold Nanoparticles. Drug Deliv. 2018, 25, 1384–1393.
(103) Wei, Z.; Wu, M.; Li, Z.; Lin, Z.; Zeng, J.; Sun, H.; Liu, X.; Liu, J.; Li, B.; Zeng, Y.
Gadolinium-Doped Hollow CeO2-ZrO2 Nanoplatform as Multifunctional MRI/CT Dual-
Modal Imaging Agent and Drug Delivery Vehicle. Drug Deliv. 2018, 25, 353–363.
(104) Zhou, B.; Xiong, Z.; Wang, P.; Peng, C.; Shen, M.; Mignani, S.; Majoral, J.-P.; Shi, X.
Targeted Tumor Dual Mode CT/MR Imaging Using Multifunctional Polyethylenimine-
Entrapped Gold Nanoparticles Loaded with Gadolinium. Drug Deliv. 2018, 25, 178–
186.
(105) He, W.; Cheng, L.; Zhang, L.; Jiang, X.; Liu, Z.; Cheng, Z.; Zhu, X. Bifunctional
Nanoparticles with Magnetism and NIR Fluorescence: Controlled Synthesis from
Combination of AGET ATRP and “click” Reaction. Nanotechnology 2014, 25, 045602.
(106) Teske, N. S.; Voigt, J.; Shastri, V. P. Clickable Degradable Aliphatic Polyesters via
Copolymerization with Alkyne Epoxy Esters: Synthesis and Postfunctionalization with
Organic Dyes. J. Am. Chem. Soc. 2014, 136, 10527–10533.
(107) Hollingsworth, J. V.; Bhupathiraju, N. V. S. D. K.; Sun, J.; Lochner, E.; Vicente, M. G.
H.; Russo, P. S. Preparation of Metalloporphyrin-Bound Superparamagnetic Silica
Particles via “Click” Reaction. ACS Appl. Mater. Interfaces 2016, 8, 792–801.
(108) Lee, S.; Kang, S.-W.; Ryu, J. H.; Na, J. H.; Lee, D.-E.; Han, S. J.; Kang, C. M.; Choe,
Y. S.; Lee, K. C.; Leary, J. F.; et al. Tumor-Homing Glycol Chitosan-Based
Optical/PET Dual Imaging Nanoprobe for Cancer Diagnosis. Bioconjug. Chem. 2014,
25, 601–610.
(109) Pérez-Medina, C.; Abdel-Atti, D.; Zhang, Y.; Longo, V. A.; Irwin, C. P.; Binderup, T.;
Ruiz-Cabello, J.; Fayad, Z. A.; Lewis, J. S.; Mulder, W. J. M.; et al. A Modular
Labeling Strategy for in vivo PET and Near-Infrared Fluorescence Imaging of
Nanoparticle Tumor Targeting. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2014, 55,
1706–1711.
(110) Jeon, J.; Kang, J. A.; Shim, H. E.; Nam, Y. R.; Yoon, S.; Kim, H. R.; Lee, D. E.; Park,
S. H. Efficient Method for Iodine Radioisotope Labeling of Cyclooctyne-Containing
Molecules Using Strain-Promoted Copper-Free Click Reaction. Bioorg. Med. Chem.
2015, 23, 3303–3308.
(111) Arranja, A. G.; Pathak, V.; Lammers, T.; Shi, Y. Tumor-Targeted Nanomedicines for
Cancer Theranostics. Pharmacol. Res. 2017, 115, 87–95.
(112) Lammers, T.; Kiessling, F.; Hennink, W. E.; Storm, G. Drug Targeting to Tumors:
Principles, Pitfalls and (Pre-) Clinical Progress. J. Controlled Release 2012, 161,
175–187.
(113) Sun, Q.; Ojha, T.; Kiessling, F.; Lammers, T.; Shi, Y. Enhancing Tumor Penetration of
Nanomedicines. Biomacromolecules 2017, 18, 1449–1459.
(114) Golombek, S. K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.;
Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses.
Adv. Drug Deliv. Rev. 2018, 130, 17–38.
77
(115) Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the Surface Modification,
Size, and Shape on Cellular Uptake of Nanoparticles. Cell Biol. Int. 2015, 39, 881–
890.
(116) Albanese, A.; Tang, P. S.; Chan, W. C. W. The Effect of Nanoparticle Size, Shape,
and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–
16.
(117) Danhier, F. To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the
Clinic, What Is the Future of Nanomedicine? J. Controlled Release 2016, 244, 108–
121.
(118) Sulthana, S.; Banerjee, T.; Kallu, J.; Vuppala, S. R.; Heckert, B.; Naz, S.; Shelby, T.;
Yambem, O.; Santra, S. Combination Therapy of NSCLC Using Hsp90 Inhibitor and
Doxorubicin Carrying Functional Nanoceria. Mol. Pharm. 2017, 14, 875–884.
(119) Junyaprasert, V. B.; Dhanahiranpruk, S.; Suksiriworapong, J.; Sripha, K.;
Moongkarndi, P. Enhanced Toxicity and Cellular Uptake of Methotrexate-Conjugated
Nanoparticles in Folate Receptor-Positive Cancer Cells by Decorating with Folic Acid-
Conjugated d-α-Tocopheryl Polyethylene Glycol 1000 Succinate. Colloids Surf. B
Biointerfaces 2015, 136, 383–393.
(120) Wu, Y.; Zhang, Y.; Zhang, W.; Sun, C.; Wu, J.; Tang, J. Reversing of Multidrug
Resistance Breast Cancer by Co-Delivery of P-Gp SiRNA and Doxorubicin via Folic
Acid-Modified Core-Shell Nanomicelles. Colloids Surf. B Biointerfaces 2016, 138, 60–
69.
(121) Singh, Y.; Durga Rao Viswanadham, K. K.; Kumar Jajoriya, A.; Meher, J. G.; Raval,
K.; Jaiswal, S.; Dewangan, J.; Bora, H. K.; Rath, S. K.; Lal, J.; et al. Click Biotinylation
of PLGA Template for Biotin Receptor Oriented Delivery of Doxorubicin
Hydrochloride in 4T1 Cell-Induced Breast Cancer. Mol. Pharm. 2017, 14, 2749–2765.
(122) Liu, R.; Zhao, J.; Han, G.; Zhao, T.; Zhang, R.; Liu, B.; Liu, Z.; Zhang, C.; Yang, L.;
Zhang, Z. Click-Functionalized SERS Nanoprobes with Improved Labeling Efficiency
and Capability for Cancer Cell Imaging. ACS Appl. Mater. Interfaces 2017, 9, 38222–
38229.
(123) Olejniczak, J.; Collet, G.; Nguyen Huu, V. A.; Chan, M.; Lee, S.; Almutairi, A.
Biorthogonal Click Chemistry on Poly(Lactic-Co-Glycolic Acid)-Polymeric Particles.
Biomater. Sci. 2017, 5, 211–215.
(124) Sun, Q.; Kang, Z.; Xue, L.; Shang, Y.; Su, Z.; Sun, H.; Ping, Q.; Mo, R.; Zhang, C. A
Collaborative Assembly Strategy for Tumor-Targeted SiRNA Delivery. J. Am. Chem.
Soc. 2015, 137, 6000–6010.
(125) Li, H.; Mu, Y.; Qian, S.; Lu, J.; Wan, Y.; Fu, G.; Liu, S. Synthesis of Fluorescent Dye-
Doped Silica Nanoparticles for Target-Cell-Specific Delivery and Intracellular
MicroRNA Imaging. The Analyst 2015, 140, 567–573.
(126) Morris, W.; Briley, W. E.; Auyeung, E.; Cabezas, M. D.; Mirkin, C. A. Nucleic Acid-
Metal Organic Framework (MOF) Nanoparticle Conjugates. J. Am. Chem. Soc. 2014,
136, 7261–7264.
(127) Wang, C.-F.; Mäkilä, E. M.; Bonduelle, C.; Rytkönen, J.; Raula, J.; Almeida, S.;
Närvänen, A.; Salonen, J. J.; Lecommandoux, S.; Hirvonen, J. T.; et al.
Functionalization of Alkyne-Terminated Thermally Hydrocarbonized Porous Silicon
Nanoparticles With Targeting Peptides and Antifouling Polymers: Effect on the
Human Plasma Protein Adsorption. ACS Appl. Mater. Interfaces 2015, 7, 2006–2015.
(128) Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 Targeted and
Cargo-Loaded Exosomes Facilitate Simultaneous Imaging and Therapy of Glioma in
vitro and in vivo. Biomaterials 2018, 178, 302–316.
(129) Ke, W.; Zha, Z.; Mukerabigwi, J. F.; Chen, W.; Wang, Y.; He, C.; Ge, Z. Matrix
Metalloproteinase-Responsive Multifunctional Peptide-Linked Amphiphilic Block
Copolymers for Intelligent Systemic Anticancer Drug Delivery. Bioconjug. Chem.
2017, 28, 2190–2198.
(130) Arriortua, O. K.; Garaio, E.; Herrero de la Parte, B.; Insausti, M.; Lezama, L.;
Plazaola, F.; García, J. A.; Aizpurua, J. M.; Sagartzazu, M.; Irazola, M.; et al.
Antitumor Magnetic Hyperthermia Induced by RGD-Functionalized Fe3O4
78
Nanoparticles, in an Experimental Model of Colorectal Liver Metastases. Beilstein J.
Nanotechnol. 2016, 7, 1532–1542.
(131) Slegerova, J.; Hajek, M.; Rehor, I.; Sedlak, F.; Stursa, J.; Hruby, M.; Cigler, P.
Designing the Nanobiointerface of Fluorescent Nanodiamonds: Highly Selective
Targeting of Glioma Cancer Cells. Nanoscale 2015, 7, 415–420.
(132) Yuan, H.; Wilks, M. Q.; El Fakhri, G.; Normandin, M. D.; Kaittanis, C.; Josephson, L.
Heat-Induced-Radiolabeling and Click Chemistry: A Powerful Combination for
Generating Multifunctional Nanomaterials. PloS One 2017, 12, e0172722.
(133) Qin, M.; Zong, H.; Kopelman, R. Click Conjugation of Peptide to Hydrogel
Nanoparticles for Tumor-Targeted Drug Delivery. Biomacromolecules 2014, 15,
3728–3734.
(134) Wang, C.-F.; Sarparanta, M. P.; Mäkilä, E. M.; Hyvönen, M. L. K.; Laakkonen, P. M.;
Salonen, J. J.; Hirvonen, J. T.; Airaksinen, A. J.; Santos, H. A. Multifunctional Porous
Silicon Nanoparticles for Cancer Theranostics. Biomaterials 2015, 48, 108–118.
(135) Wang, C.-F.; Mäkilä, E. M.; Kaasalainen, M. H.; Liu, D.; Sarparanta, M. P.;
Airaksinen, A. J.; Salonen, J. J.; Hirvonen, J. T.; Santos, H. A. Copper-Free Azide-
Alkyne Cycloaddition of Targeting Peptides to Porous Silicon Nanoparticles for
Intracellular Drug Uptake. Biomaterials 2014, 35, 1257–1266.
(136) Huang, B.; Otis, J.; Joice, M.; Kotlyar, A.; Thomas, T. P. PSMA-Targeted Stably
Linked “Dendrimer-Glutamate Urea-Methotrexate” as a Prostate Cancer Therapeutic.
Biomacromolecules 2014, 15, 915–923.
(137) Zhao, M.; Liu, Y.; Hsieh, R. S.; Wang, N.; Tai, W.; Joo, K.-I.; Wang, P.; Gu, Z.; Tang,
Y. Clickable Protein Nanocapsules for Targeted Delivery of Recombinant P53
Protein. J. Am. Chem. Soc. 2014, 136, 15319–15325.
(138) Otis, J. B.; Zong, H.; Kotylar, A.; Yin, A.; Bhattacharjee, S.; Wang, H.; Baker, J. R.;
Wang, S. H. Dendrimer Antibody Conjugate to Target and Image HER-2
Overexpressing Cancer Cells. Oncotarget 2016, 7, 36002–36013.
(139) Zhou, Z.; Badkas, A.; Stevenson, M.; Lee, J.-Y.; Leung, Y.-K. Herceptin Conjugated
PLGA-PHis-PEG PH Sensitive Nanoparticles for Targeted and Controlled Drug
Delivery. Int. J. Pharm. 2015, 487, 81–90.
(140) Lin, Q.; Yang, Y.; Hu, Q.; Guo, Z.; Liu, T.; Xu, J.; Wu, J.; Kirk, T. B.; Ma, D.; Xue, W.
Injectable Supramolecular Hydrogel Formed from α-Cyclodextrin and PEGylated
Arginine-Functionalized Poly(l-Lysine) Dendron for Sustained MMP-9 ShRNA
Plasmid Delivery. Acta Biomater. 2017, 49, 456–471.
(141) Li, X.; Zhao, L.; Liang, Q.; Liang, Q.; Ye, J.; Komatsu, N.; Zhang, Q.; Gao, W.; Xu, M.;
Chen, X. Cationic Polyarginine Conjugated Mesoporous Bioactive Glass
Nanoparticles with Polyglycerol Coating for Efficient DNA Delivery. J. Biomed.
Nanotechnol. 2017, 13, 280–289.
(142) Maity, A. R.; Stepensky, D. Efficient Subcellular Targeting to the Cell Nucleus of
Quantum Dots Densely Decorated with a Nuclear Localization Sequence Peptide.
ACS Appl. Mater. Interfaces 2016, 8, 2001–2009.
(143) Tatiparti, K.; Sau, S.; Gawde, K. A.; Iyer, A. K. Copper-Free “Click” Chemistry-Based
Synthesis and Characterization of Carbonic Anhydrase-IX Anchored Albumin-
Paclitaxel Nanoparticles for Targeting Tumor Hypoxia. Int. J. Mol. Sci. 2018, 19. 838.
(144) Calabrese, C. M.; Merkel, T. J.; Briley, W. E.; Randeria, P. S.; Narayan, S. P.; Rouge,
J. L.; Walker, D. A.; Scott, A. W.; Mirkin, C. A. Biocompatible Infinite-Coordination-
Polymer Nanoparticle-Nucleic-Acid Conjugates for Antisense Gene Regulation.
Angew. Chem. Int. Ed Engl. 2015, 54, 476–480.
(145) Collina, S. New Perspectives in Cancer Therapy: The Biotin-Antitumor Molecule
Conjugates. Med. Chem. 2014, S1, 1-8.
(146) Zwicke, G. L.; Ali Mansoori, G.; Jeffery, C. J. Utilizing the Folate Receptor for Active
Targeting of Cancer Nanotherapeutics. Nano Rev. 2012, 3, 18496.
(147) Puvvada, N.; Rajput, S.; Kumar, B. N. P.; Sarkar, S.; Konar, S.; Brunt, K. R.; Rao, R.
R.; Mazumdar, A.; Das, S. K.; Basu, R.; et al. Novel ZnO Hollow-Nanocarriers
Containing Paclitaxel Targeting Folate-Receptors in a Malignant PH-
Microenvironment for Effective Monitoring and Promoting Breast Tumor Regression.
Sci. Rep. 2015, 5, 11760.
79
(148) Wei, J.; Shuai, X.; Wang, R.; He, X.; Li, Y.; Ding, M.; Li, J.; Tan, H.; Fu, Q. Clickable
and Imageable Multiblock Polymer Micelles with Magnetically Guided and PEG-
Switched Targeting and Release Property for Precise Tumor Theranosis.
Biomaterials 2017, 145, 138–153.
(149) Zhang, Y.; Lv, T.; Zhang, H.; Xie, X.; Li, Z.; Chen, H.; Gao, Y. Folate and
Heptamethine Cyanine Modified Chitosan-Based Nanotheranostics for Tumor
Targeted Near-Infrared Fluorescence Imaging and Photodynamic Therapy.
Biomacromolecules 2017, 18, 2146–2160.
(150) Guldris, N.; Gallo, J.; García-Hevia, L.; Rivas, J.; Bañobre-López, M.; Salonen, L. M.
Orthogonal Clickable Iron Oxide Nanoparticle Platform for Targeting, Imaging, and
On-Demand Release. Chem. Weinh. Bergstr. Ger. 2018, 24, 8624–8631.
(151) Kato, D.; Oishi, M. Ultrasensitive Detection of DNA and RNA Based on Enzyme-Free
Click Chemical Ligation Chain Reaction on Dispersed Gold Nanoparticles. ACS Nano
2014, 8, 9988–9997.
(152) Mendez-Gonzalez, D.; Laurenti, M.; Latorre, A.; Somoza, A.; Vazquez, A.; Negredo,
A. I.; López-Cabarcos, E.; Calderón, O. G.; Melle, S.; Rubio-Retama, J.
Oligonucleotide Sensor Based on Selective Capture of Upconversion Nanoparticles
Triggered by Target-Induced DNA Interstrand Ligand Reaction. ACS Appl. Mater.
Interfaces 2017, 9, 12272–12281.
(153) Rambaruth, N. D. S.; Dwek, M. V. Cell Surface Glycan-Lectin Interactions in Tumor
Metastasis. Acta Histochem. 2011, 113, 591–600.
(154) Nakahara, S.; Raz, A. Biological Modulation by Lectins and Their Ligands in Tumor
Progression and Metastasis. Anticancer Agents Med. Chem. 2008, 8, 22–36.
(155) Poonthiyil, V.; Lindhorst, T. K.; Golovko, V. B.; Fairbanks, A. J. Recent Applications of
Click Chemistry for the Functionalization of Gold Nanoparticles and Their Conversion
to Glyco-Gold Nanoparticles. Beilstein J. Org. Chem. 2018, 14, 11–24.
(156) Choi, K. Y.; Yoon, H. Y.; Kim, J.-H.; Bae, S. M.; Park, R.-W.; Kang, Y. M.; Kim, I.-S.;
Kwon, I. C.; Choi, K.; Jeong, S. Y.; et al. Smart Nanocarrier Based on PEGylated
Hyaluronic Acid for Cancer Therapy. ACS Nano 2011, 5, 8591–8599.
(157) Lundquist, J. J.; Toone, E. J. The Cluster Glycoside Effect. Chem. Rev. 2002, 102,
555–578.
(158) Bezouska, K. Design, Functional Evaluation and Biomedical Applications of
Carbohydrate Dendrimers (Glycodendrimers). J. Biotechnol. 2002, 90, 269–290.
(159) Rajakumar, P.; Anandhan, R.; Vadla, G. P.; Vellaichamy, E. Synthesis and Cardio
Protective Biological Applications of Glucodendrimers by H9C2 Cell Studies.
Carbohydr. Polym. 2014, 99, 403–414.
(160) Kong, N.; Zhou, J.; Park, J.; Xie, S.; Ramström, O.; Yan, M. Quantitative Fluorine
NMR to Determine Carbohydrate Density on Glyconanomaterials Synthesized from
Perfluorophenyl Azide-Functionalized Silica Nanoparticles by Click Reaction. Anal.
Chem. 2015, 87, 9451–9458.
(161) Tollas, S.; Bereczki, I.; Borbás, A.; Batta, G.; Vanderlinden, E.; Naesens, L.;
Herczegh, P. Synthesis of a Cluster-Forming Sialylthio-D-Galactose Fullerene
Conjugate and Evaluation of Its Interaction with Influenza Virus Hemagglutinin and
Neuraminidase. Bioorg. Med. Chem. Lett. 2014, 24, 2420–2423.
(162) Keefe, A. D.; Pai, S.; Ellington, A. Aptamers as Therapeutics. Nat. Rev. Drug Discov.
2010, 9, 537–550.
(163) Ma, L.; Tu, C.; Le, P.; Chitoor, S.; Lim, S. J.; Zahid, M. U.; Teng, K. W.; Ge, P.;
Selvin, P. R.; Smith, A. M. Multidentate Polymer Coatings for Compact and
Homogeneous Quantum Dots with Efficient Bioconjugation. J. Am. Chem. Soc. 2016,
138, 3382–3394.
(164) Chen, W.-H.; Yu, X.; Cecconello, A.; Sohn, Y. S.; Nechushtai, R.; Willner, I. Stimuli-
Responsive Nucleic Acid-Functionalized Metal-Organic Framework Nanoparticles
Using PH- and Metal-Ion-Dependent DNAzymes as Locks. Chem. Sci. 2017, 8,
5769–5780.
(165) Ding, Q.; Zhan, Q.; Zhou, X.; Zhang, T.; Xing, D. Theranostic Upconversion
Nanobeacons for Tumor MRNA Ratiometric Fluorescence Detection and Imaging-
Monitored Drug Delivery. Small Weinh. Bergstr. Ger. 2016, 12, 5944–5953.
80
(166) Akiel, R. D.; Zhang, X.; Abeywardana, C.; Stepanov, V.; Qin, P. Z.; Takahashi, S.
Investigating Functional DNA Grafted on Nanodiamond Surface Using Site-Directed
Spin Labeling and Electron Paramagnetic Resonance Spectroscopy. J. Phys. Chem.
B 2016, 120, 4003–4008.
(167) Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–
280.
(168) Thumshirn, G.; Hersel, U.; Goodman, S. L.; Kessler, H. Multimeric Cyclic RGD
Peptides as Potential Tools for Tumor Targeting: Solid-Phase Peptide Synthesis and
Chemoselective Oxime Ligation. Chem. - Eur. J. 2003, 9, 2717–2725.
(169) Rehor, I.; Slegerova, J.; Kucka, J.; Proks, V.; Petrakova, V.; Adam, M.-P.; Treussart,
F.; Turner, S.; Bals, S.; Sacha, P.; et al. Fluorescent Nanodiamonds Embedded in
Biocompatible Translucent Shells. Small Weinh. Bergstr. Ger. 2014, 10, 1106–1115.
(170) Oz, Y.; Arslan, M.; Gevrek, T. N.; Sanyal, R.; Sanyal, A. Modular Fabrication of
Polymer Brush Coated Magnetic Nanoparticles: Engineering the Interface for
Targeted Cellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 19813–19826.
(171) Lindgren, M.; Hällbrink, M.; Prochiantz, A.; Langel, Ü. Cell-Penetrating Peptides.
Trends Pharmacol. Sci. 2000, 21, 99–103.
(172) Vivès, E.; Brodin, P.; Lebleu, B. A. Truncated HIV-1 Tat Protein Basic Domain
Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell
Nucleus. J. Biol. Chem. 1997, 272, 16010–16017.
(173) Liu, D.; Zhang, H.; Mäkilä, E.; Fan, J.; Herranz-Blanco, B.; Wang, C.-F.; Rosa, R.;
Ribeiro, A. J.; Salonen, J.; Hirvonen, J.; et al. Microfluidic Assisted One-Step
Fabrication of Porous Silicon@acetalated Dextran Nanocomposites for Precisely
Controlled Combination Chemotherapy. Biomaterials 2015, 39, 249–259.
(174) Han, S.-S.; Li, Z.-Y.; Zhu, J.-Y.; Han, K.; Zeng, Z.-Y.; Hong, W.; Li, W.-X.; Jia, H.-Z.;
Liu, Y.; Zhuo, R.-X.; et al. Dual-PH Sensitive Charge-Reversal Polypeptide Micelles
for Tumor-Triggered Targeting Uptake and Nuclear Drug Delivery. Small Weinh.
Bergstr. Ger. 2015, 11, 2543–2554.
(175) Brunner, K.; Harder, J.; Halbach, T.; Willibald, J.; Spada, F.; Gnerlich, F.; Sparrer, K.;
Beil, A.; Möckl, L.; Bräuchle, C.; et al. Cell-Penetrating and Neurotargeting Dendritic
SiRNA Nanostructures. Angew. Chem. Int. Ed Engl. 2015, 54, 1946–1949.
(176) Wan, J.; Brust, A.; Bhola, R. F.; Jha, P.; Mobli, M.; Lewis, R. J.; Christie, M. J.;
Alewood, P. F. Inhibition of the Norepinephrine Transporter by χ-Conotoxin
Dendrimers. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2016, 22, 280–289.
(177) Lovelace, E. S.; Armishaw, C. J.; Colgrave, M. L.; Wahlstrom, M. E.; Alewood, P. F.;
Daly, N. L.; Craik, D. J. Cyclic MrIA: A Stable and Potent Cyclic Conotoxin with a
Novel Topological Fold That Targets the Norepinephrine Transporter. J. Med. Chem.
2006, 49, 6561–6568.
(178) Hüttl, C.; Hettrich, C.; Riedel, M.; Henklein, P.; Rawel, H.; Bier, F. F. Development of
Peptidyl Lysine Dendrons: 1,3-Dipolar Cycloaddition for Peptide Coupling and
Antibody Recognition. Chem. Biol. Drug Des. 2015, 85, 565–573.
(179) Tang, W.; Becker, M. L. “Click” Reactions: A Versatile Toolbox for the Synthesis of
Peptide-Conjugates. Chem. Soc. Rev. 2014, 43, 7013–7039.
(180) Nogueira-Librelotto, D. R.; Codevilla, C. F.; Farooqi, A.; Rolim, C. M. B. Transferrin-
Conjugated Nanocarriers as Active-Targeted Drug Delivery Platforms for Cancer
Therapy. Curr. Pharm. Des. 2017, 23, 454–466.
(181) Wang, W.; Kapur, A.; Ji, X.; Zeng, B.; Mishra, D.; Mattoussi, H. Multifunctional and
High Affinity Polymer Ligand That Provides Bio-Orthogonal Coating of Quantum Dots.
Bioconjug. Chem. 2016, 27, 2024–2036.
(182) Asadian-Birjand, M.; Biglione, C.; Bergueiro, J.; Cappelletti, A.; Rahane, C.; Chate,
G.; Khandare, J.; Klemke, B.; Strumia, M. C.; Calderón, M. Transferrin Decorated
Thermoresponsive Nanogels as Magnetic Trap Devices for Circulating Tumor Cells.
Macromol. Rapid Commun. 2016, 37, 439–445.
(183) Jivan, F.; Yegappan, R.; Pearce, H.; Carrow, J. K.; McShane, M.; Gaharwar, A. K.;
Alge, D. L. Sequential Thiol-Ene and Tetrazine Click Reactions for the Polymerization
and Functionalization of Hydrogel Microparticles. Biomacromolecules 2016, 17,
3516–3523.
81
(184) Martínez-Jothar, L.; Doulkeridou, S.; Schiffelers, R. M.; Sastre Torano, J.; Oliveira,
S.; van Nostrum, C. F.; Hennink, W. E. Insights into Maleimide-Thiol Conjugation
Chemistry: Conditions for Efficient Surface Functionalization of Nanoparticles for
Receptor Targeting. J. Control. Release 2018, 282, 101–109.
(185) Estupiñán, D.; Bannwarth, M. B.; Mylon, S. E.; Landfester, K.; Muñoz-Espí, R.;
Crespy, D. Multifunctional Clickable and Protein-Repellent Magnetic Silica
Nanoparticles. Nanoscale 2016, 8, 3019–3030.
(186) Pathak, R. K.; McNitt, C. D.; Popik, V. V.; Dhar, S. Copper-Free Click-Chemistry
Platform to Functionalize Cisplatin Prodrugs. Chem. Weinh. Bergstr. Ger. 2014, 20,
6861–6865.
(187) Hui, J. Z.; Al Zaki, A.; Cheng, Z.; Popik, V.; Zhang, H.; Luning Prak, E. T.; Tsourkas,
A. Facile Method for the Site-Specific, Covalent Attachment of Full-Length IgG onto
Nanoparticles. Small Weinh. Bergstr. Ger. 2014, 10, 3354–3363.
(188) Ta, H. T.; Li, Z.; Hagemeyer, C. E.; Cowin, G.; Zhang, S.; Palasubramaniam, J.; Alt,
K.; Wang, X.; Peter, K.; Whittaker, A. K. Molecular Imaging of Activated Platelets via
Antibody-Targeted Ultra-Small Iron Oxide Nanoparticles Displaying Unique Dual MRI
Contrast. Biomaterials 2017, 134, 31–42.
(189) Colombo, M.; Sommaruga, S.; Mazzucchelli, S.; Polito, L.; Verderio, P.; Galeffi, P.;
Corsi, F.; Tortora, P.; Prosperi, D. Site-Specific Conjugation of ScFvs Antibodies to
Nanoparticles by Bioorthogonal Strain-Promoted Alkyne-Nitrone Cycloaddition.
Angew. Chem. Int. Ed Engl. 2012, 51, 496–499.
(190) Jeong, S.; Park, J. Y.; Cha, M. G.; Chang, H.; Kim, Y.-I.; Kim, H.-M.; Jun, B.-H.; Lee,
D. S.; Lee, Y.-S.; et al. Highly Robust and Optimized Conjugation of Antibodies to
Nanoparticles Using Quantitatively Validated Protocols. Nanoscale 2017, 9, 2548–
2555.
(191) Lee-Montiel, F. T.; Li, P.; Imoukhuede, P. I. Quantum Dot Multiplexing for the Profiling
of Cellular Receptors. Nanoscale 2015, 7, 18504–18514.
(192) Qiao, R.; Liu, C.; Liu, M.; Hu, H.; Liu, C.; Hou, Y.; Wu, K.; Lin, Y.; Liang, J.; Gao, M.
Ultrasensitive in vivo Detection of Primary Gastric Tumor and Lymphatic Metastasis
Using Upconversion Nanoparticles. ACS Nano 2015, 9, 2120–2129.
(193) Sanjaya, K. C.; Ranzoni, A.; Watterson, D.; Young, P.; Cooper, M. A. Evaluation of
Direct versus Multi-Layer Passivation and Capture Chemistries for Nanoparticle-
Based Biosensor Applications. Biosens. Bioelectron. 2015, 67, 769–774.
(194) Cserép, G. B.; Herner, A.; Kele, P. Bioorthogonal Fluorescent Labels: A Review on
Combined Forces. Methods Appl. Fluoresc. 2015, 3, 042001.
(195) Koo, H.; Lee, S.; Na, J. H.; Kim, S. H.; Hahn, S. K.; Choi, K.; Kwon, I. C.; Jeong, S.
Y.; Kim, K. Bioorthogonal Copper-Free Click Chemistry in vivo for Tumor-Targeted
Delivery of Nanoparticles. Angew. Chem. Int. Ed Engl. 2012, 51, 11836–11840.
(196) Lamoot, A.; Uvyn, A.; Kasmi, S.; De Geest, B. G. Covalent Cell Surface Conjugation
of Nanoparticles by a Combination of Metabolic Labeling and Click Chemistry.
Angew. Chem. 2021, 133, 6390–6395.
(197) Chen, Y.; Cordero, J. M.; Wang, H.; Franke, D.; Achorn, O. B.; Freyria, F. S.;
Coropceanu, I.; Wei, H.; Chen, O.; Mooney, D. J.; et al. A Ligand System for the
Flexible Functionalization of Quantum Dots via Click Chemistry. Angew. Chem. Int.
Ed Engl. 2018, 57, 4652–4656.
(198) Au, K. M.; Tripathy, A.; Lin, C. P.-I.; Wagner, K.; Hong, S.; Wang, A. Z.; Park, S. I.
Bespoke Pretargeted Nanoradioimmunotherapy for the Treatment of Non-Hodgkin
Lymphoma. ACS Nano 2018, 12, 1544–1563.
(199) Lu, G.; Zuo, L.; Zhang, J.; Zhu, H.; Zhuang, W.; Wei, W.; Xie, H.-Y. Two-Step Tumor-
Targeting Therapy via Integrating Metabolic Lipid-Engineering with in Situ Click
Chemistry. Biomater. Sci. 2020, 8, 2283–2288.
(200) Lee, S.; Yoon, H. I.; Na, J. H.; Jeon, S.; Lim, S.; Koo, H.; Han, S.-S.; Kang, S.-W.;
Park, S.-J.; Moon, S.-H.; et al. In vivo Stem Cell Tracking with Imageable
Nanoparticles That Bind Bioorthogonal Chemical Receptors on the Stem Cell
Surface. Biomaterials 2017, 139, 12–29.
(201) Lim, S.; Kim, W.; Song, S.; Shim, M. K.; Yoon, H. Y.; Kim, B.-S.; Kwon, I. C.; Kim, K.
Intracellular Uptake Mechanism of Bioorthogonally Conjugated Nanoparticles on
82
Metabolically Engineered Mesenchymal Stem Cells. Bioconjug. Chem. 2021, 32,
199–214.
(202) Haun, J. B.; Devaraj, N. K.; Hilderbrand, S. A.; Lee, H.; Weissleder, R. Bioorthogonal
Chemistry Amplifies Nanoparticle Binding and Enhances the Sensitivity of Cell
Detection. Nat. Nanotechnol. 2010, 5, 660–665.
(203) Haun, J. B.; Devaraj, N. K.; Marinelli, B. S.; Lee, H.; Weissleder, R. Probing
Intracellular Biomarkers and Mediators of Cell Activation Using Nanosensors and
Bioorthogonal Chemistry. ACS Nano 2011, 5, 3204–3213.
(204) Chung, H. J.; Reiner, T.; Budin, G.; Min, C.; Liong, M.; Issadore, D.; Lee, H.;
Weissleder, R. Ubiquitous Detection of Gram-Positive Bacteria with Bioorthogonal
Magnetofluorescent Nanoparticles. ACS Nano 2011, 5, 8834–8841.
(205) Budin, G.; Chung, H. J.; Lee, H.; Weissleder, R. A Magnetic Gram Stain for Bacterial
Detection. Angew. Chem. Int. Ed Engl. 2012, 51, 7752–7755.
(206) Tassa, C.; Liong, M.; Hilderbrand, S.; Sandler, J. E.; Reiner, T.; Keliher, E. J.;
Weissleder, R.; Shaw, S. Y. On-Chip Bioorthogonal Chemistry Enables
Immobilization of in Situ Modified Nanoparticles and Small Molecules for Label-Free
Monitoring of Protein Binding and Reaction Kinetics. Lab. Chip 2012, 12, 3103–3110.
(207) Ghazani, A. A.; Pectasides, M.; Sharma, A.; Castro, C. M.; Mino-Kenudson, M.; Lee,
H.; Shepard, J.-A. O.; Weissleder, R. Molecular Characterization of Scant Lung
Tumor Cells Using Iron-Oxide Nanoparticles and Micro-Nuclear Magnetic
Resonance. Nanomedicine Nanotechnol. Biol. Med. 2014, 10, 661–668.
(208) Lu, G.; Li, F.; Zhang, F.; Huang, L.-L.; Zhang, L.; Lv, Y.; Wei, W.; Xie, H.-Y.
Amplifying Nanoparticle Targeting Performance to Tumor via Diels-Alder
Cycloaddition. Adv. Funct. Mater. 2018, 1707596.
(209) Yoo, J.; Choi, S.; Son, J.; Yi, G.; Kim, E.; Koo, H. Click Chemistry-Mediated Tumor-
Targeting of SN38-Loaded Nanoparticles Using Trastuzumab. Biochem. Biophys.
Res. Commun. 2019, 515, 207–213.
(210) Agasti, S. S.; Liong, M.; Tassa, C.; Chung, H. J.; Shaw, S. Y.; Lee, H.; Weissleder, R.
Supramolecular Host-Guest Interaction for Labeling and Detection of Cellular
Biomarkers. Angew. Chem. Int. Ed Engl. 2012, 51, 450–454.
(211) O’Brien, P. J.; Elahipanah, S.; Rogozhnikov, D.; Yousaf, M. N. Bio-Orthogonal
Mediated Nucleic Acid Transfection of Cells via Cell Surface Engineering. ACS Cent.
Sci. 2017, 3, 489–500.
(212) Du, L.; Qin, H.; Ma, T.; Zhang, T.; Xing, D. In vivo Imaging-Guided
Photothermal/Photoacoustic Synergistic Therapy with Bioorthogonal Metabolic
Glycoengineering-Activated Tumor Targeting Nanoparticles. ACS Nano 2017, 11,
8930–8943.
(213) Mao, D.; Hu, F.; Kenry, J.; S.; Wu, W.; Ding, D.; Kong, D.; Liu, B. Metal-Organic-
Framework-Assisted In vivo Bacterial Metabolic Labeling and Precise Antibacterial
Therapy. Adv. Mater. 2018, 1706831.
(214) Lee, S. B.; Kim, H. L.; Jeong, H.-J.; Lim, S. T.; Sohn, M.-H.; Kim, D. W. Mesoporous
Silica Nanoparticle Pretargeting for PET Imaging Based on a Rapid Bioorthogonal
Reaction in a Living Body. Angew. Chem. Int. Ed. 2013, 52, 10549–10552.
(215) Jeong, H. J.; Yoo, R. J.; Kim, J. K.; Kim, M. H.; Park, S. H.; Kim, H.; Lim, J. W.; Do,
S. H.; Lee, K. C.; Lee, Y. J.; et al. Macrophage Cell Tracking PET Imaging Using
Mesoporous Silica Nanoparticles via in vivo Bioorthogonal F-18 Labeling.
Biomaterials 2019, 199, 32–39.
(216) Tu, Y.; Dong, Y.; Wang, K.; Shen, S.; Yuan, Y.; Wang, J. Intercellular Delivery of
Bioorthogonal Chemical Receptors for Enhanced Tumor Targeting and Penetration.
Biomaterials 2020, 259, 120298.
(217) Lee, S.; Koo, H.; Na, J. H.; Han, S. J.; Min, H. S.; Lee, S. J.; Kim, S. H.; Yun, S. H.;
Jeong, S. Y.; Kwon, I. C.; et al. Chemical Tumor-Targeting of Nanoparticles Based on
Metabolic Glycoengineering and Click Chemistry. ACS Nano 2014, 8, 2048–2063.
(218) Yoon, H. Y.; Shin, M. L.; Shim, M. K.; Lee, S.; Na, J. H.; Koo, H.; Lee, H.; Kim, J.-H.;
Lee, K. Y.; Kim, K.; et al. Artificial Chemical Reporter Targeting Strategy Using
Bioorthogonal Click Reaction for Improving Active-Targeting Efficiency of Tumor. Mol.
Pharm. 2017, 14, 1558–1570.
83
(219) Brand, C.; Iacono, P.; Pérez-Medina, C.; Mulder, W. J. M.; Kircher, M. F.; Reiner, T.
Specific Binding of Liposomal Nanoparticles through Inverse Electron-Demand Diels-
Alder Click Chemistry. ChemistryOpen 2017, 6, 615–619.
(220) Denk, C.; Svatunek, D.; Mairinger, S.; Stanek, J.; Filip, T.; Matscheko, D.; Kuntner,
C.; Wanek, T.; Mikula, H. Design, Synthesis, and Evaluation of a Low-Molecular-
Weight
11
C-Labeled Tetrazine for Pretargeted PET Imaging Applying Bioorthogonal
in vivo Click Chemistry. Bioconjug. Chem. 2016, 27, 1707–1712.
(221) van Onzen, A. H. A. M.; Rossin, R.; Schenning, A. P. H. J.; Nicolay, K.; Milroy, L.-G.;
Robillard, M. S.; Brunsveld, L. Tetrazine— Trans -Cyclooctene Chemistry Applied to
Fabricate Self-Assembled Fluorescent and Radioactive Nanoparticles for in vivo Dual
Mode Imaging. Bioconjug. Chem. 2019, 30, 547–551.
(222) Keinänen, O.; Mäkilä, E. M.; Lindgren, R.; Virtanen, H.; Liljenbäck, H.; Oikonen, V.;
Sarparanta, M.; Molthoff, C.; Windhorst, A. D.; Roivainen, A.; et al. Pretargeted PET
Imaging of Trans -Cyclooctene-Modified Porous Silicon Nanoparticles. ACS Omega
2017, 2, 62–69.
(223) Qiao, J.; Tian, F.; Deng, Y.; Shang, Y.; Chen, S.; Chang, E.; Yao, J. Bio-Orthogonal
Click-Targeting Nanocomposites for Chemo-Photothermal Synergistic Therapy in
Breast Cancer. Theranostics 2020, 10, 5305–5321.
(224) Wang, H.; Tang, L.; Liu, Y.; Dobrucka, I. T.; Dobrucki, L. W.; Yin, L.; Cheng, J. In vivo
Targeting of Metabolically Labeled Cancers with Ultra-Small Silica Nanoconjugates.
Theranostics 2016, 6, 1467–1476.
(225) Wei, R.; Dong, Y.; Tu, Y.; Luo, S.; Pang, X.; Zhang, W.; Yao, W.; Tang, W.; Yang, H.;
Wei, X.; et al. Bioorthogonal Pretargeting Strategy for Anchoring Activatable
Photosensitizers on Plasma Membranes for Effective Photodynamic Therapy. ACS
Appl. Mater. Interfaces 2021, 13, 14004–14014.
(226) Zhang, W.; Deng, W.; Zhang, H.; Sun, X.; Huang, T.; Wang, W.; Sun, P.; Fan, Q.;
Huang, W. Bioorthogonal-Targeted 1064 Nm Excitation Theranostic Nanoplatform for
Precise NIR-IIa Fluorescence Imaging Guided Efficient NIR-II Photothermal Therapy.
Biomaterials 2020, 243, 119934.
(227) Perez-Medina, C.; Abdel-Atti, D.; Zhang, Y.; Longo, V. A.; Irwin, C. P.; Binderup, T.;
Ruiz-Cabello, J.; Fayad, Z. A.; Lewis, J. S.; Mulder, W. J. M.; et al. A Modular
Labeling Strategy for In vivo PET and Near-Infrared Fluorescence Imaging of
Nanoparticle Tumor Targeting. J. Nucl. Med. 2014, 55, 1706–1711.
(228) Du, L.; Qin, H.; Ma, T.; Zhang, T.; Xing, D. In vivo Imaging-Guided
Photothermal/Photoacoustic Synergistic Therapy with Bioorthogonal Metabolic
Glycoengineering-Activated Tumor Targeting Nanoparticles. ACS Nano 2017, 11,
8930–8943.
(229) Lee, S.; Jung, S.; Koo, H.; Na, J. H.; Yoon, H. Y.; Shim, M. K.; Park, J.; Kim, J.-H.;
Lee, S.; Pomper, M. G.; et al. Nano-Sized Metabolic Precursors for Heterogeneous
Tumor-Targeting Strategy Using Bioorthogonal Click Chemistry in vivo. Biomaterials
2017, 148, 1–15.
(230) Xie, X.; Li, B.; Wang, J.; Zhan, C.; Huang, Y.; Zeng, F.; Wu, S. Bioorthogonal
Nanosystem for Near-Infrared Fluorescence Imaging and Prodrug Activation in
Mouse Model. ACS Mater. Lett. 2019, 1, 549–557.
(231) Xie, X.; Li, B.; Wang, J.; Zhan, C.; Huang, Y.; Zeng, F.; Wu, S. Tetrazine-Mediated
Bioorthogonal System for Prodrug Activation, Photothermal Therapy, and
Optoacoustic Imaging. ACS Appl. Mater. Interfaces 2019, 11, 41875–41888.
(232) Liu, Y.; Hou, W.; Sun, H.; Cui, C.; Zhang, L.; Jiang, Y.; Wu, Y.; Wang, Y.; Li, J.;
Sumerlin, B. S.; et al. Thiol–Ene Click Chemistry: A Biocompatible Way for
Orthogonal Bioconjugation of Colloidal Nanoparticles. Chem. Sci. 2017, 8, 6182–
6187.
(233) Ke, P. C.; Lin, S.; Parak, W. J.; Davis, T. P.; Caruso, F. A Decade of the Protein
Corona. ACS Nano 2017, 11, 11773–11776.
(234) Mirshafiee, V.; Mahmoudi, M.; Lou, K.; Cheng, J.; Kraft, M. L. Protein Corona
Significantly Reduces Active Targeting Yield. Chem. Commun. 2013, 49, 2557–2559.
(235) Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J. J. Antifouling Strategies for
Selective In vitro and In vivo Sensing. Chem. Rev. 2020, 120, 3852–3889.
84
(236) Moyano, D. F.; Saha, K.; Prakash, G.; Yan, B.; Kong, H.; Yazdani, M.; Rotello, V. M.
Fabrication of Corona-Free Nanoparticles with Tunable Hydrophobicity. ACS Nano
2014, 8, 6748–6755.
(237) Affonso de Oliveira, J. F.; Scheffer, F. R.; Landis, R. F.; Teixeira Neto, É.; Rotello, V.
M.; Cardoso, M. B. Dual Functionalization of Nanoparticles for Generating Corona-
Free and Noncytotoxic Silica Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10,
41917–41923.
(238) Zhang, X.; Liu, Y.; Gopalakrishnan, S.; Castellanos-Garcia, L.; Li, G.; Malassiné, M.;
Uddin, I.; Huang, R.; Luther, D. C.; Vachet, R. W.; et al. Intracellular Activation of
Bioorthogonal Nanozymes through Endosomal Proteolysis of the Protein Corona.
ACS Nano 2020, 14, 4767–4773.

85
TOC Graphic
