
Anthony Danko, Ph.D.
Naval Facilities Engineering Command (NAVFAC)
Engineering and Expeditionary Warfare Center (EXWC)
Treatment Technologies for PFAS Site Management

2
Presentation Overview
• Evaluating Remediation Technologies
• Sorption
• In Situ Technologies
• Dealing with Investigation-Derived Waste (IDW)
• Wrap-Up
FRTR 2018: PFAS Emerging Characterization and Remedial Technologies

3
Summary of Available Technologies – Drinking Water Treatment
Technology Category Technology Maturity/Availability
Sorption
Activated Carbon* Commercialized, can be purchased from vendors
Anion Exchange Resin* Commercialized, can be purchased from vendors
Biochar Field Pilot Scale, not commercially available
Zeolites/Clay Minerals Commercialized, can be purchased from vendors
Membrane Filtration
Reverse Osmosis and
Nanofiltration
+
Commercialized, can be purchased from vendors
Coagulation Specialty Coagulants Full Scale application being conducted by researchers
Redox Change Electrochemical Field Pilot Scale, not commercially available
Other Sonochemical Field Pilot Scale, not commercially available
Evaluating Remediation Technologies
* Technologies that will be discussed
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

4
Summary of Available Technologies – Soil Treatment
Technology Category Technology Maturity/Availability
Sorption and Technologies
Modified Carbon* Commercialized, can be purchased from vendors
Minerals/Modified Minerals* Commercialized, can be purchased from vendors
Excavation Disposal
To Landfill Commercialized
To Incinerator Commercialized
Thermal Field Pilot Scale, commercially available
* Technologies that will be discussed
Evaluating Remediation Technologies
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

5
Consider Effect of Prior Remediation for Co-Contaminants on PFAS
• Benzene plume
• Oxygen injections at yellow
• Elevated levels of PFAA at location of historical and present
benzene plume – lacking in areas with no O
2
injections
• Fourfold difference in Kd between PFHxA and PFOA yet
their plume overlapped – likely due to in situ transformation
of precursors
• Navy currently conducting similar study under NESDI
Reference Evidence of Remediation-Induced Alteration of Subsurface Poly- and Perfluoroalkyl Substance Distribution at a
Former Firefighter Training Area Meghan E. McGuire, Charles Schaefer, Trenton Richards, Will J. Backe, Jennifer A. Field,
Erika Houtz,, David L. Sedlak, Jennifer L. Guelfo, Assaf Wunsch, and Christopher P. Higgins
Evaluating Remediation Technologies
Plume
Extent
2002
Treatment
Building
Treatment
Discharge
Most Recent
Burn Pit Area
Direction of
Groundwater Flow
Plume
Extent
2011
IRT
Pond 001
0 ft 150 ft 300 ft 450 ft
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

6
Pump-and-Treat
• At drinking water wellhead
• At point of use
• To control plume size/spread
• At base boundary to prevent plume migration
Only practical treatment for groundwater available
Key
Point
Wellhead Treatment
Point of Entry Treatment
Sorption
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

7
Granular Activated Carbon (GAC)
Material
• Made from bituminous coal or coconut
• Highly porous, large surface area
Application
• Typically used in packed-bed flow-through vessels
• Operate in series (lead-lag) or parallel
• Virgin or Reactivated GAC
http://store.ecologixsystems.com/detail/index.cfm?nPID=294
Sorption
Reagglomeration
Coal Blend Pulverizing Agglomeration Crushing Baking Activation Screening
Finished
Product
Even Activation
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

8
Granular Activated Carbon (cont.)
Mechanism
• Adsorption on surface process, physical mass transfer
• No chemical degradation or transformation
Effectiveness
• Capable of 90 to >99% removal efficiency
• Individual PFAS have different GAC breakthrough times
–e.g., GAC capacity for PFOS>PFOA
• Influent conc. for <5 Carbon PFAS typically lower
• High DOC reduces effectiveness
Reference -Yu, Q., R. Zhang, S. Deng, J. Huang, G. Yu, 2009.
"Sorption of perfluorooctane sulfonate and perfluorooctanoate
on activated carbons and resin: Kinetic and isotherm study."
Water Research, 43, 1150-1158.
PFAS <5 carbons shorter
breakthrough times
Key
Point
Sorption
Activated Carbon
hemi-micelle
micelle
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

9
Reactivation of PFAS Contaminated Granular Activated Carbon
Thermal Reactivation Process
• Reactivation temperature 1,300°F
• PFAS pyrolysed to carbon char
• Lower CO
2
footprint than making virgin GAC
• Reactivated carbon just as effective as virgin carbon
Reactivation furnace
under negative
pressure and
nitrogen
environment
Furnace off gas
passed through after
burn to destroy
organics
Emission stream
passed through
chemical scrubber to
remove acid gases
Final treatment
through baghouse
filters to remove
particulate matter
Process is expensive and energy intensive
Key
Point
Sorption
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

10
PFBS (ppb)
RSSCT PFOA Breakthrough Curves
10 Minutes Empty Bed Contact Time (EBCT)
Removal of PFBS Using
Filtrasorb
®
vs. Coconut
1.2
1
0.8
0.6
PFOA (ppb)
0.4
0.2
0
0 20,000 40,000 60,000 80,000 100,000 120,000 140,000 160,000 180,000 200,000
0
0 20,000 40,000 60,000 80,000 100,000 120,000
200
180
160
140
120
100
80
60
40
20
Bed Volumes Treated (BV)
Bed Volumes Treated (BV)
Filtrasorb
®
Coconut 12x40
Feed
Virgin
Filtrasorb
®
Coconut
12x40
Coconut
8x30
Feed
PFOA
50% Feed
PFOA
Reagglomerated coal
significantly outperformed
coconut
Reagglomerated coal
significantly outperformed
coconut
70 ppt EPA Health Advisory Exposure Limit
Bituminous vs. Coconut Carbon
Bituminous carbon appears to perform better than coconut carbon
at this specific site
Key
Point
NEWMOA PFAS Technical Workshop – Activated Carbon
Don Ivey and John Matthis May 2017
Sorption
Background TOC 1.42 mg/L
Simulated Empty Bed
Contact Time (EBCT)
10 minutes
Concentration of PFOA 920 ng/L (ppt)
Background TOC 0.16 mg/L
Simulated Empty Bed
Contact Time (EBCT)
10 minutes
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

11
Case Study – Point of Entry Treatment – Vermont Residences
• PFOA contamination from
textile coating at
CHEMFAB
®
• 541 samples from private
wells
• Bottled water delivered to
residents
• 11 homes connected to
municipal water
• 255 POET systems
installed
Sorption
Non-Detect (ND)
20-100 ppt
100-1,000 ppt
>1,000 ppt
Landfill Monitoring
Factory
Landfill
Wastewater Treatment Plant
Town Water Sample
Water Line
Village Boundary (VCGI)
Sampling Boundary
6/22/16 Sampling Extension
5/24/16 Sampling Extension
Town Boundary
<20 ppt (VDH Advisory)
1 0
MILES
LAYERS PFOA
BOUNDARIES
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

12
Case Study – POET Vermont
• Initially sampled once per month for 3 months
• Influent, midpoint and effluent
• Influent PFOA Concentration >1,000 ppt: sample every 3 months
• Influent PFOA Concentration >200 ppt to <1,000 ppt sample every 6 months
• Influent PFOA Concentration <200 ppt every 12 months
Sorption
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

13
Case Study POET Vermont – Results
• Influent concentrations vary from <20 ppt
to 4,600 ppt
• Volume treated per unit from 50 gal over
one month to 37,000 gal over 3 months
• Pre and post filter replaced every 4 months
• UV lap replaced every 12 months
• GAC replacement assumed every 2 years
• Swap lead and lag tank then ship GAC
media to vendor
Reference: Lessons Learned on Vermont POET Installations and
Operations at Residences Impacted by PFASs. Richard Spiese.
Sorption
TOC Concentration (ppm, mg/L)
PFC Concentration (ppt, ng/L)
Simulated Days of Operation
Simulated Days of Operation vs. PFCs and TOC
PFC/TOC Customer ACT Data
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0
50
100
150
200
250
300
350
400
0.0 100.0 200.0 300.0 400.0 500.0 600.0
PFHpA PFHxA PFOA Feed PFHpA Feed PFHxA Feed PFOA TOC Removal Feed TOC
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

14
Case Study – NAS Brunswick, ME GWETS
• Former Naval Air Station in Brunswick, ME, BRAC 2011
• Treating CVOCs at GWETS using air stripping and GAC (vapor and liquid phase)
• Recovered over 500 kg VOCs since 1995; removal now limited by back diffusion rate,
asymptotic range
• 1,4-Dioxane addressed by addition of HiPOx
®
unit
• PFAS removed via liquid-phase GAC
–PFOA breakthrough determines changeout
–Shorter-chain PFAS, carboxylates, break through
earlier
Sorption
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

15
Case Study – NAS Brunswick, ME GWETS – Results
Sorption
1.00
PFOS Concentration (µg/L)
0.90
0.80
0.70
0.60
0.50
0.40
0.30
0.20
0.10
0.00
PFOS – Plant Influent
Sample Date
PFOS – Plant Effluent
PFOS – HiPOx
®
Effluent
PFOS – USEPA HA
PFOS – GAC Mid-point
Carbon change-out
11/2/2015 2/10/2016 5/20/2016 8/28/2016 12/6/2016 3/16/2017 6/24/2017 10/2/2017 1/10/2018
Carbon Change-out 11/10/15
Lead Vessel: Coconut Carbon
Lag Vessel: F600
Carbon Change-out 10/12/16
Lead Vessel: F600
Figure 1: PFOS Concentrations
GWETS Carbon Change Out PFC Monitoring
Former Naval Air Station Brunswick, Brunswick, ME
PFOA – Plant Influent
11/2/2015
3.5
3
2.5
2
1.5
1
0.5
0
PFOA Concentration (µg/L)
2/10/2016 5/20/2016 8/28/2016 12/6/2016 3/16/2017 6/24/2017 10/2/2017 1/10/2018
Sample Date
PFOA – Plant Effluent
PFOA – HiPOx
®
Effluent
PFOA – USEPA HA
PFOA – GAC Mid-point
Carbon change-out
Carbon Change-out 11/10/15
Lead Vessel: Coconut Carbon
Lag Vessel: F600
Carbon Change-out 10/12/16
Lead Vessel: F600
Figure 2a: PFOA Concentrations
GWETS Carbon Change Out PFC Monitoring
Former Naval Air Station Brunswick, Brunswick, ME
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

16
Case Study – NAS Brunswick, ME GWETS – Results (cont.)
Sorption
11/2/2015
0.160
0.140
0.120
0.100
0.080
0.060
0.040
0.020
2/10/2016 5/20/2016 8/28/2016 12/6/2016 3/16/2017 6/24/2017 10/2/2017 1/10/2018
Sample Date
PFOA–Plant Effluent
PFOA–USEPA HA
PFOA–GAC Mid-point
Carbon change-out
Carbon Change-out 11/10/15
Lead Vessel: Coconut Carbon
Lag Vessel: F600
Carbon Change-out 10/12/16
Lead Vessel: F600
Figure 2b: PFOA Concentrations (Carbon Vessels Only)
GWETS Carbon Change Out PFC Monitoring
Former Naval Air Station Brunswick, Brunswick, ME
0.000
PFOA Concentration (µg/L)
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

17
Case Study – NAS Brunswick, ME GWETS – Results (cont.)
Sorption
11/2/2015
0.160
0.140
0.120
0.100
0.080
0.060
0.040
0.020
2/10/2016 5/20/2016 8/28/2016 12/6/2016 3/16/2017 6/24/2017 10/2/2017 1/10/2018
Sample Date
PFBA – Plant Influent
PFBA – HiPOx
®
Effluent
PFBA – GAC Mid-point
Carbon change-out
Carbon Change-out 11/10/15
Lead Vessel: Coconut Carbon
Lag Vessel: F600
Carbon Change-out 10/12/16
Lead Vessel: F600
Figure 3: PFBA Concentrations
GWETS Carbon Change Out PFC Monitoring
Former Naval Air Station Brunswick, Brunswick, ME
0.000
PFOA Concentration (µg/L)
Sample Date
PFBA – Plant Effluent
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies
18
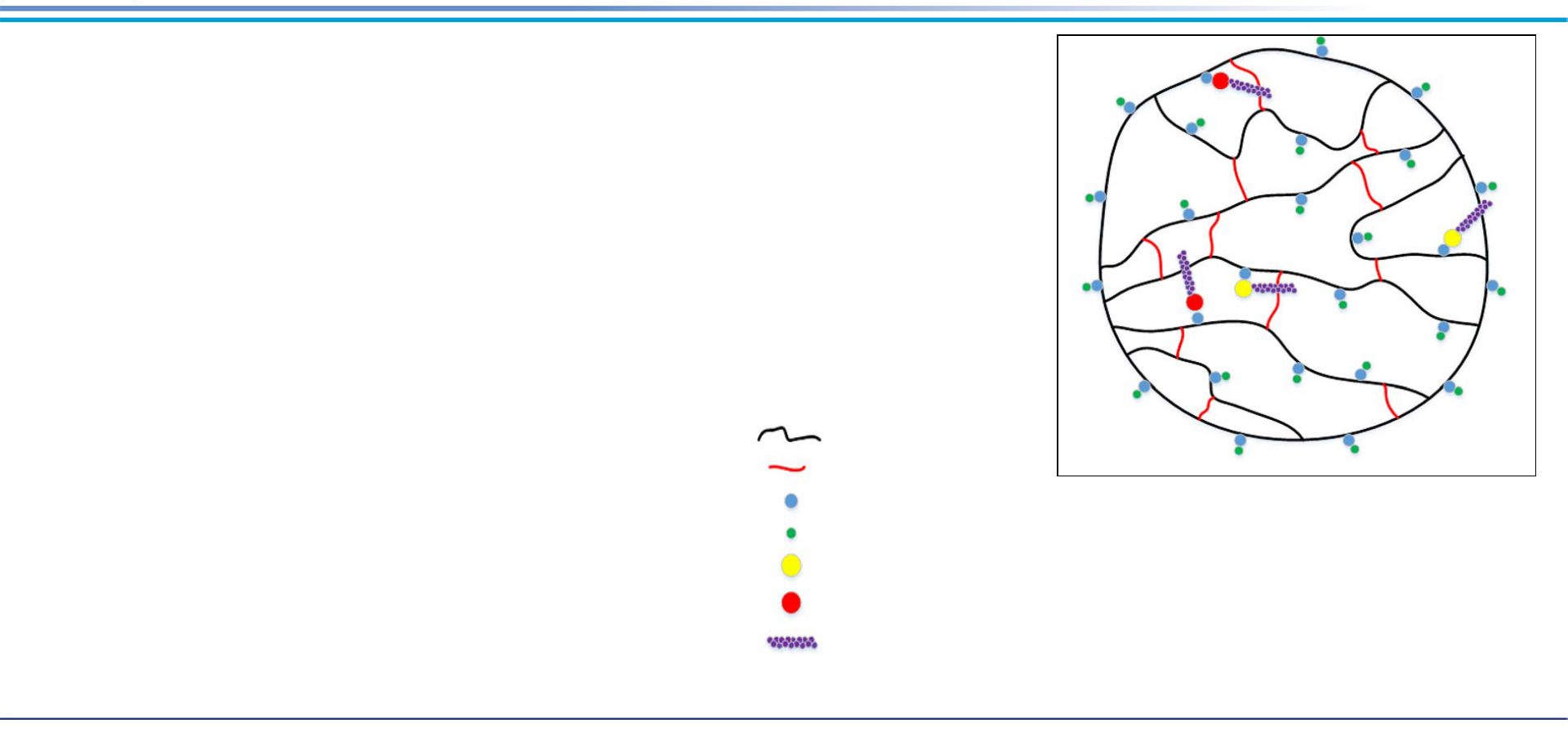
Ion Exchange
Material
• Synthetic neutral co-polymeric media (plastics)
with positively-charged exchange sites
• Can be regenerated (produces waste stream)
or single use (must be disposed of properly)
Application
• Removes anionic PFAS binding to negatively-
charged functional group
• Lead-lag including combination of single use
and regenerated
Reference: Steve Woodward John Berry Brandon Newman. 2017. Ion Exchange Resin for PFAS
Removal and Pilot Test Comparison to GAC. Remediation Journal Volume 27, Issue 3 Pages 19–27
Sorption
Polystyrene polymer chain
Fixed ion exchange group, e.g., quaternary ammonium, —
≡N
+
, for anion IEX
Divinylbenzene crosslink
Exchangeable counter ion, e.g., chloride ion, Cl-, for anion IEX
Sulfonate group, —SO
3
-
, of PFAS (e.g., PFOS), replacing exchangeable counter ion
Carboxylate group, —CO
2
-
, of PFAS (e.g., PFOA), replacing exchangeable counter ion
PFAS carbon-fluorine tail adsorbing to polystyrene polymer chain or divinylbenzene
crosslink via Van der Waals forces
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

19
Ion Exchange (cont.)
Mechanism
• Acts as ion exchange resin and adsorbent resin
• Positively charged anion exchange media
• Removes negatively-charged PFAS from water
Effectiveness
• Reaction kinetics faster than GAC
• Operating capacity higher than GAC
• Breakthrough varies for different PFAS
• Less frequent media change-outs
Sorption
% Removal from GAC (5.6 min EBCT) vs.
Ion Exchange (1.4 min EBCT)
Ion Exchange
Bituminous GAC
% Removal After Treating 146 days
PFOS PFHxS PFOA PFBS PFHpA PFHxA
Anion-Exchange Resin
hemi-micelle
micelle
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

20
Considerations When Using Ion Exchange
• Type and concentration of inorganic ions in groundwater affect PFAS capacity of resin
• Bench-scale tests recommended to determine most effective resin
• More cost-effective at higher concentrations
• Organic matter may foul resin
• Co-contaminants compete for resin site
• Site-specific testing should be performed
Sorption
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

21
Regeneration of Ion Exchange Resins
• Brine solution can desorb anionic head of PFAS from resin
• Organic solvent-like methanol or ethanol can desorb C-F tail
• Surfactants with both nonionic and anionic properties can be used as regenerants
• Most successful has been organic solvents and sodium chloride
• The solution used to regenerate may then need to be concentrated to minimize the
volume of waste
Shipped back to vendor for regeneration
Key
Point
Sorption
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

22
Case Study – Comparison of GAC with Ion Exchange at Pease AFB
• Historic use of AFFF for firefighting training
• Ion Exchange – ECT Sorbix A3F
• Note 6:2 FS 2
nd
highest concentration PFAS
• GAC – Calgon Filtrasorb
®
400 (F400)
Reference: Steve Woodard John Berry Brandon Newman. 2017 Ion Exchange Resin for PFAS Removal and Pilot Test Comparison to GAC. Remediation Journal Volume 27, Issue 3 Pages 19–27
Sorption
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

23
Case Study – Comparison of GAC with Ion Exchange at Pease AFB (cont.)
GAC
• 4 vessels in series
• Each containing 9 gal F400
• Each vessel 5 min EBCT, overall 20 min EBCT
• Samples collected at influent and after each
vessel weekly for 8 weeks
• At 1.8 gpm treated 100,486 gal water
(11,165 bed volumes)
Ion Exchange
• 3 vessels in series
• Each containing 9 gal resin
• Each vessel 2.5 min EBCT, overall 7.5 min EBCT
• At 3.6 gpm treated 422,645 gal water (46,961 BVs)
• Samples collected routinely at influent and effluent
Sorption
GROUNDWATER
IN
CARTRIDGE
FILTERS
CARTRIDGE
FILTERS
GROUNDWATER
OUT
SOLVENT
RECOVERY
REGENERANT
SUPPLY
GAC1 GAC2 GAC3 GAC4
IX1 IX2 IX3
TRANSFER
PUMP
TRANSFER
PUMP #1
TRANSFER
PUMP #2
SP
SP
SP
SP
SP SP SP SP
SP SP SP
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

24
Case Study – Comparison of GAC with Ion Exchange at Pease AFB (cont.)
GAC
Ion Exchange
Entire Pilot-Scale Setup
Sorption
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

25
Case Study – Comparison of GAC with Ion Exchange at Pease AFB (cont.)
PFOS PFOA
Sorption
Influent
Lead Resin (2.5-min EBCT)
Lag Resin (5-min EBCT)
Lead GAC (5-min EBCT)
Lag GAC (10-min EBCT)
USEPA HA: PFOA+PFOS
Bed Volumes
10,000 20,000 30,000 40,000 50,000 0
0.001
0.01
0.1
1
10
100
Concentration (µg/L)
Influent
Lead Resin (2.5-min EBCT)
Lag Resin (5-min EBCT)
Lead GAC (5-min EBCT)
Lag GAC (10-min EBCT)
USEPA HA: PFOA+PFOS
Bed Volumes
0.001
0.01
0.1
1
10
100
10,000 20,000 30,000 40,000 50,000 0
Concentration (µg/L)
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

26
Case Study – Comparison of GAC with Ion Exchange at Pease AFB (cont.)
PFBA PFBS
Sorption
Avg. Influent
Bed Volumes Treated
5,000 10,000 15,000 20,000 25,000 0
0
0.5
1
1.5
2
2.5
0.2
0.4
0.6
0.8
1
1.2
Concentration (µg/L)
Concentration (µg/L)
Resin GAC Avg. Influent Resin GAC
5,000 10,000 15,000 20,000 25,000 0
Bed Volumes Treated
0
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

27
Case Study – Comparison of GAC with Ion Exchange at Pease AFB (cont.)
• Three regeneration trials using proprietary blend of organic solvent and brine
Step 1
Purge lead vessel with
1 BV 10% brine to
prime resin for
regeneration
Step 2
Pump 10 BV
regenerant through
resin counter flow
Step 3
Pump 10 BV potable
water to rinse resin
counter flow
Step 4
Return resin vessel to
full service
TOTAL PFAS
Regenerant Solution Recovery
• Distill off solvent fraction into regenerant tank for reuse,
left with concentrated brine PFAS fraction
• OR conduct superloading – process concentrated brine
PFAS solution through adsorption media then recycle
brine solution
Sorption
1.0
2.0
3.0
4.0
5.0
6.0
Concentration (µg/L)
Lead Resin – Virgin
2,000 4,000 6,000 8,000 0
Bed Volumes Treated
0
Lead Resin – Regenerated
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

28
Case Study – Comparison of GAC with Ion Exchange at Pease AFB (cont.)
• Both GAC and Ion Exchange Resin can remove PFOS and PFOA from groundwater to
below EPA LHA
At 5 min. contact time
• Resin treated 8X more BV than GAC before breakthrough of PFOS observed
• Resin treated 6X more BV than GAC before breakthrough of PFOA observed
• Resin removed 1.66 mg PFAS per gram of resin whereas GAC removed 0.40 mg
PFAS per gram GAC
• Resin could be regenerated in the field
Sorption
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

29
In Situ Stabilization (ISS)
• Use of amendments for adsorbing and stabilizing PFAS in soil and groundwater
• GAC, stabilizers, and modified minerals (organoclays)
• Commercially available
• Additional amendments being developed
• Critical to monitor soil leachate to determine treatment effectiveness
• Limited full-scale application in U.S. (more overseas)
In Situ Technologies
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

30
Activated Carbon for In Situ Water Treatment – PlumeStop
®
Material
• Colloidal activated carbon
• 1-2 µm sized particles of carbon suspended in water
by organic polymer dispersion chemistry
Application
• In situ sorbent technology sorbs PFOS and PFOA
from aqueous phase
• Treats dissolved-phase contaminants
• Applied by low-pressure injections
In Situ Technologies
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

31
Activated Carbon for In Situ Water Treatment – PlumeStop
®
(cont.)
Mechanism
• Coats surface of soil
• Contaminants in dissolved phase then sorb to carbon
• Does not destroy PFAS, immobilizes PFAS in place
• Occupies just 0.1% soil pore volume
Effectiveness
• Reduces aqueous concentration to below 70 ng/L
• Radius of Influence can be up to 25 ft
• Can be applied as multiple barriers perpendicular to plume
In Situ Technologies
RITS 2018: PFAS Remediation: Technologies, Guidance, and Application
A Scanning Electron Microscope (SEM) Image of
Sand Grains With and Without a Coating of Carbon

32
In Situ Soil Treatment – Aluminum-Based Sorbent – Rembind Plus
®
Material
• Aluminum hydroxide, activated carbon, organic matter, and kaolinite
Application
• Apply to soil in ~2 to 5% by weight
• Adjust to 30% moisture content
• Binding occurs in 24 hours
• Pilot tested for water treatment
In Situ Technologies
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

33
In Situ Soil Treatment – Aluminum-Based Sorbent – Rembind Plus
®
(cont.)
Mechanism
• Aluminum hydroxide binds to functional head of PFAS by electrostatic interactions
• Activated carbon and organic matter binds to tail via by hydrophobic interactions and
Van der Waals forces
In Situ Technologies
Large complex organic humus molecule
consisting of chains and rings of mainly
carbon and hydrogen atoms
Organic Matter
Activated Carbon
Aluminum Hydroxide
(Amorphous)
Electrostatic
Interactions
Physical
Binding
Hydrophobic Interactions
Van der Waals
Point of zero charge > pH 9.1
Carboxyl
group
Phenolic
hydroxyl
group
Alcoholic
hydroxyl
group
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

34
Aluminum-Based Sorbent for GW Case Study – Air Force Site
• Historical use of AFFF at site
• Full-scale GAC system: two 20,000-lb GAC vessels in
operation to remove PFOS/PFOA from groundwater
• Goal of pilot study to evaluate sorption capacity of
RemBind Plus
®
In Situ Technologies
Influent Concentrations
Concentration (ng/L)
6000
5000
4000
3000
2000
1000
0
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

35
Aluminum-Based Sorbent for GW Case Study – Air Force Site (cont.)
• 30-gal batch reactor pilot test set up next to GAC system
• 30 gal of contaminated water mixed 1.135 kg aluminum-
based sorbent for one hour and allowed to settle overnight
• Next day treated GW moved to effluent tank and
contaminated GW added to tank with amendment without
replacing amendment
• Run for 2 weeks treating 280 gal water
• Monitored for 53 PFAS compounds and TOP assay
• TOC also monitored
In Situ Technologies
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

36
Aluminum-Based Sorbent for GW Case Study – Air Force Site – Results
• 18 PFASs detected frequently
• Removal ranged from 80 to 100% after 155 gal
• Slight decrease in removal beyond 155 gal
In Situ Technologies
Top Assay Influent
PFAS Concentration
(nmole/L)
Total PFOA PFOS PFSAs Precursors
35
30
25
20
15
10
5
0
PFAS precursors unable
to be directly analyzed
Inf-Pre TOPA
Inf-Post TOPA
PFOA Sorbed
PFOS Sorbed
Pre-Treatment PFOS
% PFOS Removal
Pre-Treatment Concentration (µg/L)
5.0
4.5
4.0
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0.0
3000
2500
2000
1500
1000
500
0
Sorbed Mass (µg/kg)
0 50 100 150 200 250 300
0 50 100 150 200 250 300
Groundwater Volume (gal)
Groundwater Volume (gal)
PFOS Removal (%)
100
95
90
85
80
75
70
65
60
55
50
% PFOS Removal
Cumulative Removed PFOA and PFOS
(µg/kg-RemBind Plus
®
) from Groundwater
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

37
Types of IDW
Liquid Waste
• Purge water from groundwater sampling
• Concentrated AFFF
Solid Waste
• Well installation waste (soil cuttings)
• Soil cuttings from core sampling
• Spent GAC
• Spent ion exchange resin
• Soil from excavations
Dealing with Investigation-Derived Waste (IDW)
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

38
Challenges with Handling IDW
• PFAS are considered non-hazardous (can be disposed of in any landfill)
• Landfill refusal to accept PFAS waste
• Potential for future liability
• Risk of landfill leachate
Dealing with Investigation-Derived Waste (IDW)
Consideration should be given to taking liquid waste to existing onsite
GWETS if available
Key
Point
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

39
Considerations for Liquid IDW
• If PFAS concentrations are below regulatory levels, water may be considered to be
disposed to sanitary sewer/POTW
• At sites where there is a PFAS GWETS, purge water should be considered to be
treated in that system with operator approval
• Purge water may be considered to be passed through a drum of GAC, held in a
receiving tank pending analysis
• If below regulatory values, GW may be able to be discharged to the sanitary
sewer/POTW
• Purge water may be able to be sent to an off-site treatment facility willing to accept it
Dealing with Investigation-Derived Waste (IDW)
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

40
Considerations for Liquid IDW
• Currently sending to a landfill or a treatment facility may be the only choice
• As treatment becomes more common, the soil cuttings may be treatable on-site
(e.g., thermal)
• PFAS waste is non hazardous*, so 90 day rule does not apply
• Option – retain material on site as treatment approaches and policies are developed
• EXWC conducting research on treatment for IDW and source zone soils
Dealing with Investigation-Derived Waste (IDW)
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

41
Key Points
• GAC may be the only practical treatment for groundwater to date
• PFAS <5 carbons much shorter breakthrough times
• Bituminous carbon appears to perform better than coconut carbon
• Ion exchange resin may be better at removing PFAS and can be regenerated but may be more
expensive
• In situ treatment technologies PlumeStop
®
, RemBind Plus
®
and MatCARE™
limited field
demonstrations in U.S.
Wrap-Up
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

42
Select References
• ITRC PFAS Remediation Factsheet
• PFAS Remediation Whitepaper (Internal Navy Document)
• Andres Arias Espana, Victor, Megharaj Mallavarapu, and Ravi Naidu. 2015. “Treatment technologies
for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA): A critical review with
an emphasis on field testing,” Environmental Technology and Innovation, 4, 168-181.
• Du, Ziwen, Shubo Deng, Yue Bein, Qian Huang, Bin Wang, Jun Huang, and Gang Yu. 2014.
“Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents – A
review,” Journal of Hazardous Materials, 274, 443-454.
• Zhu, Runliang, Qingze Chen, Qing Zhou, Yunfei Xi, Jianxi Zhu, and Hongping He. 2016. “Adsorbents
based on montmorillonite for contaminant removal from water: A review,” Applied Clay Science, 123,
239-258.
• Merino, Nancy, Yan Qu, Rula Deeb, Elisabeth L. Hawley, Michael R. Hoffmann, and Shaily
Mahendra. 2016. “Degradation and Removal Methods for Perfluoroalkyl and Polyfluoroalkyl
Substances in Water,” Environmental Engineering Science, 33, 615-649.
Wrap-Up
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

44
Questions and Answers
Wrap-Up
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

45
Backup Slides
Wrap-Up
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

46
Mechanism of Sorption – Electrostatic Interaction
• Interaction between negative and positive charges
• Strong negative charged shell around CF chain due to fluorine atoms and functional
group
• Electrostatic bond mainly at functional group sue to stronger negative charge
• To promote electrostatic bond increase ionic strength, ensure pH is not too alkaline
• Example seen in organoclays
Reference Du, Ziwen, Shubo Deng, Yue Bein, Qian Huang, Bin Wang,
Jun Huang, and Gang Yu. 2014. “Adsorption behavior and mechanism of
perfluorinated compounds on various adsorbents – A review,”
Journal of Hazardous Materials, 274, 443-454.
Sorption
Electrostatic Attraction
PFC molecule
Divalent cation
Positively charged site
Negatively charged site
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

47
Mechanism of Sorption – Hydrophobic Interactions
• Occurs at the electronegative CF chain
• Longer chain more hydrophobic
• Leads to formation of micelles
• Is often stronger than electrostatic repulsion (between negatively-charged tail and
negatively-charged sorbent)
Sorption
Hydrophobic Interaction
PFC molecule
Positively
charged site
Electrostatic Repulsion
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

48
Property MatCARE™
Bulk Density (kg m
-3
) 608
Particle Density (kg m
-3
) 1,677
Porosity (%) 40
Pore Volume (kg m
-3
) —
Particle Size 77.4% between 2,000 and 1,180 µm
Surface Area (m
2
g
-1
) 31.91
Reversible Swelling (%) 2.5
Moisture Holding Capacity (%) 50.28
In Situ Soil Treatment Modified Organoclay Sorbent – MatCARE™
Material
• Palygorskite-based material modified with
oleylamine, i.e., amine modified clay sorbent
Application
• Applied to soil at 10% w/w
• Water content of soil 60%
In Situ Technologies
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

49
In Situ Soil Treatment Modified Organoclay Sorbent – Soil Treatability Studies
• Four soils from fire training areas at overseas Air Force Bases
• Air-dried, homogenized, and passed through 2-mm sieve
• pH, organic carbon content, and PFOS concentration
• 1 kg of each soil adjusted to 60% moisture, amendment added at 10 g per 100 g soil
• PFOS-spiked treatment also included (10 ml of PFOS stock solution) then mixed
• 10 g sample, 3x/yr
• Water extraction
In Situ Technologies
Physico-Chemical Properties of the Soil
Soils pH TOC (%) PFOS (nmol g
-1
) Texture
Solvent Extracted Water Extracted Sand (%) Silt (%) Clay (%) Textural Class
A 4.8 0.96 3.66 0.52 52.63 25.62 21.74 Sandy clay loam
B 4.9 1.97 148.72 21.13 43.21 21.42 35.37 Clay loam
C 8.1 0.29 32.33 4.72 75.15 9.11 15.74 Sandy loam
D 6.5 2.03 18.52 1.86 57.04 10.93 32.03 Sandy clay loam
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies
50
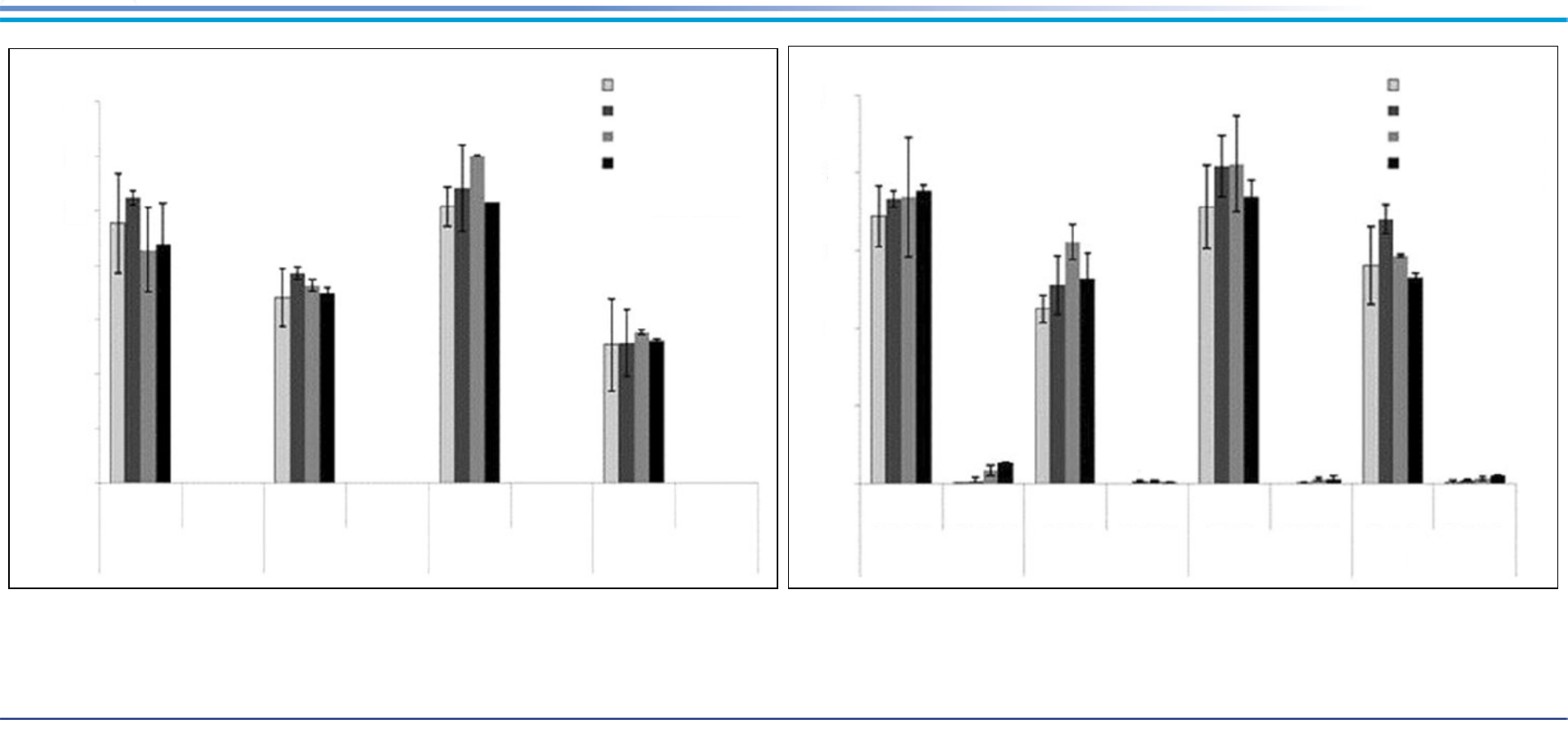
In Situ Soil Treatment Modified Organoclay Sorbent – Results
In Situ Technologies
25
°
C
no spike
25
°
C
spike with 0.2 mmol/kg PFOS
Control
Sorbent
Control
Sorbent
Control
Sorbent
Control
Sorbent
Soil A
Soil B
Soil C
Soil D
Control
Sorbent
Control
Sorbent
Control
Sorbent
Control
Sorbent
Soil A
Soil B
Soil C
Soil D
% Release
% Release
14
12
10
8
6
4
2
0
25
20
15
10
5
0
a
a
1st quarter
2nd quarter
3rd quarter
4th quarter
1st quarter
2nd quarter
3rd quarter
4th quarter
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies


52
Aluminum-Based Sorbent for GW Case Study – AF Site – Future Work
Verify amendment sorption capacity
Optimize dosage to meet EPA Health Advisory
Monitor effectiveness on short-chain PFAS and PFAA precursors
Conduct regeneration trials using proprietary wash solutions
In Situ Technologies
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

53
Aluminum-Based Sorbent for Full-Scale Soil Treatment Case Study
• Airport contaminated with PFAS
• Replacing asphalt – excavated 900 tons of PFAS-contaminated soil
In Situ Technologies
Aviation Rescue and Fire Fighting Services
Damaged Asphalt
Aircraft Taxiway
Damaged
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

54
Aluminum-Based Sorbent for Full-Scale Soil Treatment Case Study (cont.)
• 900 tons of contaminated soil
• PFOS total concentration <5.7 mg/kg
• PFOS leachable concentration <180 µg/L (by USEPA Method 1311)
In Situ Technologies
Aircraft Taxiway
Construction of New Apron PFAS-Contaminated Soil
~900 tonnes
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

55
Aluminum-Based Sorbent for Full-Scale Soil Treatment Case Study (cont.)
• Transported 900 tonnes of soil to municipal waste landfill site
• Treated hotspots with 10% RemBind
®
• Validated samples at accredited lab
• Obtained EPA approval for disposal in a purpose-built burial cell
In Situ Technologies
RemBind
®
Capping
RemBind
®
Layer
Waste
2500
10500
2000
Soil Disposal Area
1
2
1
2
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

56
Aluminum-Based Sorbent for Full-Scale Soil Treatment Case Study (cont.)
Laying the Amendment Capping Layer Finished Lined Burial Cell
In Situ Technologies
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies
57
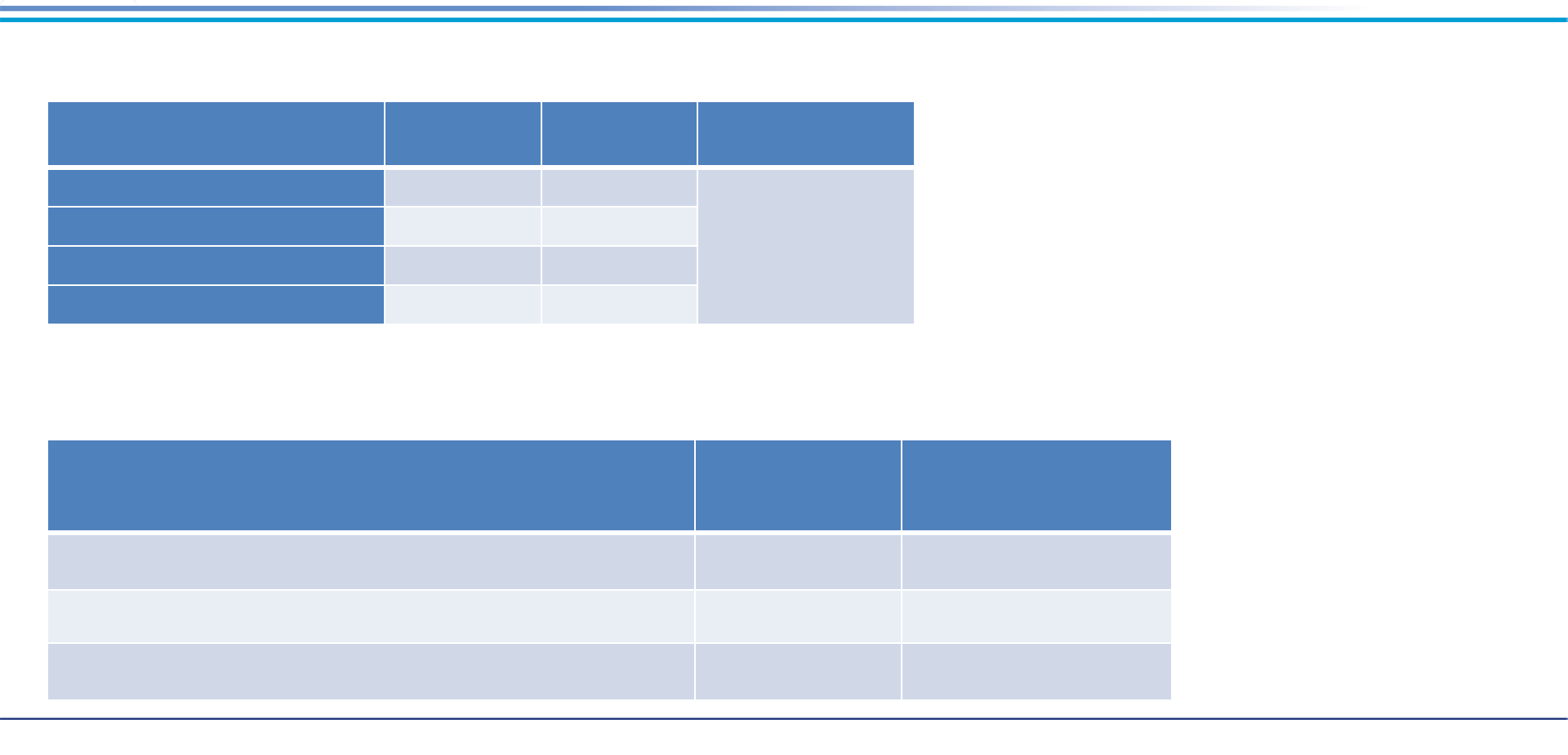
Aluminum-Based Sorbent for Full-Scale Soil Treatment Case Study (cont.)
• Soil Leachate after Treatment
Activity
Approximate
Cost (US)
Cost per Ton
(900 Tons)
Landfill disposal fees $63,500 $67
Investigation, bench trials, mixing, and reagent supply
$47,500 $50
Total $111,000 $117
In Situ Technologies
Hotspot 1
(µg/L)*
Hotspot 2
(µg/L)*
Compliance Limit
(µg/L)*
PFOS <0.01 <0.01
0.2
PFOA <0.01 <0.01
6:2 Fluorotelomer sulfonate <0.1 <0.1
8:2 Fluorotelomer sulfonate <0.2 <0.2
*Soil leachate concentrations as measured by TCLP at pH 5
• Project Costs
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

58
Aluminum-Based Sorbent for Full-Scale Soil Treatment Case Study (cont.)
• A water authority in Cape
Cod, MA treated soil with
amendment in the bottom
of an excavation before
backfilling to mitigate the
risk of PFAS leaching in
a drinking water source
In Situ Technologies
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies

59
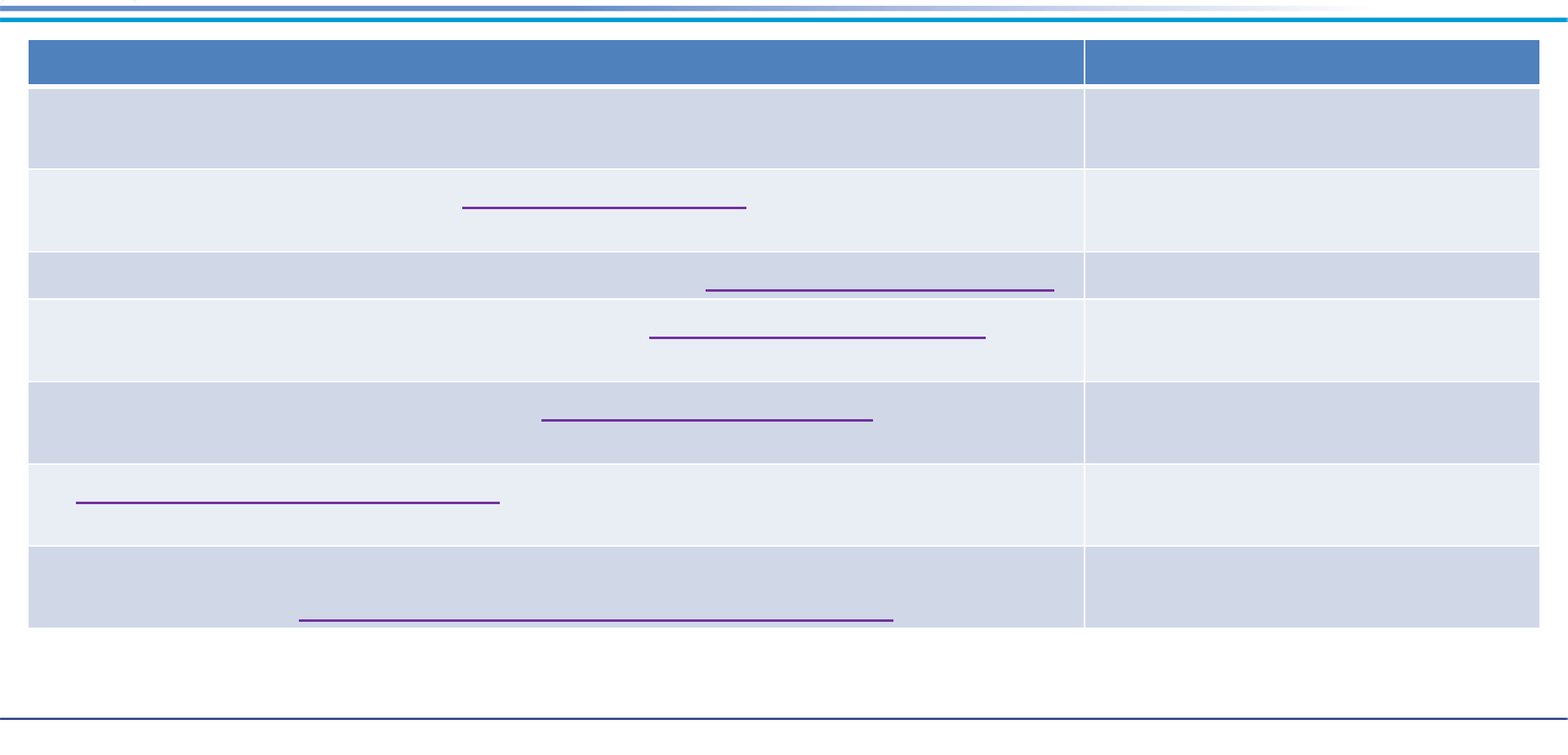
96% reduction
PFOS
90% reduction
PFOS
99% reduction
PFOS
98% reduction
PFOS
In Situ Technologies
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies
60
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies
SERDP PFAS Projects*
Project PI
Field Demonstration and Life Cycle Comparison of Ex-Situ Treatment Technologies for Poly- and
Perfluoroalkyl Substances (PFASs) in Groundwater
Alice Fulmer, Water Research
Foundation
Rational Design and Implementation of Novel Polymer Adsorbents for Selective Uptake of
PFASs from Groundwater
Dr. Damian Helbling, Cornell University
Ex Situ Treatment of PFAS Contaminated Groundwater Using Ion Exchange with Regeneration
Dr. Mark Fuller, CB&I Federal Services
Remediation of PFAS Contaminated Groundwater Using Cationic Hydrophobic Polymers as
Ultra-High Affinity Sorbents
Dr. Reyes Sierra-Alvarez, University of
Arizona
Regenerable Resin Sorbent Technologies with Regenerant Solution Recycling for Sustainable
Treatment of PFASs
Dr. Timothy Strathmann, Colorado
School of Mines
An Electrocoagulation and Electrooxidation Treatment Train to Degrade Perfluoroalkyl
Substances and Other Persistent Organic Contaminants in Groundwater
Dr. Dora Chiang, AECOM
Treatment of Legacy and Emerging Fluoroalkyl Contaminants in Groundwater with Integrated
Approaches: Rapid and Regenerable Adsorption and UV-Induced Defluorination
Dr. Jinyong Liu, University of California,
Riverside
*Not a complete list

61
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies
SERDP PFAS Projects*
Project PI
Removal of Complex Mixtures of Perfluoroalkyl Acids from Water Using Molecularly Engineered
Coatings on Sand and Silica
Dr. Paul Edmiston, The College of
Wooster
Combined In Situ/Ex Situ Treatment Train for Remediation of PFAS Contaminated Groundwater
Dr. Michelle Crimi, Clarkson University
Electrochemical Oxidation of Perfluoroalkyl Acids in Still Bottoms from Regeneration of Ion
Exchange Resins
Dr. Qingguo Huang, University of
Georgia
Electrically Assisted Sorption and Desorption of PFASs
Dr. Douglas Call, North Carolina State
University
Development of Coupled Physicochemical and Biological Systems for In Situ Remediation of
Perfluorinated Chemical and Chlorinated Solvent Groundwater Plumes
Dr. Kurt Pennell Brown University
Molecular Design of Effective and Versatile Adsorbents for Ex Situ Treatment of AFFF-Impacted
Groundwater
Dr. Mandy Michalsen, U.S. Army Corps
of Engineers
In situ Remediation of Aqueous Film Forming Foams and Common Co-Contaminants with the
Dual Approach of Chemical Oxidation and Bioremediation
Dr. Lisa Alvarez-Cohen
University of California at Berkeley
*Not a complete list

62
FRTR 2018: PFAS Emerging Contaminants and Remediation Technologies
ESTCP PFAS Projects*
Project PI
Field Demonstration to Enhance PFAS Degradation and Mass Removal Using Thermally-Enhanced
Persulfate Oxidation Followed by Pump-and-Treat
Dr. John Kornuc
NAVFAC EXWC
Characterization of the Nature and Extent of Per- and Polyfluoroalkyl Substance (PFASs) in
Environmental Media at DoD Sites for Informed Decision-Making
Dr. John Kornuc
NAVFAC EXWC
*Not a complete list

