
INNOVATION
OUTLOOK
RENEWABLE
METHANOL
in partnership with

© IRENA 2021
Unless otherwise stated, material in this publication may be freely used, shared, copied, reproduced, printed and/or stored, provided that appropriate
acknowledgement is given of IRENA as the source and copyright holder. Material in this publication that is attributed to third parties may be subject to
separate terms of use and restrictions, and appropriate permissions from these third parties may need to be secured before any use of such material.
ISBN 978-92-9260-320-5
CITATION IRENA AND METHANOL INSTITUTE (2021),Innovation Outlook : Renewable Methanol,
International Renewable Energy Agency, Abu Dhabi.
About IRENA
The International Renewable Energy Agency (IRENA) is an intergovernmental organisation that supports countries in
their transition to a sustainable energy future and serves as the principal platform for international co-operation, a centre
of excellence and a repository of policy, technology, resource and financial knowledge on renewable energy. IRENA
promotes the widespread adoption and sustainable use of all forms of renewable energy, including bioenergy, geothermal,
hydropower, ocean, solar and wind energy, in the pursuit of sustainable development, energy access, energy security and
low-carbon economic growth and prosperity. www.irena.org
About METHANOL INSTITUTE
The Methanol Institute (MI) is the global trade association for the methanol industry, representing the world’s leading
producers, distributors, and technology companies. Founded in 1989 in Washington DC, MI now represents its members
from five oces around world in Washington DC, Beijing, Brussels, Delhi, and Singapore. MI serves its members as the
voice of the methanol industry, representing companies within the membership to governments and businesses around
the world to promote the sustainable growth of the industry. MI focuses on advancing the utilisation of methanol as a
clean fuel in energy-related applications such as land & marine transport, power generation, fuel cells, industrial boilers,
and cook stoves. MI also supports sustainable and renewable process to produce methanol as a carbon-neutral chemical
and fuel. www.methanol.org
Acknowledgements
This report was jointly prepared by the International Renewable Energy Agency (IRENA) and the Methanol Institute (MI).
It was developed under the guidance of Dolf Gielen (IRENA) and Greg Dolan (MI). The contributing authors are Seungwoo
Kang and Francisco Boshell (IRENA), Alain Goeppert and Surya G. Prakash (University of Southern California), and Ingvar
Landälv (Fuels & Energy Consulting) with valuable additional contributions from Paul Durrant (IRENA).
The authors appreciate thetechnicalreviewprovided byDeger Saygin (ShuraEnergy TransitionCenter), Tue Johansson
(A.P. Moller - Maersk), Florian Ausfelder (Dechema), Alexandra Ebbinghaus (Shell), Christopher Kidder (International DME
Association), Choon Fong Shih (University of Chinese Academy of Sciences), Mark Berggren (MMSA), Andrew Fenwick
(Johnson Matthey), Tore Sylvester Jeppersen (Haldor Topsoe), Peter J. Nieuwenhuizen (Enerkem), Acya Yalcin and Jason
Chesko (Methanex).
Valuable review and feedback were also provided by IRENA and MI colleagues, including Herib Bianco, Ricardo Gorini,
Paul Komor, Toshimasa Masuyama, Emanuele Taibi (IRENA), and Tim Chan (Methanol Institute).
The chapters in this outlook were edited by Justin French-Brooks.
Available for download: www.irena.org/publications
For further information or to provide feedback, please contact IRENA atinf[email protected]g
This publication and the material herein are provided “as is”. All reasonable precautions have been taken by IRENA to verify the reliability of the material
in this publication. However,neither IRENA nor any of itsocials, agents, data or other third-party content providers provides a warranty of any kind,
either expressed or implied, and they accept no responsibility or liability for any consequence of use of the publication or material herein.
The information contained herein does not necessarily represent the views of all Members of IRENA. The mention of specific companies or certain
projects or products does not imply that they are endorsed or recommended by IRENA in preference to others of a similar nature that are not mentioned.
The designations employed and the presentation of material herein do not imply the expression of any opinion on the part of IRENA concerning the legal
status of any region, country, territory, city or area or of its authorities, or concerning the delimitation of frontiers or boundaries.
Disclaimer


INNOVATION OUTLOOK: 4
1. Methanol:
•
Methanol is a key product in the chemical industry.
It is mainly used for producing other chemicals such
as formaldehyde, acetic acid and plastics. Around
98million tonnes (Mt) are produced per annum, nearly
all of which is produced from fossil fuels (either natural
gas or coal).
•
The life-cycle emissions from current methanol
production and use are around 0.3gigatonnes (Gt)
CO
2
per annum (about 10% of total chemical sector
emissions).
•
Methanol production has nearly doubled in the past
decade, with a large share of that growth being in
China. Under current trends, production could rise to
500 Mt per annum by 2050, releasing 1.5 Gt CO
2
per
annum if solely sourced from fossil fuels.
•
The cost of producing fossil fuel-based methanol is in
the range of USD 100-250 per tonne (t).
2. Renewable methanol:
•
Renewable methanol can be produced using renewable
energy and renewable feedstocks via two routes:
•
Bio-methanol is produced from biomass. Key
potential sustainable biomass feedstocks
include: forestry and agricultural waste and
by-products, biogas from landfill, sewage,
municipal solid waste (MSW) and black liquor
from the pulp and paper industry.
•
Green e-methanol is obtained by using CO
2
captured from renewable sources (bioenergy
with carbon capture and storage [BECCS] and
direct air capture [DAC]) and green hydrogen,
i.e. hydrogen produced with renewable
electricity.
•
Less than 0.2 Mt of renewable methanol is produced
annually, mostly as bio-methanol. The methanol
produced by either route is chemically identical to
methanol produced from fossil fuel sources.
•
Interest in renewable methanol is being driven by
the need to mitigate climate change by substantially
reducing or eliminating CO
2
emissions, and in particular
by the growing focus on holding the average global
temperature rise to no more than 1.5°C. This implies
achieving net carbon neutral emissions across all
sectors of the economy by mid-century.
•
Low-emission methanol could play a larger role in
decarbonising certain sectors where options are
currently limited – particularly as a feedstock in
the chemical industry or as a fuel in road or marine
transport.
3. Production costs of bio-methanol:
•
Since production is currently low, limited data are
available on actual costs, meaning that potential costs
need to be estimated. The bio-methanol production
cost will depend on the bio-feedstock cost, investment
cost and the efficiency of the conversion processes.
KEY FINDINGS
Methanol plays an important role in the chemical industry, and is an emerging energy fuel
currently mostly produced from fossil fuels. A transition to renewable methanol – derived
from biomass or synthesised from green hydrogen and carbon dioxide (CO
2
) – could expand
methanol’s use as a chemical feedstock and fuel while moving industrial and transport sectors
toward net carbon neutral goals. The cost of renewable methanol production is currently high
and production volumes are low. But with the right policies, renewable methanol could be cost-
competitive by 2050 or earlier.

RENEWABLE METHANOL 5
Biomass and MSW feedstock costs vary between
USD0 and USD17 per gigajoule (GJ).
•
With a lower feedstock cost range of up to USD6/GJ,
the cost of bio-methanol is estimated to be in the range
USD 320/t and USD 770/t, with the range influenced
by differences in the specific projects – including
differences in CAPEX, OPEX and conversion efficiency.
•
With process improvements, the cost range could be
reduced to between USD 220/t and USD 560/t for the
lower feedstock price range up to 6 USD/GJ, with a
correspondingly higher range for the higher feedstock
price range.
•
Production of bio-methanol from the waste streams
of other industrial processes (e.g. black liquor from
paper mills and MSW) in particular offer opportunities
to simplify the feedstock logistics and improve overall
plant economics. Co-production of heat, electricity
or other chemicals could also potentially improve the
economics of bio-methanol production.
•
In the short term biomass could be co-fed into a coal-
based gasifier, or biogas fed into a natural gas-based
methanol plant, so allowing for the gradual introduction
of biomass as a feedstock and making methanol
production more sustainable at a potentially lower cost.
4. Production costs of green e-methanol:
•
The cost of e-methanol depends to a large extent
on the cost of hydrogen and CO
2
. The cost of CO
2
depends on the source from which it is captured, e.g.
from biomass, industrial processes or DAC.
•
The current production cost of e-methanol is estimated
to be in the range USD800-1600/t assuming CO
2
is
sourced from BECCS at a cost of USD10-50/t. If CO
2
is
obtained by DAC, where costs are currently USD300-
600/t, then e-methanol production costs would be in
the range USD1200-2400/t.
•
The future cost of green hydrogen production mainly
depends on the combination of further reductions
in the cost of renewable power generation and
electrolysers, and gains in efficiency and durability.
•
With anticipated decreases in renewable power
prices, the cost of e-methanol is expected to decrease
to levels between USD250-630/t by 2050.
•
As in the case of bio-methanol, co-production of
brown/grey (fossil) and green e-methanol could allow
the gradual introduction of green e-methanol at a
reasonable cost.
5. Benefits and challenges for renewable
methanol:
•
Renewable methanol can be produced from a variety
of sustainable feedstocks, such as biomass, waste
or CO
2
and hydrogen. Its use in place of fossil fuels
can reduce greenhouse gas (GHG) emissions and in
some cases can also reduce other harmful emissions
(sulphur oxides [SOx], nitrogen oxides [NOx],
particulate matter [PM] etc.)
•
It is a versatile fuel that can be used in internal combustion
engines, and in hybrid and fuel cell vehicles and vessels.
It is a liquid at ambient temperature and pressures, and
so is straightforward to store, transport and distribute.
It is compatible with existing distribution infrastructure
and can be blended with conventional fuels.
•
Production of methanol from biomass and from CO
2
and H
2
does not involve experimental technologies.
Almost identical proven and fully commercial
technologies are used to make methanol from fossil
fuel-based syngas and can be used for bio- and
e-methanol production.
•
Currently the main barrier to renewable methanol
uptake is its higher cost compared to fossil fuel-based
alternatives, and that cost differential will persist for
some time to come. However, its value is in its emission
reduction potential compared to existing options.
•
Addressing process differences and facilitating the
scale-up of production and use can help reduce costs,
but will require a variety of policy interventions. With
the right support mechanisms, and with the best
production conditions, renewable methanol could
approach the current cost and price of methanol from
fossil fuels.

INNOVATION OUTLOOK: 6
CONTENTS
KEY FINDINGS .................................................................................................................................................................... 4
CONTENTS ...........................................................................................................................................................................6
ABBREVIATIONS .............................................................................................................................................................. 11
SUMMARY FOR POLICY MAKERS ...............................................................................................................................12
1. CURRENT PRODUCTION AND APPLICATIONS OF METHANOL ................................................................22
1.1. Methanol as a raw material ...................................................................................................................................... 22
1.2. Methanol as a fuel ........................................................................................................................................................25
1.3. Storage, transport and distribution of methanol ........................................................................................... 29
2. PRODUCTION PROCESS AND TECHNOLOGY STATUS .................................................................................32
2.1. Low-carbon methanol ................................................................................................................................................ 33
2.2. Renewable methanol ................................................................................................................................................. 34
Bio-methanol from biomass and MSW ................................................................................................................................... 34
Bio-methanol from biogas ........................................................................................................................................................... 40
Bio-methanol from the pulping cycle in pulp mills ............................................................................................................. 41
Methanol from CO
2
(e-methanol) ............................................................................................................................................. 42
Combination of bio- and e-methanol production .............................................................................................................. 50
3. PERFORMANCE AND SUSTAINABILITY ..............................................................................................................53
3.1. Performance and eciency .................................................................................................................................... 53
Bio-methanol ......................................................................................................................................................................................53
E-methanol ......................................................................................................................................................................................... 54
3.2. Renewable methanol vs alternatives.....................................................................................................................57
3.3. Emissions and sustainability ................................................................................................................................... 59
Emissions ............................................................................................................................................................................................ 59
Sustainability and carbon neutrality ........................................................................................................................................ 63

RENEWABLE METHANOL 7
4. CURRENT COSTS AND COST PROJECTIONS ................................................................................................... 65
4.1. Bio-methanol costs ..................................................................................................................................................... 65
Methanol production from biomass and MSW via gasification .................................................................................... 65
Methanol production from biogas .............................................................................................................................................73
Methanol as by-product from wood pulping ........................................................................................................................75
4.2. E-methanol costs ........................................................................................................................................................ 76
E-methanol production costs – A literature review ............................................................................................................76
4.3. Summary of renewable methanol costs today and in the future ............................................................. 84
5. POTENTIAL AND BARRIERS ..................................................................................................................................87
5.1. Demand .............................................................................................................................................................................87
5.2. Sustainable feedstock ................................................................................................................................................90
Biomass ...............................................................................................................................................................................................90
CO
2
and hydrogen ...........................................................................................................................................................................90
5.3. Impact of renewable methanol on the energy sector .................................................................................... 91
5.4. Drivers ................................................................................................................................................................................ 91
5.5. Barriers ............................................................................................................................................................................. 92
Bio-methanol ..................................................................................................................................................................................... 92
E-methanol ......................................................................................................................................................................................... 93
5.6. Policies and recommendations ..............................................................................................................................94
REFERENCES AND FURTHER INFORMATION ..................................................................................................... 99
ANNEXES ..........................................................................................................................................................................110
Annex 1. Some of the pros and cons of methanol and renewable methanol ..............................................110
Annex 2. Overview of major methanol production processes from various carbon sources. .............. 116
Annex 3. Comparison of renewable methanol with other fuels on a price per unit
of energy basis ....................................................................................................................................................117
Annex 4. Overview of existing or planned facilities and technology providers for
e-methanol and bio-methanol production ............................................................................................. 118

INNOVATION OUTLOOK: 8
Figures
Figure 1. Global methanol demand and production capacity (2001-2019) ................................................ 12
Figure 2. Principal methanol production routes ..................................................................................................... 13
Figure 3. Current and future production costs of bio- and e-methanol. .................................................... 15
Figure 4. Comparison of renewable methanol with other fuels on a price
per unit of energy basis ................................................................................................................................ 16
Figure 5. Global methanol demand in 2019 ..............................................................................................................17
Figure 6. The feedstocks and applications of methanol .................................................................................... 23
Figure 7. Global methanol demand and production capacity (2001-2019) ............................................... 24
Figure 8. Historical methanol sale price (1995-2020) ......................................................................................... 24
Figure 9. Fleet of M100 fuelled taxis in Guiyang City, Guizhou province, China ...................................... 26
Figure 10. Geely M100 truck (2019) in China and M100 truck in Israel (2020). .......................................... 26
Figure 11. Gumpert Nathalie, methanol-fuelled hybrid fuel cell supercar .....................................................27
Figure 12. Palcan hybrid methanol reformer/proton-exchange
membrane fuel cell passenger bus in China .........................................................................................27
Figure 13. Methanol-powered Stena Germanica 50000 DWT ferry
operating between Gothenburg and Kiel ............................................................................................. 28
Figure 14. Ocean-going vessel powered by methanol ......................................................................................... 29
Figure 15. Methanol stations in China ..........................................................................................................................30
Figure 16. M15 dispensing pump alongside gasoline and diesel fuel dispensers
at a filling station, and M100 dispensing pump in Israel .................................................................30
Figure 17. DME filling station and pump in Shanghai, China in 2008 .............................................................. 31
Figure 18. Bio-DME filling station in Sweden in 2011 .............................................................................................. 31
Figure 19. Proposed classification of methanol from various feedstocks ................................................... 32
Figure 20. Gasification-based methanol plant – general scheme ................................................................... 35
Figure 21. Enerkem’s MSW to biofuels (methanol and ethanol) plant in Alberta, Canada. ................... 39
Figure 22. Reformer-based methanol plant – general scheme ......................................................................... 41
Figure 23. Types of hydrogen according to production process ...................................................................... 42
Figure 24. Approaches to e-methanol production through electrolysis and
electrochemical processes ......................................................................................................................... 43
Figure 25. CO
2
feedstock for the production of e-methanol .............................................................................. 44
Figure 26. The “George Olah Renewable CO
2
-to-Methanol Plant” of CRI in Iceland ................................46

RENEWABLE METHANOL 9
Figure 27. 1000 t/y e-methanol demonstration plant in Lanzhou, Gansu Province,
Northwestern China ......................................................................................................................................46
Figure 28. Combined bio- and e-methanol scheme with biomass or MSW as feedstock .................... 51
Figure 29. Combined bio- and e-methanol scheme with biogas as feedstock ........................................ 52
Figure 30. Example of estimates for global renewable CO
2
availability from dierent sources
by the middle of the 21st century ..........................................................................................................56
Figure 31. Volumetric energy content of various fuels ......................................................................................... 58
Figure 32. GHG emissions of methanol produced from various feedstocks (from feedstock
extraction to final use, values from Table 11) .......................................................................................63
Figure 33. Anthropogenic carbon cycle for a circular economy ....................................................................... 64
Figure 34. Global supply curve for primary biomass, 2030................................................................................69
Figure 35. Estimated costs of bio-methanol up to 2050 ..................................................................................... 72
Figure 36. Potential production cost reduction for bio-methanol from biomass
within a 15 to 20 year timeframe .............................................................................................................. 73
Figure 37. Potential production cost reduction for bio-methanol from MSW
within a 15 to 20 year timeframe .............................................................................................................. 73
Figure 38. Production cost for biomethane via gasification and via anaerobic digestion ......................74
Figure 39. Cost of methanol as a function of hydrogen and CO
2
cost ............................................................ 81
Figure 40. Estimated costs of renewable e-methanol up to 2050 depending
on the renewable CO
2
.................................................................................................................................. 83
Figure 41. Current and future production costs of bio- and e-methanol ....................................................85
Figure 42. Comparison of renewable methanol with other fuels on a price
per unit of energy basis ...............................................................................................................................86
Figure 43. Fleet of Geely Emgrand 7 cars operating in Iceland and powered by 100%
renewable methanol, in front of the CRI CO
2
-to-methanol production plant ........................88
Figure 44. Swedish car powered by an M56 mix (56% methanol in gasoline) with
bio-methanol from the LTU Green Fuels plant (in the background) ......................................... 88
Figure 45. Chemrec bioDME pilot plant and Volvo DME-fuelled truck ..........................................................88
Figure 46. Passenger ship MS innogy on Lake Baldeney (Germany) powered by a
hybrid fuel cell system fuelled by renewable methanol ..................................................................88
Figure 47. Current and future methanol production by source ........................................................................89
Figure 48. A hypothetical CFD smoothing returns in a volatile market .........................................................96

INNOVATION OUTLOOK: 10
Tables
Table 1. Pros and cons of methanol and renewable methanol ..........................................................................18
Table 2. Examples of syngas conditioning and cleaning processes ............................................................... 36
Table 3. Gasifier design principles ................................................................................................................................ 37
Table 4. Gasification technologies and their application ..................................................................................... 38
Table 5. Methanol plants co-fed with a mix of natural gas and biomethane .............................................40
Table 6. By-product bio-methanol from wood pulping .......................................................................................41
Table 7. Overview of existing or planned facilities and technology providers for
e-methanol production ....................................................................................................................................47
Table 8. Energy conversion eciencies for certain process units ...................................................................53
Table 9. Selection of renewable and non-renewable sources of CO
2
............................................................55
Table 10. Comparison of various fuel properties .....................................................................................................57
Table 11. GHG emissions of methanol from various sources, ordered by feedstock type .......................61
Table 12. Capital cost for bio-methanol plants .........................................................................................................66
Table 13. Capital cost for gasification-based plants for other products .........................................................67
Table 14. Capital cost element in production cost ...................................................................................................68
Table 15. Feedstock cost element in production cost ............................................................................................69
Table 16. OPEX (excluding feedstock) cost element in production cost ........................................................70
Table 17. Total production cost for bio-methanol from biomass and MSW ...................................................71
Table 18. Total production cost for bio-methanol after potential cost reduction ....................................... 72
Table 19: Impact of feedstock price in production of methanol from methane/biomethane ................75
Table 20. Approximate production cost for bio-methanol from wood pulping ...........................................75
Table 21. Production costs and production capacity of e-methanol reported in the literature ............77
Table 22. Cost of green hydrogen today and in the futures .................................................................................79
Table 23. Cost of CO from various sources .............................................................................................................. 80
Table 24. Estimated costs of renewable methanol up to 2050 ..........................................................................82
Table 25. Capital cost for CO-to-methanol plants ................................................................................................. 84

RENEWABLE METHANOL 11
AGR Acid gas removal
ASU Air separation unit
BECCS Bioenergy with carbon capture and
storage
BECCU Bioenergy with carbon capture and use
BEV Battery electric vehicle
BTX Benzene, toluene and xylenes
(aromatics)
CAPEX Capital expenditure
CCS Carbon capture and storage
CCU Carbon capture and use
CFD Contract for dierence
CH
3
OH Methanol
CI Carbon intensity
CNG Compressed natural gas
CO Carbon monoxide
CO
2
Carbon dioxide
CO
2
-eq Carbon dioxide equivalent
COS Carbonyl sulphide
CPP Coal power plant
CRI Carbon Recycling International
DAC Direct air capture
DME Dimethyl ether
DMFC Direct methanol fuel cell
DWT Deadweight tonnage
ECA Emission Control Areas
e-fuel Electrofuel
EU European Union
EV Electric vehicle
FCV Fuel cell vehicle
FEED Front-end engineering design
FFV Flexible fuel vehicle
FT fuels Fischer-Tropsch fuels
GHG Greenhouse gas
H
2
Hydrogen
HCl Hydrogen chloride
HF Hydrogen fluoride
HF Hydrogen fluoride
HHV Higher heating value
ICE Internal combustion engine
IMO International Maritime Organization
IRR Internal rate of return
LCA Life-cycle analysis
LCFS Low Carbon Fuel Standard
LCM Low-carbon methanol
LHV Lower heating value
LNG Liquefied natural gas
LPG Liquefied petroleum gas
MDI Methylenebis (4-phenyl isocyanate)
MMA Methyl methacrylate
MSW Municipal solid waste
MTBE methyl tert-butyl ether
MTG Methanol-to-gasoline
MTO Methanol-to-olefins
NOx Nitrogen oxides
n/k Not known
OMEs Oxymethylene ethers
OPEX Operating expenditure
PEM Polymer electrolyte membrane
PM Particulate matter
PV Photovoltaic
RED Renewable Energy Directive
RES Renewable energy source
SGAB Sub Group on Advanced Biofuels
SNG Synthetic natural gas
SOx Sulphur oxides
TRL Technology readiness level
TTW Tank-to-wheel
US United States
WGS Water gas shift
WTT Wheel-to-tank
WTW Wheel-to-wheel
UNITS OF MEASURE
EJ Exajoule
GJ Gigajoule
Gt Gigatonne
kg Kilogram
km Kilometre
kt/y Thousand tonnes per year
kW Kilowatt
kWh Kilowatt hour
L Litre
L/d Litres per day
MJ Megajoule
Mt Million tonnes
MtCO
2
Million tonnes of carbon dioxide
MW Megawatt
MWh Megawatt hour
MW
t
Megawatt thermal
m
3
Cubic metre
t Tonne
t/d Tonnes per day
t/y Tonnes per year
ABBREVIATIONS

Figure 1. Global methanol demand and production capacity (2001-2019)
Source: Based on data from MMSA (2020).
INNOVATION OUTLOOK: 12
Methanol is one of the four critical basic chemicals –
alongside ethylene, propylene and ammonia – used to
produce all other chemical products. About two-thirds
of methanol is used to produce other chemicals, such
as formaldehyde, acetic acid and plastics. Methanol use
for the production of polyethylene and polypropylene in
particular has grown significantly, going from almost zero
ten years ago to 25Mt in 2019. The remaining methanol is
mainly used as a fuel for vehicles, ships, industrial boilers
and cooking. Methanol’s use as a fuel – either by itself, as
a blend with gasoline, for the production of biodiesel, or in
the form of methyl tert-butyl ether (MTBE) and dimethyl
ether (DME) – has also grown rapidly since the mid-2000s.
Most methanol is currently produced from natural gas or
coal, with estimated annual life-cycle emissions of 0.3 Gt
CO
2
, around 10% of the total chemical and petrochemical
sector’s CO
2
emissions. Addressing emissions from
methanol production is therefore a key component of
the decarbonisation of the chemical sector and could
contribute to the transport sector where the methanol
can be used as a fuel.
Market status and production process
Worldwide annual production of methanol nearly doubled
over the past decade to reach about 98Mt in 2019. A large
part of that growth came from China through methanol
production from coal. Methanol demand is expected to
continue increasing to reach more than 120 Mt by 2025
(MMSA, 2020; Berggren, 2019) and 500 Mt by 2050 in
IRENA’s Transforming Energy Scenario.
SUMMARY FOR POLICY MAKERS

Figure 2. Principal methanol production routes
Renewable CO
2
: from bio-origin and through direct air capture (DAC)
Non-renewable CO
2
: from fossil origin, industry
While there is not a standard colour code for the dierent types of methanol production processes; this illustration of various types of methanol
according to feedstock and energy sources is an initial proposition that is meant to be a basis for further discussion with stakeholders
RENEWABLE METHANOL 13
This is in line with the “well-below 2°C” Paris climate goal
(Saygin and Gielen, forthcoming). Most of the growth
until 2028 is expected to come from the Chinese market,
mainly to be used in the production of olefins, with a
smaller share for gasoline blending, formaldehyde, acetic
acid and MTBE production.
Renewable methanol
Currently, methanol is produced almost exclusively from
fossil fuels. However, methanol can also be made from
other feedstocks that contain carbon, including biomass,
biogas, waste streams and CO
2
(for example captured
from flue gases or through DAC).
Renewable methanol can be produced using renewable
energy and renewable feedstocks via two routes:
•
Bio-methanol is produced from biomass. Key potential
sustainable biomass feedstocks include: forestry and
agricultural waste and by-products, biogas from
landfill, sewage, MSW and black liquor from the pulp
and paper industry.
•
Green e-methanol is obtained from CO
2
captured
from renewable sources (e.g. via BECCS or DAC)
and green hydrogen, i.e. hydrogen produced with
renewable electricity.
To qualify as renewable, all feedstocks and energy
used to produce the methanol need to be of renewable
origin (e.g. biomass, solar, wind, hydro, geothermal).
The methanol produced by either route is chemically
identical to methanol produced from fossil fuel sources.
R
R
e
e
n
n
e
e
w
w
a
a
b
b
l
l
e
e
e
e
l
l
e
e
c
c
t
t
r
r
i
i
c
c
i
i
t
t
y
y
N
N
a
a
t
t
u
u
r
r
a
a
l
l
g
g
a
a
s
s
C
C
o
o
a
a
l
l
Reforming
Gasification
Electrolysis
Carbon capture
and storage (CCS)
CH
3
OH
Blue methanol
CH
3
OH
Grey methanol
CH
3
OH
Brown methanol
Syngas
Syngas
H
2
Blue Hydrogen
CO
2
Non-renewable
B
B
i
i
o
o
m
m
a
a
s
s
s
s
Gasification/
reforming
CH
3
OH
Green methanol
Syngas
CO
2
Renewable
High
carbon
intensity
Low carbon
intensity
Bio-methanol
E-methanol
Bio-
e-methanol
Renewable
CO
2
Non-renewable
Renewable
Non-
renewable
Renewable CO
2
: from bio-origin and through direct air capture (DAC)
Non-renewable CO
2
: from fossil origin, industry
H
2
Green hydrogen

INNOVATION OUTLOOK: 14
Current progress on renewable
methanol production
Less than 0.2 Mt of renewable methanol is produced
annually, from only a handful of plants. Those renewable-
methanol commercial facilities and demonstration
projects focus mainly on using waste and by-product
streams from other industrial processes, which oer the
best economics at present. Suitable feedstocks include:
MSW and low-priced biomass, biogas, waste streams,
and black liquor from the pulp and paper industry.
For example, a commercial-scale plant producing
bio-methanol from bio-methane is in operation in the
Netherlands and a plant producing bio-methanol from
MSW is operating in Canada. In Iceland, e-methanol
is produced by combining renewable hydrogen and
CO
2
from a geothermal power plant. The current
projects benefit from favourable conditions, such as
low feedstock cost (e.g. biogas), strong integration with
conventional industrial processes (e.g. pulp and paper
industry), or very inexpensive renewable electricity
(e.g. geothermal and hydro energy in Iceland).
Depending on appropriate local conditions, there are
other early or niche opportunities for bio-methanol
and e-methanol production (e.g. integrated production
with bio-ethanol from sugarcane, co-feeding biomass
feedstock and fossil fuels, and co-production of heat,
electricity and other chemicals).
The co-feeding of renewable feedstock (e.g. biomass,
CO
2
, green hydrogen, renewable electricity) into natural
gas- or coal-based methanol production facilities
could be a strategy to gradually introduce renewable
methanol production, and reduce the environmental
impact and carbon intensity of conventional methanol
production. The output of these hybrid plants is
sometimes called low-carbon methanol (LCM).
This demand could help with the early scale-up of
electrolysers for hydrogen production, CO
2
capture
processes and other technologies for later large-scale
renewable methanol deployment.
Cost competitiveness of renewable methanol
Renewable methanol production costs are significantly
higher than those of today’s natural gas- and coal-based
methanol production (whose production costs are in
the range of USD 100-250/t). With the lowest-cost
feedstocks and with improvements in production
processes, the cost of producing renewable methanol
from either the gasification of biomass or MSW, or
using CO
2
and renewable hydrogen, could approach the
current cost and price of methanol from fossil fuels, as
illustrated in Figure 3 and Figure 4.
Improving the competitiveness
of bio-methanol
Technology maturity and cost reduction. The gasification
of oil and coal is a well-proven technology with multiple
large units in operation. The application of gasification
technologies to various biomass types and MSW is,
however, in the early commercialisation phase and
requires further development before reaching full
commercial status. In the optimum cases, bio-methanol
is close to competing on cost with fossil fuel-generated
methanol, but it is more expensive, in many cases, by
a factor of up to two. As the cost of the feedstock is
not expected to decrease significantly in the future,
reducing CAPEX will be the largest contributor to
lowering production costs, through economies of
scale and learning curve mechanisms such as process
improvements, improved and more (cost-) eective
plant configurations and plant size.
Sustainable and low-cost biomass feedstocks. The
scale-up of bio-methanol production will depend on the
availability of low-cost biomass feedstock (the share of
feedstock cost in the total production cost can be as high
as 50%). Bio-methanol production requires reliable and
consistent supplies of feedstock. While in some cases
biomass feedstock supplies can be provided locally, many
other projects require more extensive supply chains.
The biomass must be sustainably sourced. Sustainability
assessments and monitoring are needed to consider and
manage the risks of adverse economic, environmental
and social impacts (IRENA, 2020a). The gross maximum
availability of sustainable biomass in the world is
estimated to be 147exajoules in 2030 (IRENA, 2014).
Biomass feedstock costs around the world can vary by up
to 17 USD/GJ depending on the type and the location. The
lowest-cost feedstocks – i.e. below USD6/GJ (EUR20/
megawatt hour) are mainly MSW and residues, and the
availability of these feedstocks is limited. As biomass has
the potential for use in a wide range of options for energy
purposes and for materials, bio-methanol production will
be competing with other applications.

Figure 3. Current and future production costs of bio- and e-methanol
1
Notes: MeOH = methanol. Costs do not incorporate any carbon credit that might be available. Current fossil methanol cost and price are from
coal and natural gas feedstock in 2020. Exchange rate used in this figure is USD1 = EUR0.9.
RENEWABLE METHANOL 15
Improving the competitiveness of e-methanol
Abundant and low-cost green hydrogen. Large-scale
production of e-methanol will depend on the availability
of inexpensive green hydrogen and CO
2
, as well as the
capital cost of the plant. From a cost perspective the
main drivers will be the cost of the renewable power
needed to generate the required H
2
, as well as plant
utilisation rates (especially the electrolysers). Currently,
e-methanol remains costly to produce from these
sources. However, the cost of renewable electricity
produced from wind and solar, which is already
competitive with fossil fuel-generated electricity in most
markets, is predicted to continue decreasing over the
next decades (IRENA, 2020b; IRENA, 2020c). The cost of
e-methanol should therefore also decrease significantly
over the same period. Economies of scale and innovation
in electrolysers will also help reduce costs.
A sustainable and aordable source of carbon. The
necessary CO
2
can be captured from various sources
including power plants and industrial exhaust streams
(e.g. iron, steel and cement production). However, to
be renewable and sustainable, CO
2
has to be obtained
USD/tonne
2 400
1 400
2 200
1 200
2 000
1 000
400
1 800
800
200
1 600
600
0
Current fossil
methanol price
Current fossil
methanol cost
E-methanol - CO
2
from combined
renewable source
E-methanol - CO
2
from DAC only
Bio-methanol < USD 6/GJ
feedstock cost
Bio-methanol USD 6-15/GJ
feedstock cost
Current production
cost levels
Mature production
cost levels
Current production
cost levels
Mature production
cost levels
1 013
884
455
355
764
327
553
227
1620
820
1120
2380
290
630
630
250
A carbon credit of USD 50/t CO
2
would
lower renewable methanol production cost
by about USD 80/t MeOH

Figure 4. Comparison of renewable methanol with other fuels on a price per unit of energy basis
Notes: Exchange rate used in this figure USD1 = EUR0.9. Fuel costs and prices are averaged over 10years. See Annex 3 for details.
INNOVATION OUTLOOK: 16
from renewable sources such as biomass combustion,
distilleries and biogas. CO
2
capture from these sources
needs to be expanded. The production of e-methanol
from renewable CO
2
sources, especially the least
expensive but most limited ones, might also be in
competition with other carbon capture, use and storage
applications. Ultimately, the capture of CO
2
from air
(DAC) oers the largest potential, but its costs need to
decrease substantially.
The combination of bio- and e-methanol production in
a single facility could be very beneficial. In such a hybrid
plant, the excess CO
2
generated in the production of bio-
methanol can serve as the CO
2
source for the production
of e-methanol with green hydrogen.
Outlook for renewable methanol.
With current global demand for methanol at close to
100 Mt per year and growing, there is a large potential
market for renewable methanol. Methanol, whether from
fossil fuels or renewable sources, has the same chemical
structure: CH
3
OH. As such, renewable methanol could
directly replace fossil methanol in any of its current
uses, e.g. as a feedstock for the production of various
chemicals, materials, plastics and products, and as a fuel
for transport, shipping, cooking, heating and electricity
production. The current expansion of fossil methanol as
a fuel in some applications could also ease the gradual
transition to renewable methanol as the distribution and
transport infrastructure would remain the same.
USD/GJ
0
Current fossil
methanol price
Bio-methanol E-methanol
70
60
100
50
20
90
40
10
80
30
Current production
cost levels
Mature production
cost levels
Gasoline (US Gulf Coast)
Diesel (US Gulf Coast)
Heating Oil No. 2 (New York Harbor)
Jet Fuel (US Gulf Coast)
Gasoline (average US)
Diesel (average US)
Gasoline (average
EU)
Diesel (average EU)
Retail with tax
Before tax

Figure 5. Global methanol demand in 2019
1
Source: Based on data from MMSA (2020)
RENEWABLE METHANOL 17
In addition to existing methanol use, renewable green
methanol could also replace most petroleum-based
hydrocarbons and petrochemicals, either directly or
through methanol derivatives, for a potential market
requiring billions of tonnes of methanol per year.
Production of plastics and aromatics (BTX) from
renewable methanol could, for example, be greatly
expanded. This would facilitate the transition to a
sustainable circular green economy where renewable
methanol is uniquely positioned as a future-proof
chemical feedstock and fuel.
While the expansion of renewable methanol is currently
held back by its higher production cost when compared
to natural gas- and coal-based methanol, renewable
methanol is one of the easiest-to-implement sustainable
alternatives available, especially in the chemical and
transport sectors.
Table 1 summarises the benefits and challenges of
scaled-up renewable methanol use. A more detailed
discussion of the pros and cons of methanol can be
found in Annex 1.
98
million
tonnes
Gasoline blending
14%
Methyl tert-butyl ether (MTBE)
11%
Biodiesel
3%
Dimethyl ether (DME)
3%
Methanol-to-olefins
25%
Formaldehyde
25%
Methyl chloride (chloromethane)
2%
Methylamines
2%
Methanethiol (methyl mercaptan)
1%
Methyl methacrylate (MMA)
2%
Acetic acid
8%
Others
4%

INNOVATION OUTLOOK: 18
Table 1. Pros and cons of methanol and renewable methanol
Pros Cons
+
Can be produced on an industrial scale
from various carbon-containing feedstocks.
Natural gas and coal today; biomass, solid
waste and CO
2
+ H
2
tomorrow
+
Already used to produce hundreds of
everyday industrial chemicals and consumer
products
+
Methanol is a liquid at atmospheric
conditions. This makes it easy to store,
transport and distribute by ship, pipeline,
truck and rail
+
Only relatively inexpensive and minor
modification to existing oil infrastructure
needed for methanol storage and
distribution
+
Versatile fuel for internal combustion
engines, hybrid (fuel/electric) systems and
fuel cells, turbine engines, cookstoves, and
boilers
+
Potential liquid hydrogen carrier
+
Low pollutant emissions: no soot (PM), no
SO
x
, low NO
x
. Low-carbon and renewable
methanol also reduces CO
2
emissions
+
No inherent technical challenges in scaling
up the production of methanol to meet the
needs of the transport or chemical industry
sectors
+
Methanol is readily biodegradable
×
Production of renewable methanol remains
more expensive than fossil methanol
×
Production of renewable methanol needs
to be scaled up
×
Competition for renewable feedstock
(biomass, CO
2
, renewable power, green
hydrogen) with other renewable alternatives
×
Renewable methanol requires investment
support, technology-neutral public policy,
and removal of barriers to access affordable
renewable electricity, CO
2
and biomass
feedstocks
×
Fuel standards for methanol need to be
expanded to allow for wider use in more
countries and for more applications
×
Only about half the volumetric energy
density of gasoline and diesel fuel
×
Corrosive to some metals and incompatible
with some plastics and materials
×
Highly flammable and can lead to explosion
if handled improperly, like gasoline, ethanol
or hydrogen
×
Toxic; can be lethal if ingested

RENEWABLE METHANOL 19
Action areas to foster renewable
methanol production
As with any other alternative to fossil fuels, for renewable
methanol to take o in the chemical sector and as a
renewable fuel, demand and supply have to be stimulated
by suitable policies, regulations and mandates. These
could include, among others, renewable fuel standards,
incentives, carbon taxes, cap-and-trade schemes, long-
term guaranteed price floors, contracts for dierence
(CfD), lower taxes on renewable fuels and feedstocks/
products, information campaigns and eco-labelling.
Life-cycle analyses (LCAs) and other benchmarks will
be needed to weigh up the benefits of each process,
material and fuel.
In the transition to fully renewable methanol production,
the co-production of green and conventional products
with proportionate credit should also be allowed. These
include, for example, LCM technologies where green
hydrogen and CO
2
are added to the process of methanol
production from natural gas.
This would allow for a gradual greening of the methanol
produced while keeping costs low. Once the technologies
(e.g. electrolyser, CO
2
capture) are scaled up and the
cost of renewable power low enough, the share of green
methanol, and credits, could increase.

INNOVATION OUTLOOK: 20
Box 1. How to facilitate the transition to renewable methanol:
Recommendations for industry and governments
1
Ensure systemic investment throughout the value chain, including
technology development, infrastructure and deployment. Methanol
can be utilised in existing internal combustion engines as well as in
more advanced powertrains and chemical production processes.
Conventional grey and blue methanol can be used today, with greater
substitution of green methanol over time. Economies of scale and
improved technologies for renewable methanol production will lead
to competitive pricing for multiple sectors, and must be supported
by targeted investment support in the form of direct subsidies and
loan guarantees for production CAPEX (electrolysers, CO
2
capture, and
synthesis equipment). Industry and government also need to partner
on major cost-lowering and risk-mitigation pilot projects and fuel
infrastructure deployment.
2
Create a level playing field through public policy to facilitate
sector-coupling. Drive investment in renewable electricity from the
power sector and biomass utilisation from the agriculture/forestry sector
that can be scaled up to reduce the OPEX production costs of renewable
methanol. Investment will also be needed in renewable/captured CO
2
through BECCS or DAC. The methanol produced can be used in the
transport and industrial sectors. Each sector may find a dierent pathway
to carbon neutrality, and public policy should encourage synergies by
sector-coupling.
3
Support market forces in the chemical sector, focusing on carbon
intensity in consumer products. Renewable methanol can be an essential
building block for hundreds of products that touch our daily lives,
contributing to a circular economy, benefiting from carbon footprinting
and premium pricing mechanisms.
4
Acknowledge how renewable methanol can contribute to carbon
neutrality in “green deals”, COVID-19 economic recovery packages
and hydrogen strategies. The criteria used to define support strategies for
carbon neutrality must follow inclusive frameworks that include low-carbon
liquid fuels and chemical feedstock such as renewable methanol.
5
Translate the political will for carbon reduction into regulatory
measures and support to facilitate long-term growth. Regulatory
measures for fuel standards/quotas should account for the carbon
intensity of the targeted market, facilitating pricing incentives to provide
stability for sustained growth and investment.

RENEWABLE METHANOL 21
6
Encourage international co-operation on trade strategies to
create jobs and foster competitive new industries for e-methanol in
both producing and consuming regions.
As an e-fuel and e-chemical,
e-methanol can be produced in regions with ample resources of renewable
electricity, using carbon as a carrier in the form of an easily transportable
liquid molecule. Investing in e-methanol production capacity in dierent
countries around the world will diversify energy and feedstock supply and
reduce political risks.
7
Institute policy instruments to ensure equitable tax treatment and
a long-term guaranteed price floor for renewable methanol and other
promising fuels. Fuel excise and other taxes should be based on energy
content and not volume (e.g. USD per kWh, not USD per litre). Energy
tax reductions can be provided for renewable fuels, including renewable
methanol – both bio-methanol and e-methanol. Taxation policy can “make
or break” alternative fuels. A meaningful production support system that
could motivate investment is a contract for dierence (CfD) scheme, in
which advanced renewable fuel production projects bid for, and the winners
are awarded, CfDs in so-called reverse auctions (lowest bid wins).

INNOVATION OUTLOOK: 22
Methanol (CH
3
OH) is a colourless water-soluble liquid
with a mild alcoholic odour. It freezes at -97.6°C, boils
at 64.6°C and has a density of 0.791kilograms (kg) per
cubic metre at 20°C. Methanol is an important organic
feedstock in the chemical industry, with worldwide
annual demand nearly doubling over the past decade to
reach about 98million tonnes (Mt) in 2019 (Figure 6 and
Figure 7), while global production capacity has reached
about 150 Mt (MI, 2020a; MMSA, 2020).
Since 1995, the average contract price for methanol in
Europe has been fluctuating roughly between USD200
and USD400 per tonne (t) when adjusted for inflation
(see Figure 8). Production costs are about USD100 to
USD250/t depending on the feedstock (natural gas or
coal) and the price of that feedstock.
1.1. Methanol as a raw material
Methanol occurs naturally in fruits, vegetables, fermented
food and beverages, the atmosphere and even in space.
Historically methanol was commonly referred to as
wood alcohol because it was first produced as a minor
by-product of charcoal manufacturing, by destructive
distillation of wood. In this process, onetonne of wood
generated only about 10–20litres (L) of methanol (along
with other products).
At the beginning of the 1830s, methanol produced in
this way was used for lighting, cooking and heating
purposes, but was later replaced in these applications
by cheaper fuels, especially kerosene. Interestingly,
up until the 1920s wood was the only source for
methanol. From that point on, industrial production
of methanol from coal was introduced followed by
production from natural gas starting in the 1940s.
This shift to fossil resources allowed for a dramatic
increase in methanol production capacity.
Fast-forward to 2019, of the almost 100 Mt of methanol
produced per year (125 billionL), more than 60% was used
to synthesise chemicals such as formaldehyde, acetic acid,
methyl methacrylate, and ethylene and propylene through
the methanol-to-olefin (MTO) route. These base chemicals
are then further processed to manufacture hundreds
of products that touch our daily lives, from paints and
plastics, to building materials and car parts.
Formaldehyde remains the largest-volume chemical
product derived from methanol and is mainly used to
prepare phenol-, urea- and melamine-formaldehyde
and polyacetal resins, as well as butanediol and
methylenebis(4-phenyl isocyanate) (MDI). MDI foam
is, for example, used as insulation in refrigerators,
in doors, and in motor car dashboards and fenders.
The formaldehyde resins are then predominantly
employed as adhesives in the wood industry in a wide
variety of applications, including the manufacture of
particle boards, plywood and other wood panels.
Among new uses of methanol, the MTO process, as an
alternative to the more traditional production of ethylene
and propylene through petrochemical routes, has seen
tremendous growth in the past 10 years in China for
the production of polyethylene and polypropylene.
From essentially no production through this route in
2010, MTO now accounts for about 25% of global
methanol consumption (MMSA, 2020).
Methanol has many other uses, including as a solvent,
antifreeze, windscreen washer fluid and for denitrification
at wastewater treatment plants (Olah, 2018).
1. CURRENT PRODUCTION AND
APPLICATIONS OF METHANOL

Figure 6. The feedstocks and applications of methanol
1
Sources: Chatterton (2019); Dolan (2020); MMSA (2020).
RENEWABLE METHANOL 23
FeedstockConversionDerivativesMarkets
Natural gas
~65%
Coal
~35%
Biomass & renewables
<1%
Other 5%
DME 3%
Biodiesel 3%
Gasoline blending
and combustion
MMA 2%
Acetic acid 8%
Methylamines 2%
MTBE 11%
Chloromethanes 2%
MTO 25%
Formaldehyde 25%
Methanol synthesis
Appliances
Automotive
Construction Electronics
Fuel Paint Pharma Marine
14%

Figure 7. Global methanol demand and production capacity (2001-2019)
1
Source: Based on data from MMSA (2020).
Figure 8. Historical methanol sale price (1995-2020)
1
Note: Western Europe contract average realised price, FOB Rotterdam.
Source: Based on data from MMSA (2020).
INNOVATION OUTLOOK: 24
Prices
Prices adjusted for inflation (in 2020 $)

RENEWABLE METHANOL 25
1.2. Methanol as a fuel
The use of methanol as a fuel, either by itself, in a blend with
gasoline, for the production of biodiesel, or in the form of
methyl tert-butyl ether (MTBE) and dimethyl ether (DME),
has also grown rapidly since the mid-2000s. Together
these fuel uses now represent about 31% of methanol
consumption. MTBE has been used as an oxygenated
anti-knock fuel additive in gasoline since the 1980s. While
MTBE has been banned in some countries such as the
UnitedStates because of groundwater contamination
issues, its use has been increasing in other regions
including Asia and Mexico. Biodiesel can be obtained by
reacting methanol with fats and oils. However, direct use
of methanol as a fuel has seen the largest growth; from
less than 1% in 2000, the share of methanol consumption
for that purpose has now increased to more than 14%.
Due to its high octane rating, methanol can be used as an
additive or substitute for gasoline in internal combustion
engines (ICEs). Methanol can also be used in modified
diesel engines (Bromberg and Cohn, 2009; Bromberg and
Cohn, 2010), and advanced hybrid and fuel cell vehicles.
Notably, methanol has only about half the volumetric
energy density of gasoline and diesel. If pure methanol
is used as a fuel, adjustments to the tank size have to be
made if a similar range is to be achieved. Direct methanol
fuel cells (DMFCs) can also convert the chemical energy
in methanol directly into electrical power at ambient
temperature (McGrath et al., 2004).
Because methanol does not produce soot, fumes or
odour, it is also widely used in cook stoves (over 5 Mt
in 2018 in China alone) (Dolan, 2020). DME, produced
from methanol by simple dehydration, is a gas that can
be liquefied at moderate pressure, much like liquefied
petroleum gas (LPG). DME as a diesel fuel substitute with
a high cetane rating and producing no soot emissions
(particulate matter [PM]) has also attracted much interest
(Semelsberger et al., 2006; Arcoumanis et al., 2008).
DME can also replace LPG in applications such as
heating and cooking. Up to 20% DME can be blended
with LPG with no or very limited modifications to
existing equipment. Methanol can also be used as a
fuel to produce heat and steam in industrial boilers,
and for electric power generation in gas turbines
(Temchin, 2003; Basu and Wainwright, 2001). More than
1000boiler units in China consumed 2 Mt of methanol
in 2018 (Dolan, 2020).
Methanol has historically been a candidate as an
alternative to conventional crude oil-based fuels. This
was initially the case at the time of crude oil supply
constraints in the 1970s and 1980s. Methanol (fossil) has
a high octane rating, and during the 1980s and 1990s was
widely tested both as a low blend component and as a
pure fuel in large test fleets in many countries, mainly
with the goal of reducing air pollution. This interest was
driven by the knowledge that methanol is relatively
cheap to produce from coal and natural gas, and that it
can be used with only minor modification to the existing
vehicle fleets and distribution infrastructure.
By the late 1990s, various technological advances were
achieving wide acceptance in the automobile industry:
direct fuel injection, three-way catalytic converters,
reformulated gasoline, etc. These reduced dramatically
the emission problems associated with gasoline-powered
vehicles, but decreased at the same time the benefits of
methanol-based fuels. Simultaneously oil prices remained
low meaning that despite being a technical success,
methanol was not a commercial success (Olah et al., 2018).
While the interest in methanol-powered vehicles diminished
in developed countries, China has recently been active in
promoting methanol as a transport fuel, largely to decrease
its dependence on imported fuel. Numerous Chinese
automotive manufacturers are oering methanol-powered
vehicles, including cars, vans, trucks and buses able to run
on M85 (85% methanol, 15% gasoline) and M100 (pure
methanol), as well as methanol/gasoline blends with lower
methanol content (SGS, 2020). Flexible-fuel vehicles able
to run on various mixtures of methanol and gasoline, or
so called GEM fuels (gasoline/ethanol/methanol), are also
available (IRENA, 2019a; Olah et al., 2018; Schröder et al.,
2020). These vehicles cost a similar amount to regular cars.
Methanol can also be used in diesel engines, either by
co-feeding with a small amount of diesel pilot fuel, the
addition of ignition improver (MD95), or the installation
of glow plugs. Use of engines specifically optimised for
methanol that allow for higher compression ratios are
also possible (Schröder et al., 2020). Examples of a fleet
of methanol-fuelled taxis and heavy-duty trucks can be
seen in Figure 9 and Figure 10. China currently consumes
4.8 Mt of methanol per year for road transport (Dolan,
2020). Methanol as a road fuel is also attracting growing
interest in other parts of the world, including Israel, India
and Europe, as well as for other applications such as
trains and heavy machinery (Landälv, 2017).

Figure 9. Fleet of M100 fuelled taxis in Guiyang City, Guizhou province, China
Figure 10. Geely M100 truck (2019) in China and M100 truck in Israel (2020).
Source: Geely (2020); DOR Group (2020).
INNOVATION OUTLOOK: 26
Source: Geely (2020).

Figure 11. Gumpert Nathalie, methanol-fuelled hybrid fuel cell supercar
Figure 12. Palcan hybrid methanol reformer/proton-exchange membrane fuel cell passenger bus in China
Source: Gumpert Aiways (2020).
Source: Palcan Energy Corp. (2020).
RENEWABLE METHANOL 27
While methanol can be used in conventional ICE vehicles,
it can also be a fuel for advanced hybrid and fuel cell
vehicles. In that case methanol is reformed on board a
vehicle to hydrogen, which is fed to a fuel cell to charge
batteries in an electric vehicle (EV) or provide direct
propulsion in a fuel cell vehicle (FCV).
The use of liquid methanol avoids the need for costly
on-board systems able to store and transfer hydrogen
gas safely under extreme pressure (350-700 bar) in
FCVs. To date, methanol is the only liquid fuel that has
been demonstrated on a practical scale in fuel cell-based
transport applications.
The potential for on-board methanol reformers to power
FCVs has been demonstrated in numerous prototypes
constructed and tested by various car companies in the
1990s and 2000s, including Ford, General Motors, Honda,
Mazda, Mitsubishi, Nissan and Toyota (Olah et al., 2018).
In the early 2000s, Daimler introduced the NECAR 5
methanol-powered FCV, which in 2002 was the first FCV
to drive 5000kilometres (km) across the UnitedStates
from coast to coast (Daimler, 2020). Newer models of car
developed by Gumpert Aiways and Palcan Energy are
shown in Figure 11 and Figure 12 (Gumpert Aiways, 2020;
Palcan Energy Corp., 2020), expanding the range of EVs
or FCVs from 300km to over 1000km on a 3-minute
fill-up of methanol fuel.

Source: Stena Line (2020).
INNOVATION OUTLOOK: 28
Maritime transport is another sector that has shown
a growing interest in methanol. Currently more than
20 large ships in operation or on order are powered
by methanol (DNV GL, 2020). The shipping sector is
currently responsible for about 3% of all GHG emissions
and 9% of the GHG emissions associated with the
transport sector (IRENA, 2019b). Maritime shipping
represents 80-90% of international trade. The traditional
marine fuel used in ships is diesel bunker fuel, which is
relatively high in sulphur.
Even with new regulations set by the International
Maritime Organization to reduce the sulphur limit in
marine fuels from 3.5% to 0.5%, ships will still emit
large amounts of sulphur oxides (SO
x
), nitrogen
oxides (NO
x
) and PM into the atmosphere. In addition,
the proliferation of emission control areas (ECAs)
around the world, where emission limits are even
more stringent, requires the use of very low sulphur
fuel oil or marine gasoil, which are much more costly
than traditional heavy fuel oil. Because these are far
costlier to produce, the shipping industry has been
looking for alternatives, among which methanol is a
prime candidate.
Methanol, due to its production process, is sulphur-free
and when burned produces almost no PM (due to the
absence of carbon-carbon bonds) and low amounts of
NOx. A number of demonstration projects have been
looking into methanol for marine use (SGS, 2020).
Conversion of existing large and small ships to methanol
can be achieved easily at a moderate cost (Haraldson,
2015). For new builds, the investment cost is similar to
traditional ships.
Operating on methanol is already economical, especially
in ECAs. Examples of ships running on methanol are
shown in Figure 13 and Figure 14 (MI, 2020b). One
example is the Stena Germanica, a 50000 t, 32000
horsepower ferry operating between Germany and
Sweden that was retrofitted in less than three months to
run on methanol. The world’s largest methanol producer
and distributor, Methanex, also operates part of its fleet
of 50000 deadweight tonnage (DWT) chemical tankers
on dual-fuel MAN engines that can operate on diesel fuel
or methanol. Projects to introduce methanol-powered
fuel cell systems for ship propulsion are also under way
to improve eciency and emissions compared to ICEs
(Chatterton, 2019; Fastwater, 2020).
Figure 13. Methanol-powered Stena Germanica 50000 DWT ferry operating between Gothenburg and Kiel

RENEWABLE METHANOL 29
For aviation purposes methanol could be converted to
kerosene-type aviation fuels using a process similar to
the methanol-to-gasoline (MTG) process (Wang et al.,
2016; Wormslev and Broberg, 2020). Methanol itself is
not usually considered the most suitable fuel due to its
lower volumetric energy density compared to kerosene.
However, methanol could possibly be a candidate for
more advanced hybrid planes using a combination of fuel
cell and battery to run electric turbofans or turboprops
(Soloveichik, 2018). This type of hybrid electric aircraft
would have a number of advantages, including less
pollution, noise and emissions, with energy usage
reduction in the range of 40-60%. This would somewhat
counterbalance the lower energy density of methanol.
This type of hybrid aircraft would be especially suited to
regional flights. Methanol has already been introduced in
drone-type devices to considerably increase their range
and flight time. A tiny methanol combustion motor
charges the battery during flight, allowing for longer
flight times and instant refuelling. DMFCs have also been
successfully tested in unmanned aerial vehicles.
1.3. Storage, transport and
distribution of methanol
In most applications, a liquid energy storage medium
such as methanol would be preferable to a gaseous one.
In the transport sector in particular, a transition from
liquid fossil fuel-derived products (gasoline, diesel fuel,
kerosene etc.) to a renewable and sustainable liquid fuel
would be highly desirable. This would enable the use of
the existing infrastructure with only minor modifications
and at a low cost.
Methanol is already a globally available commodity with
extensive distribution and storage capacity in place.
Millions of tonnes of methanol are transported each month
Source: Waterfront Shipping/MOL (2020).
Figure 14. Ocean-going vessel powered by methanol

Figure 15. Methanol stations in China
Figure 16. M15 dispensing pump alongside gasoline and diesel fuel dispensers at a
filling station, and M100 dispensing pump in Israel
Source: Methanol Institute. Source: Palcan Energy Corp (2020).
Source: Dor Group (2020).
INNOVATION OUTLOOK: 30
to diverse and scattered users, by ship, barge, rail and truck.
Methanol can also be transported through pipelines, much
like oil and its products. Refuelling stations dispensing
methanol for cars, buses and trucks are essentially
identical to current filling stations, requiring very little
change in consumer habits (Figure 15 and Figure 16).
In most cases the same tanks can be used. Minor changes
to the refuelling lines, gaskets, etc. might be needed to
accommodate methanol. Rather than gasoline or diesel
fuel, the consumer simply fills their tank at the local service
station with a dierent liquid fuel. Methanol pumps can
be placed alongside existing gasoline or diesel dispensing
pumps. According to a study in the UnitedStates
(Chatterton, 2019), the cost of a methanol filling station is
also the same as a gasoline/diesel one, and much cheaper
than hydrogen refuelling stations that each cost in excess
of USD2 million for only a small fraction of the capacity of a
methanol station. Methanol refuelling infrastructure is also
much cheaper than liquefied natural gas (LNG) stations,
which are currently receiving special attention in Europe
as a result of the so-called Alternative Fuel Infrastructure
Directive 2014/94/EU from 2014 (EU, 2014).

Figure 17. DME filling station and pump in Shanghai, China in 2008
Figure 18. Bio-DME filling station in Sweden in 2011
Source: IDA (2020). Source: Chemrec (2020).
RENEWABLE METHANOL 31
Methanol bunkering for ships is both easy and clean.
Because methanol is a liquid at atmospheric pressure, it
can be stored much like bunker fuel. The infrastructure cost
to store methanol is therefore low, especially compared
to LNG or hydrogen alternatives (MI, 2020c). Methanol is
already available in over 100 major ports today. It is also
readily biodegradable (MI, 2020c; Clary, 2013).
Methanol derivative DME has physical properties
similar to LPG fuels and can use existing land-based
LPG infrastructure. Since there are numerous LPG filling
stations, a transition to DME using the same technologies
could be less costly than building completely new
infrastructure (Figure 17 and Figure 18).

Figure 19. Proposed classification of methanol from various feedstocks
INNOVATION OUTLOOK: 32
Global methanol consumption reached 98.3 Mt in 2019,
and is expected to reach more than 120 Mt by 2025
(MMSA, 2020; Berggren, 2019) and 500 Mt by 2050
(Saygin and Gielen, forthcoming). As the world’s largest
methanol producer and consumer, China accounted
for more than half of total global demand, consuming
around 55 Mt of methanol in 2018, a quarter of which
was utilised in fuel applications. This was followed by the
rest of Asia (excluding China), Europe, North America
and South America. In the next ten years, most future
growth in demand is expected to arise in China, with the
expansion of applications such as transport and heating
fuels, and MTO plants (Berggren, 2019).
2. PRODUCTION PROCESS AND
TECHNOLOGY STATUS
Methanol can be produced from concentrated carbon
sources, such as natural gas, coal, biomass, by-product
streams, or even carbon dioxide (CO
2
) from various
sources including industrial flue gases or direct air capture
(DAC) (Olah et al., 2018; Bertau et al., 2014). A simplified
overview of the steps involved in methanol production
is given in Annex 2. However, for mostly economic
reasons methanol is still almost exclusively produced
from fossil fuels. About 65% of methanol production
is based on natural gas reformation (grey methanol),
while the rest (35%) is largely based on coal gasification
(brown methanol) (Dolan, 2020). Currently, only about
0.2% comes from renewable sources (green methanol).
R
R
e
e
n
n
e
e
w
w
a
a
b
b
l
l
e
e
e
e
l
l
e
e
c
c
t
t
r
r
i
i
c
c
i
i
t
t
y
y
N
N
a
a
t
t
u
u
r
r
a
a
l
l
g
g
a
a
s
s
C
C
o
o
a
a
l
l
Reforming
Gasification
Electrolysis
Carbon capture
and storage (CCS)
CH
3
OH
Blue methanol
CH
3
OH
Grey methanol
CH
3
OH
Brown methanol
Syngas
Syngas
H
2
Blue Hydrogen
CO
2
Non-renewable
B
B
i
i
o
o
m
m
a
a
s
s
s
s
Gasification/
reforming
CH
3
OH
Green methanol
Syngas
CO
2
Renewable
High
carbon
intensity
Low carbon
intensity
Bio-methanol
E-methanol
Bio-
e-methanol
Renewable
CO
2
Non-renewable
Renewable
Non-
renewable
Renewable CO
2
: from bio-origin and through direct air capture (DAC)
Non-renewable CO
2
: from fossil origin, industry
H
2
Green hydrogen

RENEWABLE METHANOL 33
Most methanol production capacity using coal is located in
China, where vast coal reserves are available. Production
from natural gas is the norm in the rest of the world.
Depending on the feedstock and associated carbon
emissions, methanol can be categorised as high or low
carbon intensity (Figure 19). Methanol produced from
coal and natural gas without carbon capture or renewable
power input is generally considered high carbon intensity
(brown and grey methanol). Methanol production based
on the use of renewable energy in various forms, fossil
fuel with carbon capture, or a combination thereof are
considered to have lower carbon intensity (low-carbon
methanol, blue and green methanol; see Figure 19).
Methanol can also be classified as renewable and non-
renewable. To qualify as renewable, all feedstocks used
to produce the methanol need to be of renewable origin
(biomass, solar, wind, hydro, geothermal, etc.).
To produce methanol, natural gas and coal first have
to be converted to synthesis gas (syngas), a mixture
of carbon monoxide (CO), hydrogen (H
2
) and carbon
dioxide (CO
2
). In the case of coal, syngas is obtained by
gasification that combines partial oxidation and steam
treatment at high temperature (800-1800°C depending
on the process and feedstock) (Bell et al., 2010).
To produce syngas from natural gas a number of
processes are available including steam reforming,
partial oxidation dry reforming, autothermal reforming
or a combination thereof. These are high-temperature
processes (>800°C). The syngas obtained by coal
gasification requires much more pretreatment,
conditioning and adjustment to remove impurities
and contaminants (tars, dust, inorganic substances) to
optimise its composition for methanol synthesis.
Ideally, the syngas after conditioning should have a H
2
to CO ratio of at least two to one for optimal synthesis
of methanol. Due to the low hydrogen/carbon (H/C)
ratio of coal, the obtained syngas is rich in carbon oxides
(CO and CO
2
) and deficient in hydrogen. Before being
sent to the methanol unit, the syngas must thus be
subject to a water-gas shift (WGS) reaction to enhance
the amount of hydrogen formed. Some of the CO
2
produced in the process must also be separated, and
is generally simply vented to the atmosphere. Natural
gas has fewer impurities, which are easier to separate,
and a much higher H/C ratio, meaning that much less
syngas conditioning is needed. Due to its higher H/C
ratio, the CO
2
emissions associated with the production
of methanol from natural gas are also substantially
lower than from coal (about 0.5 kg of carbon dioxide
equivalent [CO
2
-eq] per kg methanol for natural gas
compared to 2.6-3.8 kg CO
2
-eq/kg methanol for coal
[Kajaste et al., 2018; MI, 2020c]).
After conditioning, the syngas is converted into methanol
by a catalytic process generally based on copper, zinc
oxide and aluminium oxide catalysts (Bertau et al.,
2014; Olah et al., 2018). Distillation of crude methanol
follows to remove the water generated during methanol
synthesis and any by-products.
A typical world-scale methanol plant using natural gas as
the feedstock has a production capacity of about 3000-
5000t per day or 1-1.7 Mt per year (Sheldon, 2017).
2.1. Low-carbon methanol
To reduce the carbon intensity of methanol production
from natural gas, a number of companies have
developed low-carbon methanol (LCM) processes.
There are several ways to reduce CO
2
emissions while
using natural gas. One option is to inject CO
2
from
some other process into the methanol synthesis loop.
Another is to decarbonise the first step in methanol
production from natural gas, which is the reforming
step to syngas. This step is very energy-intensive,
requiring part of the natural gas feedstock to be
burned to generate the heat for the reforming of the
natural gas at a temperature >800°C, generating
at the same time CO
2
. By reforming natural gas via
electrical heating with renewable power, these CO
2
emissions can be eliminated. Combining these CO
2
emissions with hydrogen produced by electrolysis of
water with renewable energy in the methanol synthesis
loop is yet another way to lower the carbon intensity
of methanol production from natural gas. These and
various other combinations of grey/blue and green
methanol production constitute hybrid solutions that
could facilitate the progressive introduction of green
methanol and allow methanol production facilities to
reduce their carbon emissions.
Methanex Corporation produces LCM at its Medicine
Hat (Canada) plant by injecting CO
2
captured from
a neighbouring industrial facility into the methanol
synthesis loop. This process significantly reduces
GHG emissions when the LCM is utilised as a fuel.

INNOVATION OUTLOOK: 34
According to Methanex, a car that relies entirely on LCM
would emit 30% less CO
2
per kilometre, from well-to-
wheel, compared to a gasoline-powered car (Hobson
and Márquez, 2018; Methanex, 2018).
Other methanol producers, including Qatar Fuel
Additives Company Limited, have implemented CO
2
recovery plants to extract the CO
2
from their flue gas and
re-inject it into the methanol synthesis loop, reducing
both GHG emissions and water consumption (QAFAC,
2020; Hobson and Márquez, 2018).
In China, Baofeng Energy has started the construction
of a green hydrogen generation plant that will be
powered by a 200megawatt (MW) photovoltaic
(PV) power plant and produce about 13000 t of H
2
per year (160 million cubic metres [m
3
]) (Hill, 2020).
The green hydrogen obtained will be fed into a coal-
based methanol plant to increase capacity and reduce
carbon emissions. The oxygen co-produced in the
electrolysis step will replace part of the air-separated
oxygen used for coal gasification, reducing the cost of
hydrogen production. This plant is expected to start
producing green hydrogen in 2021.
In Canada, Advanced Chemical Technologies is planning
to build a 5000 tonne per day methanol plant based
on natural gas, waste CO
2
from adjacent industries, and
H
2
produced by a large 660 MW electrolyser powered
by hydroelectricity. Thus, this plant will emit no CO
2
and in addition recycle some CO
2
emitted by industry
into e-methanol – methanol produced from renewable
electricity (AChT, 2020). The advantage is also that
an entire plant dedicated only to renewable methanol
is not needed, reducing the cost of the renewable
methanol produced.
There are also other large-scale technologies for
producing LCM from natural gas that yield similar
emission reductions. Among others, Johnson Matthey
has developed a process called Leading Concept
Methanol that incorporates a gas-heated reformer in
combination with an autothermal reformer (GHR+ATR).
This produces LCM by utilising renewable electricity for
all of the compressor drives, including the air separation
unit air compressors. Haldor Topsoe is developing a
compact fully electrified methane steam reformer
named eSMR (electric steam methane reforming)
(Wismann et al., 2019).
2.2. Renewable methanol
Growing concern about global climate change due
to anthropogenic GHG emissions has prompted
governments, policy makers, industry and scientists
to start actively looking for ways to “green” their
activities. In this context, renewable methanol produced
sustainably can be part of the pathway to eventually
achieve the decarbonisation of the chemical and
transport sectors. Ultra-low-carbon or net carbon-
neutral renewable methanol can be produced from a
variety of sources. Renewable methanol produced from
biomass such as forestry and agricultural waste and by-
products, biogas, sewage, municipal solid waste (MSW)
and black liquor from the pulp and paper industry is
normally called bio-methanol. By comparison, when
obtained from carbon dioxide and green hydrogen
produced with renewable electricity, it is generally
called “e-methanol”.
Bio-methanol and e-methanol from renewable sources
and processes are chemically identical to fossil fuel-
based methanol, but give rise to significantly lower GHG
emissions during the entire life cycle. In addition, the use
of renewable methanol can reduce dependency on fossil
energy imports and stimulate local economies. A number
of companies are already producing bio-methanol
and e-methanol across the world. In addition, more
companies and institutions have built prototype and
demonstration units or have active R&D in that field. A
list of existing and planned renewable methanol facilities
and demonstrations can be found in this chapter and
also in Annex 4.
Bio-methanol from biomass and MSW
The technologies used in the production of methanol
from biomass and MSW are relatively well-known since
they are similar to or the same as technologies used
in the commercial gasification-based industry, where
feedstocks are usually coal, heavy residual oil and natural
gas. However, the gasification aspect diers in feedstock
preparation. Scaling-up from advanced demonstration
plants to full-scale application still lies ahead for a
majority of technologies, but some large plants are up
and running or close to being ready for start-up. The
main processes in a conventional methanol plant are:
feedstock pretreatment, gasification, WGS, gas cleaning,
methanol synthesis and purification.

Figure 20. Gasification-based methanol plant – general scheme
* Of various kinds, including corn stover, straw and black liquor.
Notes: H
2
S = hydrogen sulphide; MeOH = methanol.
RENEWABLE METHANOL 35
In the gasifier, the feedstock is gasified into synthesis
gas (syngas), a mixture of mainly carbon monoxide
(CO) and hydrogen (H
2
), as well as CO
2
and water (H
2
O).
Depending on the type of gasifier, the syngas will also
contain low levels of hydrocarbons and traces of various
components originating from the feedstock or formed
during gasification. Gasification can be characterised
as a partial (under- stoichiometric) combustion.
The oxidising agent is pure oxygen (typically 99-99.5%)
in order to avoid a dead load of inert molecules in
the produced syngas. The presence of inerts aects
the efficiency and yield in the methanol synthesis,
and increases the size of the whole syngas handling
system, increasing plant costs. The exact ratio between
feedstock and oxygen is dependent on several factors
where feedstock reactivity, gasifier temperature,
feedstock slag behaviour and syngas composition all
are important parameters. Using a minimum amount
of oxygen is always of interest, because it will reduce
the cost of operation and maximise the syngas yield.
Theoretically, there is a trade-off between oxygen
purity, plant costs, product yield and electricity cost
(aects purity of oxygen). Commercial plants are run
with high-purity oxygen, which is a clear indicator of
where the optimum purity, in most cases, is expected
to be found.
The raw untreated syngas leaving the gasification step
needs to be cleaned and conditioned to meet the quality
level stipulated by the methanol synthesis provider.
These process steps vary considerably depending on
feedstock and gasifier technology. Syngas cleaning can
include units for the removal of, for example, tars, dust
and other trace components, and an acid gas removal
unit for CO
2
and sulphur components. Gas conditioning
normally includes adjustment of the H
2
/CO ratio to
around 2 to 1 for optimal methanol synthesis and
methane reforming in order to maximise the syngas yield
and avoid energy loss in the form of methane leaving
the methanol synthesis unit as a purge stream. Methane
reforming is not usually needed in current commercial
technologies gasifying oil and coal as they have
gasification units operating at such a high temperature
that methane formation is low – normally under 0.5%.
The various process units are described further below.
A general scheme for a gasification-based methanol
plant utilising various biomass materials or MSW is
shown in Figure 20. When utilising renewable feedstock
the first three blocks in the process scheme of Figure
20 are dierent compared to a plant fed with coal or
heavy residual oil. These are (a) the pretreatment of
the feedstock, (b) gasification and (c) gas conditioning/
cleaning. Typical biomass gasification schemes were
described by Hannula and Kurkela (2013) and by GTI
(2019). The unit adjusting the H
2
to CO ratio (the WGS)
and the acid gas removal (AGR) unit cleaning the syngas
of most of its CO
2
and of all its sulphur components are
the same as or very similar to commercial technologies
used extensively today.
Pretreatment Gasication
ASU
Gas conditioning
incl. WGS
MeOH
synthesis
MeOH
distillation
Acid gas
removal
CO
2
O
2
Biomass*
MSW
Methanol
H
2
S

INNOVATION OUTLOOK: 36
This is even more the case for the methanol synthesis
unit, because when the syngas reaches this unit its
components are virtually the same regardless of origin.
A
feedstock pretreatment
Most feedstocks for the bio-methanol plant are solid in
nature and need to be homogenised in some way before
being fed into the gasifier. This is important from both the
process control and feeder system design perspectives.
The technological challenge of pushing solids at an even
flowrate against pressure leads to a gasifier pressure
that is kept comparably low, at 5-10 bar. An inert gas
may be needed to make the feed system work properly
and safely. However, minimising this flow of inert gas
is important to minimise the level of investment in the
overall syngas system and for plant eciency. If the feed
is in liquid form, as with black liquor from pulp and paper
mills, the feeding system is simpler and in line with a
heavy residual oil feeding system. These feeding systems
can pressurise the gasification unit to high pressure,
30-60 bar.
B
gasification
The heart of the gasification unit is the gasifier. This is
a high-temperature converter of feedstock into syngas
(including various impurities) where the necessary heat
for reactions is usually provided by the combustion of a
fraction of the feedstock with pure oxygen.
Alternatively, the required heat for gasification can be
supplied indirectly through some kind of heat exchange.
Both versions are practised for biomass- and MSW-type
feedstocks, while commercial processes, with a few
exceptions, use partial oxidation with oxygen.
Gasifier technology can be classified into two categories,
non-slagging and slagging, where the first is the
common variant for renewable feedstocks, while the
latter is, with few exceptions, used for gasification
of fossil feedstocks. Non-slagging means that inert
material present in the feedstock is not allowed to smelt
in the gasifier (it would clog the vessel with severe
consequences), while slagging gasifiers run above the
smelting point of the slag. The gasifier then produces a
floating slag. The maximum non-slagging temperature
is 800-900°C, while the slagging temperature typically
is above 1000°C. The hot zone in a non-slagging gasifier
cannot have hot spots (which would lead to local melting
of slag) and there is thus no flame. As a consequence,
certain gasification reactions are less complete than
reactions occurring in a slagging gasifier where the
local temperature in the flame can be very high, towards
2000°C. The former has a hot bed where most of the
reactions take place, while the latter has a very hot flame
through which the feedstock needs to pass. As a result
of the non-slagging mode, methane and tars form in
the gasifier, which need to be handled downstream. The
slagging gasifier has very little formation of methane
and tars.
Table 2. Examples of syngas conditioning and cleaning processes
Impurities to be
removed
Process More (M) or less (L) common
Particles Particulate filter M
Tar and methane Reform for tar and/or methane decomposition M
COS COS hydrolysis converting COS to H
2
S L
Chlorine
and fluorine
components
HCl and HF removal L
Sulphur
components
AGR process either with CO
2
removal or separately M
CO
2
AGR process either with H
2
S removal or separately M
Notes: COS = carbonyl sulphide; HCl = hydrogen chloride; HF = hydrogen fluoride.

RENEWABLE METHANOL 37
C
gas conditioning and cleaning
Aftertreatment is dierent depending on the type of
gasifier. Feedstock composition, MSW and different
types of biomass material may also aect aftertreatment
requirements because certain feedstocks introduce
species that are unwanted in downstream processes.
These types of processes are mainly required for non-
slagging gasifiers. Most common impurities and how to
handle them are listed in Table 2. An example on how
conditioning and cleaning can be achieved is described
in NextChem (2020a).
Gasification-based projects
and developments
From a technological viewpoint the key to successful
commercialisation is to convert the feedstock to the
syngas quality specified by the technology providers
of the methanol synthesis unit. Syngas quality
requirements are similar regardless of the synthesis
technology placed upstream. Therefore, technology
capable of generating such high-quality syngas as
that used with Fischer-Tropsch technology for the
production of various hydrocarbon type fuels (e.g.
gasoline, diesel, kerosene) can be utilised in methanol
production plants.
Gasifier technologies can be grouped depending on
the design principles they utilise. Table 3 classifies each
technology with respect to two characteristics. One
deals with how the gasifier reactor is heated, and the
other describes the gasification principle, in brief.
In Table 4 various gasification technologies are named
by the technology owner or by the licensor developing
and commercialising the process.
The gasifier unit often consists of two or more parallel
trains that are identical in design. There are three
reasons for this: (1) the degree of scaling-up from
a previous design (maybe a demonstration stage)
becomes too great, (2) the plant as a whole has a
(part-load) redundancy in case one of the gasifier
trains needs to be shut down, and (3) gasification
technology is often more maintenance-intensive,
making parallel trains preferable. For other units in the
total process set-up, single units are common, meaning
that economies of scale in the rest of the plant have a
positive eect on production costs.
Table 4 provides information on where, when and how
the various gasification technologies are currently
applied or intended for use. Further information
regarding performance is covered in Chapter 3.
Table 3. Gasifier design principles
Heating principle
DO
2
Directly (D) heated via partial combustion with oxygen (O
2
)
IH Indirectly heated (IH), can be in dierent ways
Gasifier type
BB Bubbling bed (BB) principle
UO
2
Updraft (U), oxygen (O
2
) injected together with steam
EF Entrained flow (EF) (fuel and O
2
injected together in a burner device)
U-IH Updraft (U), indirectly heated (IH)

INNOVATION OUTLOOK: 38
Table 4. Gasification technologies and their application
1
Gasification
technology
Name/owner
Heating
principle
Type
Feedstock
Project, reference
Project
phase
Product
Plant
capacity
(unit varies)
kt/year
SES
Gasification
Technology
(U-Gas)
DO
2
BB
Biomass/
MSW
Trans World Energy,
Florida (US) (Trans
World Energy, 2020)
FEED done,
start-up Q2
2023
Methanol 875 kt/y
NextChem
Technology DO
2
UO
2
MSW
ENI Refinery,
Livorno, Italian (IT)
(NextChem, 2020b)
Basic
engineering
ready Q3
2020
Methanol 115 kt/y
MSW/
waste
wood
LowLand Methanol
(NL) (LowLands
Methanol, 2020)
Start-up
early 2023
Methanol 120 kt/y
PDQ/
Thyssenkrupp
DO
2
EF
Biomass
(torrefied)
BioTfueL Demo
Project (FR)
(BioTfuel, 2020)
Operational
FT products
(slipstream
based)
15 MWt of
biomass
HTW/
Thyssenkrupp
DO
2
BB Biomass
Värmlands-
metanol (SE)
(Värmlandsmetanol,
2017)
Planning Methanol 100 kt/y
TRI IH BB MSW
Fulcrum (US) (TRI,
2020)
Start-up Q4
2020
FT products 40 000 m
3
/y
Bioliq/KIT DO
2
EF
Pyrolysis
oil from
straw
Bioliq Demo project
(DE) (KIT, 2020)
Operational
Gasoline via
DME
5 MW
t
of
biomass
Chemrec DO
2
EF
Black
liquor
BioDME demo plant
(SE) (Chemrec,
2020)
Idling
DME (via
methanol)
4 t/d
Enerkem
(Enerkem,
2020a)
DO
2
BB MSW Edmonton (CA) Operational
Ethanol (via
methanol)
30 kt/y
DO
2
BB MSW Quebec (CA)
Announced
construction
Ethanol (via
methanol)
35 kt/y
DO
2
BB MSW Rotterdam (NL) Engineering Methanol 215 kt/y
DO
2
BB MSW Saragossa (SP) Engineering Methanol 215 kt/y
Sungas and GTI
(U-Gas)
DO
2
BB Biomass
GTI demo, Chicago
(US) (SunGas
Renewables, 2020)
Operational Syngas
5 MW
t
of
biomass
TCG Global IH U-IH Biomass
Red Rock Biofuels
(Red Rock Biofuels,
2020)
Under
Construction
Start-up
2021
FT products 58 000 m
3
/y
Notes: FEED = front-end engineering design; FT = Fischer Tropsch; kt/y = thousand tonnes per year; MWt = megawatt thermal; t/d = tonnes
per day.

So far, there is little long-term operational experience of
large plants gasifying biomass or MSW and producing
syngas for further synthesis into a product. However, there
are (not described in this report) plants gasifying MSW or
biomass and generating gas for combustion for heat and
power. The dierences between these two applications
are substantial, but are currently being bridged through a
number of advanced projects under way.
Enerkem has gasified MSW in its Edmonton, Canada,
facility for several years (Figure 21). It has experienced
operational issues for a number of reasons, but operations
are improving. In 2019, 60kt of MSW feedstock was gasified
compared to a nameplate capacity of 100kt/y. During 2019,
Enerkem had two scheduled down-time periods, which
aected the result and explains part of the dierence. From
start-up in 2015 until the end of 2019, the plant completed
more than 10000 operational hours and produced
4millionL of methanol. A methanol to ethanol conversion
unit was installed in 2017 and 2018, and was brought on
stream late in 2018. The plant has since produced ethanol.
Four of the projects listed in Table 4 use externally
produced H
2
instead of having a WGS unit to adjust the
H
2
/CO ratio to about 2. They are three Enerkem projects
(Quebec, Rotterdam, Saragossa) and the LowLands
Methanol project. Specifically, the Enerkem plant in Quebec
is planning to incorporate dedicated green hydrogen from
an 87 MW electrolyser and expects to increase the total
bio-methanol capacity up to 100 kt/y. These projects
demonstrate the combined bio-methanol/e-methanol
process described below under “Combination of bio- and
e-methanol production”.
Projects under way as per Table 4 have been ranked as
achieving technical readiness level (TRL) 8 or 9, where TRL
8 stands for “First of a kind commercial system” and TRL 9
“Full commercial operation.”
Figure 21. Enerkem’s MSW to biofuels (methanol and ethanol) plant in Alberta, Canada.
Source: Enerkem (2020b).

INNOVATION OUTLOOK: 40
Bio-methanol from biogas
Biogas production is common in the world. For example,
Europe had almost 18000 production units in operation
in 2019 (Wellinger et al., 2019a). Of these units, 540 (3%)
were upgrading biogas to biomethane of pipeline quality
to be able to inject the gas into the natural gas grid. Europe
has approximately 3570 compressed natural gas (CNG)
filling stations (Wellinger et al., 2019b), and 420 of them
deliver pure biomethane (not mixed with natural gas).
The remaining biogas production plants (97%) use the
biogas (with minimum upgrading) for local heat and power
generation. In 2019, Europe had 10500 MW of power
capacity installed using biogas as feedstock. In some
locations biomethane is used as a co-feed with natural
gas in existing methanol production facilities (see Table 5).
Since 2018, the German chemical company BASF has
been using biomethane as a co-feed with natural gas in
its existing methanol production facility in Ludwigshafen,
Germany (BASF, 2018). As a result, GHG emissions are
reduced by at least 50% compared with conventionally
produced methanol. The renewable part of the product
is certified according to the REDcert standard (REDcert,
2020), which is a standard for biofuels recognised by
the European Commission under the Renewable Energy
Directive (RED).
Since 2009, the Dutch methanol producer OCI/BioMCN
has, in a similar way to BASF, produced bio-methanol as
Table 5. Methanol plants co-fed with a mix of natural gas and biomethane
1
Technology Feedstock Project, reference
Project
phase
Product
Plant
capacity
Steam reforming
Natural gas/
biomethane
BASF, Ludwigshafen
(DE)
Operational Methanol
480 kt/y*
(2018)
Steam reforming
Natural gas/
biomethane
OCI/BioMCN
Groningen (NL)
Operational Methanol
60 kt/y**
(2017)
Steam reforming
Natural gas/
biomethane
OCI Beaumont Texas
(US)
Operational Methanol
1075 kt/y
(2020)***
* Plant capacity (Saygin and Gielen, forthcoming). Bio-methanol share is around 15%.
** Bio-methanol part (Compagne, 2017).
*** Plant capacity (OCI, 2020). Bio-methanol share not given.
part of its methanol production (Compagne, 2017). The
bio-methanol has been certified by DEKRA according
to International Sustainability and Carbon Certification.
Besides exchanging part of their natural gas feedstock
with biomethane, they have also used glycerine and
renewable CO
2
as renewable feedstocks. BioMCN
bio-methanol production capacity is approximately
60000tonnes per year (t/y). Another OCI plant can
be found in Texas. That plant’s overall capacity reached
around 1075kt/y in 2019 and plans to increase the bio-
methanol share of its output (OCI, 2020).
A general scheme for a biogas-based methanol plant is
shown in Figure 22. This is a simplification of schemes
that can be found in literature, for example by Pedersen
and Schultz (2012). Biogas needs to be pretreated to
attain the same quality as fossil natural gas before
being fed into the methane reformer. CO
2
from such
pretreatment may be fed back into the produced
syngas depending on the type of methane reformer
being used. Alternatively, methane can be reformed
together with part of the CO
2
. Linde has developed
a concept utilising so called “dry reforming” (Linde,
2020), where part of the steam has been replaced with
CO
2
. In line with this development, Linde has together
with BASF presented a new way to produce DME
where dry reforming is combined with a novel DME
synthesis process (Brudermüller, 2019). The latter new
development comprises direct synthesis of DME from
syngas.

Figure 22. Reformer-based methanol plant – general scheme
* Of various kinds, such as manure and water treatment sludge.
RENEWABLE METHANOL 41
Bio-methanol from the pulping cycle in
pulp mills
When wood pulp is converted into pulp for further
processing into various qualities of paper, raw methanol
is formed in the digester where wood chips react with
the cooking chemicals (mostly sodium hydroxide and
sodium sulphide). The degree of production depends on
the type of wood and the nature of the cooking cycle (Zhu
et al., 2000). The methanol by-product contains various
impurities and in almost all mills is used as an internal
fuel for heat and power. It can, however, be treated and
upgraded into saleable chemical-grade bio-methanol.
Recently (Q2, 2020), a large mill in Sweden started up
such a plant, the world’s first unit to produce grade
AA methanol from this type of source (Södra, 2020a).
Its production capacity is 5250 t/y. Södra claims 98% GHG
reduction for their new methanol product.
Table 6. By-product bio-methanol from wood pulping
Technology Feedstock Project Project phase Product Plant capacity
Andritz
By-product
from wood
pulping
Södra Mill,
Mönsterås (SE)
Operational
Bio-
methanol
5.25 kt/y
Not known
By-product
from wood
pulping
Alberta Pacific (CA) Operational
Bio-
methanol
3 kt/y
Biogas plant Pretreatment
Methane
reformer
MeOH
synthesis
MeOH
distillation
Syngas
compression
CO
2
Biogas
feedstock*
Methanol
Sulphur
components
O
2
Steam

Figure 23. Types of hydrogen according to production process
INNOVATION OUTLOOK: 42
Alberta Pacific has produced purified methanol for
internal bleaching purposes at its Boyle Mill, Alberta,
since 2012. Recently, California-based Oberon Fuels
was awarded a USD2.9 million grant from the California
Energy Commission to support the upgrade of its
DME production facility to double its current capacity
to 17000L of renewable DME per day, and to test
renewable methanol from a pulp mill as feedstock. The
methanol will be supplied by Alberta Pacific (Oberon
Fuels, 2020).
For this report a review of all pulp mills in Europe was
undertaken using Eurostat data, starting in 2016 and
taking into account the feedstock and pulping cycle. It
concluded that approximately 220000t/y of methanol
could be produced using the method applied by Södra.
If prorated using global pulp production, that would
lead to a potential production capacity of 1.1-1.2Mt/y of
methanol. This number is on the low side because wood
pulping globally is based more on hardwood compared
to Europe, which utilises more softwood. The pulping of
hardwood generates more methanol per tonne of pulp
than softwood pulping.
Methanol from CO
2
(e-methanol)
E-methanol is a liquid product easily obtainable from
CO
2
and green hydrogen through a one-step catalytic
process. Produced through a Power-to-X technology,
e-methanol is considered an electrofuel (e-fuel) and
electrochemical. The dierence between green hydrogen
and other types of hydrogen is illustrated in Figure 23.
Currently, most hydrogen is still produced from fossil fuels
(brown and grey hydrogen). About 48%, 30% and 18%
is produced from natural gas, oil and coal, respectively
(IRENA, 2018). Only about 4% of hydrogen is obtained
by electrolysis using either electric power from the grid
or a renewable source (green hydrogen). Hydrogen is
currently used by a diverse range of industries, including
the chemical (for methanol, ammonia and polymers),
refining (for hydrocracking and hydrotreating), metal
processing, aerospace, glass and food industries. Interest
in green hydrogen as a renewable fuel is also growing.
There are a number of ways to produce e-methanol through
electrochemical processes (Figure 24). The simplest
and most mature method is to make hydrogen through
the electrolysis of water using renewable electricity,
Renewable
electricity
H
2
Green hydrogen
Natural gas
Coal
Steam reforming
Gasification
Electrolysis
Carbon capture
and storage (CCS)
H
2
Blue hydrogen
H
2
Grey hydrogen
Non-
renewable
Renewable
High carbon
intensity
Low carbon
intensity
H
2
Brown hydrogen

Figure 24. Approaches to e-methanol production through electrolysis and electrochemical processes
Source: Ellis et al. (2019).
RENEWABLE METHANOL 43
followed by catalytic reaction with CO
2
to form
e-methanol. Another approach is to produce both
components of syngas, CO and H
2
, through electrolysis,
followed by conversion of the syngas to e-methanol as
practised for conventional methanol production. While
this route could achieve a higher conversion eciency,
it is less developed than water electrolysis (conventional
water electrolysis is in the megawatt range, while
this co-electrolysis route is at the lab, kilowatt scale).
Direct electrochemical conversion of CO
2
and water to
methanol is also being studied, but so far only limited
eciency and yield have been achieved at a laboratory
scale (Goeppert et al., 2014; Olah et al., 2018).
Because the reaction of CO
2
with H
2
from water
electrolysis is currently the only practical method to
produce e-methanol, the following discussion focuses
on that method.
In general, each molecule of CO
2
entering the process
will exit as a methanol molecule. However, each CO
2
molecule requires three molecules of hydrogen and
1
Electrolysis of water to hydrogen followed by catalytic methanol synthesis
Electrolyser
H
2
OgH
2
+O
2
E-methanol
production
E-methanol
H
2
O
O
2
H
2
CO
2
H
2
O
Renewable
electricity
2
Electrolysis of water and carbon dyoxide to syngas followed by catalytic methanol synthesis
Electrolyser
H
2
OgH
2
+O
2
CO
2
gCO+O
2
E-methanol
E-methanol
production
H
2
O
CO
2
O
2
CO
2
Syngas
H
2
/CO
H
2
O
Renewable
electricity
3
Direct electrocatalytic synthesis of methanol form water and carbon dioxide
E-methanol
Electrolyser
H
2
OgCO
2
CH
3
OH+O
2
O
2
Renewable
electricity
H
2
O
CO
2

Figure 25. CO
2
feedstock for the production of e-methanol
INNOVATION OUTLOOK: 44
will produce one molecule of water for each molecule
of methanol. Accordingly, to produce one tonne of
methanol, about 1.38 t of CO
2
and 0.19 t of hydrogen (~1.7
t of water) are needed. About 10-11 MWh of electricity
are required to produce one tonne of e-methanol;
most of it for the electrolysis of water (assuming CO
2
is provided). With a 100 MW electrolyser, about 225 t/d
of e-methanol could be produced. Such electrolysers,
although large, are already available from Thyssenkrupp
(Thyssenkrupp, 2020a). For a large 1000t/d e-methanol
plant, an electrolyser of at least about 420 MW would
be necessary. To replace a conventional mega-methanol
plant with production capacity of 2500t/d, an
electrolyser in the gigawatt range would be needed.
Production capacity for such large electrolysers still
needs to be developed.
The technology for the e-methanol synthesis step is very
similar to the one for the production of methanol from
fossil fuel-based syngas and is therefore mature (TRL 8-9).
The traditional CuO/ZnO/Al2O3 catalyst used has only to
be slightly modified to accommodate the generation of
larger amounts of water during synthesis of e-methanol.
Such catalysts are already available commercially from
a number of vendors including Haldor Topsoe, Johnson
Matthey and Clariant. The reaction is operated at
temperatures between 200°C and 300°C and pressures
of 50-100 bar. Catalysts able to operate under milder
conditions are also under development.
Carbon dioxide feedstock: The CO
2
feedstock for
e-methanol production can be divided into two broad
categories depending on its origin (Figure 25):
•
CO
2
from various industrial sources, including
power plants, and steel and cement factories. In
this instance, the CO
2
would most likely come from
the burning of fossil fuels. Even though recycled,
it would still amount to fossil-based CO
2
, which is
non-renewable and makes the overall process net
CO
2
positive. However, given that the CO
2
from
these sources would otherwise be released into the
atmosphere, using it one more time for the production
of methanol with green hydrogen would result in a
low-carbon methanol.
•
CO
2
obtained from the atmosphere either directly
by direct air capture (DAC) or through biomass.
To be renewable, sustainable and net CO
2
neutral,
biogenic sources of CO
2
will increasingly have to be
used, such as from distilleries, fermentation units,
MSW, biogas and other units such as power plants
that produce electricity by burning biomass. These
sources of CO
2
are normally treated as off-gases and
therefore emitted to the atmosphere (usually at high
CO
2
concentrations but atmospheric pressure). When
the CO
2
from these units is captured either for storage
or utilisation, the process is usually referred to as bio-
energy with carbon capture and storage (BECCS)
Fossil fuels
DAC
Power generation
with carbon
capture
Renewable
CO
2
Non-
renewable
CO
2
Methanol
Methanol
Net CO
2
neutral
Net CO
2
positive
CO
2
CO
2
Biomass conversion
with carbon capture
Industrial uses with
carbon capture
CO
2
CO
2
Atmosphere
Geosphere
Biomass

RENEWABLE METHANOL 45
or bio-energy with carbon capture and utilisation
(BECCU) (Consoli, 2019). Combining e-methanol
and bio-methanol production in a single BECCU plant
offers a number of advantages further described
in this report’s section entitled “Combination of
bio- and e-methanol production”. To complement
photosynthetic CO
2
capture from air in biomass,
anthropogenic CO
2
capture from the atmosphere
is also becoming a possibility as technologies for
DAC are now being developed and commercialised
(Goeppert et al., 2014; Sanz-Pérez et al., 2016). BECCS,
BECCU and DAC allow for a net carbon-neutral cycle
in the production of e-methanol.
Hydrogen feedstock: The electrolysis of water to
produce hydrogen has taken off in recent years
to grow in scale from kilowatts to gigawatts using
existing and well-established technologies. However,
further electrolyser technology improvements and
cost reductions are required to enable the mass
production of cost-competitive green hydrogen (IRENA,
2020c). The electricity needed for the process can
be generated by any form of energy. However, to be
sustainable it needs to come from renewable sources.
For the large-scale deployment of sustainable
electrolysis, wind and solar PV have the greatest potential
due to their increasing availability and decreasing costs.
They are the world’s fastest-growing sources of energy,
providing clean and aordable electricity.
The first contemporary commercial CO
2
-to-methanol
recycling plant using locally available, cheap
geothermal energy has been operated in Iceland
by Carbon Recycling International (CRI) since 2011.
This commercial demonstration plant, with a design
from Johnson Matthey/Jacobs and an annual capacity
of 4000t of methanol (~12 t/d), is based on the
conversion of geothermal CO
2
and the readily available
local geothermal energy (hot water and steam) sources
(Figure 26) (CRI, 2020). The necessary H
2
is produced
by water electrolysis using cheap geothermal electricity.
Iceland embarked on this development as a means to
exploit and possibly export its cheap and clean electrical
energy. The produced methanol, called Vulcanol,
is currently mixed with gasoline, used for biodiesel
production and for waste-water denitrification.
In China, the Dalian Institute of Chemical Physics
recently started operations at a 1000t/y e-methanol
demonstration project (Figure 27) (AAAS, 2020). In this
plant, the alkaline electrolysers for the production of the
necessary hydrogen (1000 normal cubic metres per hour
of H
2
) use the electricity produced by a 10 MW solar PV
plant. After initial testing and ramping up, full operation
is expected to start in October 2020. The project is the
first to demonstrate the production methanol from solar
power on an industrial scale.
Other e-methanol commercial plants are being planned
around the world with production capacities ranging
from 8000t/y to 180000 t/y e-methanol (Table 7).
If all the commercial projects in Table 7 come to fruition,
in excess of 700000 t/y of e-methanol capacity would
be available. Plants from Liquid Wind in Sweden,
ABEL in Australia, Swiss Liquid Future/Thyssenkrupp
in Norway, and RH
2
C in Canada will all use renewable
H
2
and CO
2
from either industrial or biogenic sources
(Swiss Liquid Future, 2020a; Liquid Wind, 2020;
ABEL Energy, 2020; RH
2
C, 2020). Other consortia are
planning the construction of e-methanol plants in the
ports of Antwerp and Ghent in Belgium, as well as in the
Netherlands (Nouryon, 2020; aet, 2019; INOVYN, 2020).
In Denmark, a sustainable fuel project aims to achieve
electrolyser capacity of 10 MW in 2023, 250 MW in 2027,
and 1.3 GW in 2030 respectively. The green hydrogen
generated will be combined with CO
2
captured from the
combustion of MSW or biomass to produce renewable
methanol for maritime vessels and renewable jet fuel for
planes (e-kerosene) (Maersk, 2020).
The recycling of both CO
2
and H
2
obtained as the by-
products of industrial processes is also an option in some
cases. In China, Henan Shuncheng Group/CRI recently
started the construction of an emission-to-liquid plant
that will convert H
2
from coke oven gas and CO
2
from a
lime kiln to 110000t/y of methanol (CRI, 2020).
An increasing number of technology providers are also
developing and licensing e-methanol solutions, including
entire plants, e-methanol synthesis units, catalysts and
larger electrolysers able to provide sucient hydrogen.
They include among others CRI, Thyssenkrupp/Swiss
Liquid Future, bse engineering/BASF (FlexMethanol),
Haldor Topsoe (eMethanol), and Johnson Matthey (HT,
2019a, bse engineering, 2019; CRI, 2020; JM, 2020,
Thyssenkrupp, 2020b).
Numerous institutions, companies, universities
and collaborative efforts are also developing CO
2
-
to-methanol technologies and testing them in

INNOVATION OUTLOOK: 46
demonstration and pilot plants. In Aalborg, Denmark,
the Power2Met project is producing about 800litres per
day (L/d) of e-methanol from biogas CO
2
and hydrogen
obtained by the electrolysis of water using wind and
solar energy (REintegrate, 2020; Energy Supply, 2020).
Plans are to increase the capacity to about 10000m
3
per year by 2022 (Jensen, 2019). In Luleå, Sweden, the
e-methanol technology developed by CRI was used
to produce methanol from CO
2
/CO and H
2
recovered
from an industrial blast furnace at a steel manufacturing
plant as part of the FresMe project under EU’s Horizon
2020 programme (FReSMe, 2020). The necessary H
2
is
complemented with H
2
obtained by the electrolysis of
water. The two sources of H
2
enable maximum use of
the current residual energy content of blast furnace gas
for a methanol production capacity of up to 1t/d from
blast furnace gases. This project benefited from another
EU-funded project entitled MefCO
2
aimed at improving
the technology to produce methanol from CO
2
(MefCO
2
,
2020). This test plant built in Germany had a capacity
of 1 t/d of methanol from 1.5 t/d of CO
2
captured from
the emissions of an RWE coal-fired power plant and 0.19
t/d of green hydrogen. On the same site a CO
2
-to-DME
process with a capacity of 50 L of DME per day is also
being tested in the frame of the ALIGN-CCUS project
(ALIGN-CCUS, 2020; Moser et al., 2018).
Figure 26. The “George Olah Renewable CO
2
-to-Methanol Plant” of CRI in Iceland
Source: CRI (2020).
Figure 27. 1000 t/y e-methanol demonstration plant
in Lanzhou, Gansu Province, Northwestern China
Source: Courtesy of Prof. Can Li from the Dalian
Institute of Chemical Physics.

RENEWABLE METHANOL 47
As part of the Carbon2Chem project, a cross-industrial
network funded by Germany, production of methanol
from steel mill gases complemented by H
2
from water
electrolysis will be studied at a Thyssenkrupp steel
mill (Carbon2Chem, 2020). In Japan, Mitsui Chemicals
operated for 4500hours a pilot plant with a 100 t/y
methanol capacity, using CO
2
and H
2
with a catalyst
developed by RITE (Mitsui Chemicals, 2009, 2010).
In Korea, KIST developed the CAMERE process, an
alternative two-step route from CO
2
to methanol (Joo
et al., 2004). Zero Emission Fuels, a company in the
Netherlands, is aiming to develop fully automated
modular micro-plants to produce methanol from CO
2
captured from the air and renewable H
2
produced from
solar power (ZEF, 2020). In Germany, a consortium of
30 partners named C3Mobility is aiming to develop
methods to produce renewable methanol from various
feedstocks, and use this methanol as a fuel or platform
chemical for the preparation of other transport fuels
(DME, MTGetc.) (C3 Mobility, 2020).
Besides the electrolysis route to producing hydrogen,
and subsequent conversion with CO
2
to methanol, some
institutions and companies are also exploring other
routes, such as high-temperature thermochemical
conversion using solar heat or direct electrochemical
conversion of CO
2
and water to e-methanol using
direct sunlight (artificial photosynthesis concept [JCAP,
2020]). Synhelion, in Switzerland, uses high-temperature
solar heat in excess of 1000°C to convert CO
2
and
water to CO and H
2
in a thermochemical process. The
obtained syngas (H
2
+ CO) can then be converted to
methanol using standard methanol synthesis technology
(Synhelion, 2020).
Besides methanol and DME, the production of
oxymethylene ethers (OMEs) from CO2 and hydrogen is
also being considered. OMEs are a diesel fuel substitute
with a high cetane number, which burn soot-free and
with very low pollutant emissions. The addition of OMEs
to diesel fuel was also found to decrease significantly
the PM and soot emissions (Lumpp et al., 2011; Wang
et al., 2015). However, the production of e-OMEs was
determined to be less energy-ecient than other e-fuels,
including e-methanol and e-DME (Held et al., 2019,
Kramer, 2018).
Table 7. Overview of existing or planned facilities and technology providers for e-methanol production
Country Company
Start-up
year
Capacity
(t/y)
Product Feedstock Source
Iceland CRI 2011 4000
e-methanol
(Vulcanol)
Geothermal CO
2
and H
2
from water
electrolysis
CRI, 2020
China
Dalian Institute
of Chemical
Physics
2020 1000 e-methanol
CO
2
and H
2
from
water electrolysis
(PV)
AAAS, 2020
Sweden Liquid Wind
2023
(plan for 6
facilities by
2030)
45000 e-methanol
Upcycled industrial
CO
2
and H
2
from
water electrolysis
Liquid Wind,
2020
Australia
(Tasmania)
ABEL 2023 60000 e-methanol
Biogenic CO
2
and
H
2
from water
electrolysis
ABEL Energy,
2020

INNOVATION OUTLOOK: 48
China
Henan
Shuncheng
Group/CRI
2022 110000 methanol
(a)
CO₂ from limekiln
and H₂ from coke
oven gas
CRI, 2020
Norway
Swiss Liquid
Future/
Thyssenkrupp
n/k 80000 e-methanol
CO₂ from
ferrosilicon plant
and H₂ from
water electrolysis
(hydropower)
Swiss Liquid
Future, 2020a,
Swiss Liquid
Future, 2020b
Norway
Consortium of
companies/
CRI
2024 100000 e-methanol
CO₂ and H₂ from
water electrolysis
Stefánsson,
2019
Canada
Renewable
Hydrogen
Canada (RH₂C)
n/k 120000 e-methanol
CO₂ and H₂ from
water electrolysis
(hydro)
RH₂C, 2020
Belgium
Consortium
at the port of
Antwerp
n/k 8000 e-methanol
CO₂ and H₂ from
water electrolysis
INOVYN, 2020
Belgium
Consortium
at the port of
Ghent
n/k
46000-
180000
e-methanol
Industrial CO₂ and
H₂ from water
electrolysis
aet, 2019
The
Netherlands
Consortium
Nouryon/
Gasunie/
BioMCN and 3
others
n/k 15000 e-methanol
CO₂ and H₂ from
water electrolysis
Nouryon, 2020
Germany Dow n/k ~ 200000 e-methanol
CO₂ and H₂ from
water electrolysis
Schmidt, 2020
Denmark
Consortium of
companies
2023-2030 n/k e-methanol
CO₂ from MSW
and biomass.
H₂ from water
electrolysis
(oshore wind).
Up to 1.3 GW
electrolyser
capacity by 2030
Maersk, 2020
Germany Consortium n/k n/k e-methanol
CO₂ from cement
plant and H₂ from
water electrolysis
(wind)
Westküste 100,
2020

RENEWABLE METHANOL 49
Technology demonstration plants (past and current)
Country Company
Start-up
year
Capacity Product Feedstock Source
Sweden FreSMe 2019 1 t/d
e-methanol
(b)
CO₂ and H₂ waste
stream from steel
manufacturing
and H₂ from water
electrolysis
FReSMe, 2020
Germany MefCO₂ 2019 1 t/d e-methanol
Power plant flue
gas CO₂ and
H₂ from water
electrolysis
MefCO₂, 2020
Denmark
Power2Met
Danish
Consortium
2019 800 L/d e-methanol
CO₂ from biogas
and H₂ from water
electrolysis (wind
and solar)
REintegrate,
2020
Germany Carbon2Chem 2020 50 L/d
e-methanol
(b)
CO₂/CO/H₂ from
steel mill gases
and H₂ from water
electrolysis
Carbon2Chem,
2020
Germany
ALIGN-CCUS
Project DME
from CO₂
2020 50 L/d e-DME
CO₂ from power
plant flue gas and
H₂ from water
electrolysis
ALIGN-CCUS,
2020
Switzerland
Swiss Liquid
Future
2012 75 L/d e-methanol
CO₂ and H₂ from
water electrolysis
Swiss Liquid
Future, 2020a
Germany
Total/Sunfire
e-CO₂Met
project
2022 1.5 t/d e-methanol
CO₂ from a refinery
and H₂ from water
electrolysis
Total, 2020
Germany
Bse
Engineering /
Institute for
Renewable
Energy
Systems (IRES)
2020 28 L/d e-methanol
CO₂ and H₂ from
water electrolysis
(wind)
bse
Engineering,
2020
Japan Mitsui 2009 100 t/y e-methanol
CO₂ and H₂ from
water electrolysis
Mitsui
Chemicals,
2009, 2010
Korea
Korean
Institute of
Science and
Technology
(KIST) /
CAMERE
process
2004 100 kg/d e-methanol
CO₂ from power
plant flue gas and
H₂ from water
electrolysis
Joo, 2004

INNOVATION OUTLOOK: 50
Selected technology providers
Iceland CRI
Technology
provider
50000-
100000
e-methanol
CO₂ and H₂ from
water electrolysis
CRI, 2020
Germany
Thyssenkrupp/
Uhde/Swiss
Liquid Future
Technology
provider
3600-
72000
e-methanol
CO₂ and H₂ from
water electrolysis
Thyssenkrupp,
2020a
Germany
Bse
Engineering /
BASF
Technology
provider
8200-
16400
e-methanol
CO₂ and H₂ from
water electrolysis
bse
Engineering,
2020
Denmark Haldor Topsoe
Technology
provider
Variable e-methanol
CO₂ and H₂ from
water electrolysis
HT, 2019a
United
Kingdom
Johnson
Matthey
Technology
provider
Variable
100000-
1700000
e-methanol
CO₂ and H₂ from
water electrolysis
JM, 2020
1
Notes: (a) Hydrogen obtained from coke oven gas and not from water electrolysis.
(b) Part of the hydrogen obtained from the waste stream of steel manufacturing.
n/k = not known.
Combination of bio- and e-methanol
production
The production of methanol from biomass is carried
out in a similar way to its production from coal and
heavy residual oil. CO
2
is generated in the gasifier due
to the endothermic nature (energy-consuming) of the
gasification reactions. In addition to that, due to their
chemical composition, these feedstocks produce a
syngas mixture with a low H
2
/CO ratio. For methanol
synthesis, the optimal H
2
/CO ratio is close to 2. To adjust
this ratio, part of the CO in the syngas is converted with
water to H
2
through the WGS reaction. This also creates
excess CO
2
, which is separated and generally simply
vented to the atmosphere. Because the production
of methanol from biomass generates a lot of CO
2
, the
apparent conversion rate of biomass into methanol
is reduced (Reschetilowski, 2013). The overall carbon
eciency in this type of scheme is around 50%, meaning
that only about 50% of the carbon in the feedstock ends
up in methanol; the rest is in the emitted CO
2
.
An attractive possibility to increase the carbon utilisation
rate is to react the normally emitted CO
2
with hydrogen
from some other source to produce more methanol
(Specht et al., 1999). This can be achieved by combining
the bio-methanol scheme and part of the e-methanol
scheme into a hybrid process where nearly 100% of the
carbon in the biomass ends up as carbon in the methanol
product, as illustrated in Figure 28. The hydrogen is
provided by water electrolysis using renewable power.
The elimination of CO
2
emissions, or in other words the
use of all available bio-carbon, can take place in two steps.
The first includes injection of hydrogen to accomplish
a H
2
/CO ratio of about 2, thus eliminating the need for
the WGS. The second step is to inject enough H
2
to react
the remaining CO
2
to methanol. Figure 28 illustrates this
two-step process conducted in two separate methanol
synthesis units, after which the two raw methanol streams
are combined for downstream processing.
Commercially proven catalysts are available for both
methanol synthesis from H
2
/CO and from H
2
/CO
2
.
Catalyst providers have also developed alternatives to
this approach and shown that CO, CO
2
and H
2
can be
combined into the same synthesis unit and still keep the
eciency of the conversion at a high level (Bertau et al.,

Figure 28. Combined bio- and e-methanol scheme with biomass or MSW as feedstock
* Of various kinds, including
corn stover, straw and black liquor.
RENEWABLE METHANOL 51
2014). In such a case, the two methanol synthesis units
inside the dashed rectangle in Figure 28 are combined
into one, and the AGR unit becomes a cleaning unit only
for sulphur components and other contaminants. CO
2
is
left in the main syngas stream.
Elimination of the WGS unit has a number of direct
advantages such as:
•
No investment in a WGS unit.
•
No high-pressure steam injection into the syngas
needed for the WGS reaction.
•
No loss of boiler feed water (reaction water) in the WGS.
•
No loss of green carbon due to CO becoming CO
2
in
the WGS unit.
•
No loss of syngas energy (normally 3-5% loss) in the
exothermic WGS reaction.
•
Increased syngas production in the order of 45-55%
compared to the case with a WGS unit (depending on
H
2
/CO ratio in the raw syngas upstream WGS).
•
Lower operating cost for the gas-cleaning plant due
to lower CO
2
load.
•
Lower relative investment in the syngas and methanol
generation parts of the plant due to economies of
scale. The gasification plant will stay the same.
Hydrogen injection in this way does not have any
foreseeable negative effects on the process. There
are, however, some additional overall benefits to those
already listed, such as
•
The air separation unit may no longer be required
(depends on the H
2
/CO ratio in the raw syngas)
because the oxygen produced by the electrolysis of
water produces pure oxygen, which can replace O
2
from an air separation unit. The required investments
are similar, and removing the air separation unit helps
to offset the power needs of the electrolyser.
•
All the CO
2
is easy to track and will come from a
renewable source if biomass materials make up the
feedstock.
•
The CO
2
is already in situ in the plant, at pressure and
ready to be synthesised with hydrogen to methanol.
•
If for some reason CO
2
is separated in the AGR, as
Figure 28 indicates, it is still at a high concentration
and thus highly suited as feedstock for another
“e-product” unit.
Pretreatment Gasication
Gas
conditioning
MeOH
synthesis
MeOH
synthesis
MeOH
distillation
Acid gas
removal
CO
2
O
2
O
2
from over
the fence?
H
2
Biomass*
MSW
Methanol
H
2
S
Electrolysis
Green power
generation

Figure 29. Combined bio- and e-methanol scheme with biogas as feedstock
* Of various types, such as manure and water treatment sludge.
Combining the conventional bio-methanol process
according to Figure 20 with e-methanol as described
above allows for all the carbon in the biomass to be
utilised, increasing the production potential from a given
amount of biomass from around 60% to about 140%.
Starting with 1MWh of biomass results in 1.4MWh of
methanol. This, however, also requires the necessary
renewable energy for the generation of H
2
.
Analogous to the combination of solid biomass and
electrolysis technologies shown in Figure 28, a methanol
production scheme based on biogas is also possible.
Figure 29 shows how CO
2
generated during biogas
pretreatment can either (a) be part of the reactions in
the reformer together with methane, steam and O2, or
(b), depending on the required balance between the
gases in the methane reformer, bypass the reformer
and be added later in the process chain downstream of
the methanol reformer. Additional H
2
is then needed to
create an optimum gas composition for the methanol
synthesis. A way to further decrease carbon emissions
from the process would be to heat the reformer with
renewable electricity, a process under development
by, for example, Haldor Topsoe. In late 2020, Perstorp
announced the plan to build the methanol plant using
this technology in Stenungsund, Sweden under Project
AIR. The plant aims to replace 200,000 tons of fossil
methanol and start producing renewable methanol from
2025 (Perstorp, 2020).
With the previously mentioned process alterations being
part of the process solution, virtually all carbon from
the feedstock can end up as carbon in the produced
methanol, resulting in a substantial increase in production
capacity from a given amount of biogas. From a carbon
utilisation point of view, it would be more ecient than
the current use of biogas in power and heat generation
or as a vehicle fuel in the form of biomethane.
INNOVATION OUTLOOK: 52
Biogas plant Pretreatment
Methane
reformer
Electrolysis
Green power
generation
MeOH
synthesis
MeOH
distillation
Syngas
compression
CO
2
Biogas
feedstock*
Methanol
Sulphur
components
O
2
Steam

RENEWABLE METHANOL 53
3.1.Performance and eciency
Outside China, the world’s methanol production
uses natural gas as feedstock with a few exceptions
where coal is utilised instead. Production from coal
is overwhelmingly based in China. The overall energy
conversion eciency for a large, modern natural gas-
based plant is around 70%. For coal to methanol the
energy conversion eciency is in the order of 50-60%
depending on technology selection.
The performance of renewable methanol plants
(amount of methanol produced in a given period, e.g.
a year) depends on many factors, such as the plant
set-up (e.g. feedstock, co-products, technology) and
local conditions (e.g. brownfield or greenfield site,
availability of feedstock or renewable electricity).
Assessing real-life performance is dicult as only a
limited number of commercial plants are currently
in operation (Table 4 and Table 7). Dierent models
based on various assumptions can be used to
investigate dierent plant configurations in specific
locations. This leads to a range of estimates for
eciency and environmental impact that are often
dicult to compare.
Bio-methanol
There are still only a relatively limited number of
commercial bio-methanol plants in operation (Table 4).
Nevertheless, a number of qualified actors have carried
out a considerable amount of planning and front-end
engineering for projects at an advanced stage of
construction, and advanced demonstration plants have
logged operational time resulting in a more secure base
for upscaling. Gasification-based plants for methanol
and other products like FT fuels are under construction.
A combination of input data from plants in operation,
under construction and at an advanced stage of planning
provide a more securely based set of data, oering a
more accurate picture with respect to performance
and eciency. An approximate overall estimate of the
conversion eciency of a specific process route can be
reached by multiplying the energy conversion eciency
of each process unit involved in the conversion chain.
Initially three units degrade the chemically bounded energy,
and their respective energy eciency can be multiplied
together to reach an approximate overall conversion
eciency. Table 8 describes these three process operations.
3. PERFORMANCE AND SUSTAINABILITY
Table 8. Energy conversion eciencies for certain process units
Process operation
Energy
eciency
Comment
Gasification of feedstock 0.7-0.8(+)
The wide range depends on feedstock characteristics
such as level of inerts and moisture, and overall gasifier
temperature
WGS 0.95-0.97
The higher the H
2
/CO ratio is in the feed gas, the less
water-gas shifting is required, thus the lower the energy
losses
Methanol synthesis 0.79-0.8
With a stoichiometric syngas and limited amount of inert
gases
Overall 0.53-0.62

INNOVATION OUTLOOK: 54
Multiplying these three efficiency numbers leads to
an overall conversion eciency of 0.53 to 0.62. There
may be a few percentage points to gain with further
optimisation, especially in the gasification unit.
The calculated interval coincides well with all the data
received from various technology providers and project
developers. Around 60% overall energy conversion is
typical for biomass conversion, while MSW conversion
is at the lower end of the interval.
Biogas can replace natural gas in current methanol
production plants based on fossil natural gas. After
being upgraded to pipeline quality, biomethane can be
part of the feedstock, as is done by BASF and BioMCN
(BASF, 2018; BioMCN, 2020). Large sources of biogas
upgraded and purified to clean biomethane and fed
into a reformer-based methanol production process will
have the same conversion eciency as a corresponding
natural gas-fed plant.
E-methanol
For the production of e-methanol the process is quite
straightforward. Three main parts have to be considered:
1
H
2
generation by water electrolysis,
2
CO
2
capture,
and
3
methanol synthesis.
1
The electrolysis of water to hydrogen and oxygen
is a mature technology with current efficiencies
(higher heating value [HHV] of H
2
) of roughly 75-85
% for alkaline and PEM-based electrolysers (IRENA,
2018). Alkaline electrolysers are the most common
and are inexpensive. Modular units of 10 to 20 MW are
available that can be combined to produce plants with
sizes over 100 MW and a lifetime of over 30 years with
98% availability (Thyssenkrupp, 2020a). PEM-type
electrolysers allow for a higher H
2
output pressure to
be delivered (30 bar and higher), which could reduce
the cost of pressurisation for the downstream methanol
synthesis. However, these are more expensive than
alkaline electrolysers (IRENA, 2020c). Solid oxide
electrolysers are also being developed that could
oer higher eciency by operating at much higher
temperatures (>700°C). Some hydrogen storage
capacity will also be needed to allow for continuous
operation of the methanol synthesis unit. On a large
scale, the production cost of renewable H
2
is mainly
dictated by the cost of renewable electricity.
2
Worldwide more than 37 billion tonnes of co
2
related
to human activity are released into the atmosphere every
year, of which 34 billion tonnes are energy-related (Olivier
and Peters, 2019; IRENA, 2020b). These CO
2
emissions
originate from electricity generation, cement and
fermentation plants, industry, the transport sector, heating
and cooling of buildings, and other activities. However,
while sources of CO
2
are plentiful, sources of captured
CO
2
that are currently available for recycling into fuels
and material are not. The cost associated with capturing
CO
2
depends greatly on its origin (Table 9). Facilities at
which the capture of CO
2
is easiest are those that already
produce concentrated streams of CO
2
, such as natural gas
purification and production of fertiliser and bio-ethanol
(Irlam, 2017). However, the amount of CO
2
available from
these plants is limited. Other sources with lower CO
2
concentrations include fossil fuel power plants (coal, natural
gas, oil), iron and steel plants and cement production.
The removal and capture of CO
2
from gas streams can be
achieved by a range of separation techniques depending
on factors such as CO
2
concentration, pressure and
temperature. These separation technologies are based
on various physical and chemical processes, including
absorption into a liquid solution system, adsorption onto
a solid, cryogenic separation and permeation through
membranes. The technologies for large-scale carbon
capture from fossil fuel power plants and industrial
processes are relatively mature, but have yet to be
applied on the enormous scale needed for the Power-
to-X sector. One has also to keep in mind that most of
these sources are not renewable or sustainable sources
of CO
2
; they still rely on fossil fuels.
Biomass can provide some of the needed renewable
CO
2
though BECCS and BECCU plants. Due to the ease
of obtaining inexpensive high-purity CO
2
, bio-ethanol
production facilities currently represent most of the BECCS
and BECCU units in operation (Consoli, 2019). However,
as can be seen in Figure 30, which displays estimates for
CO
2
capacity from various renewable sources, the amount
of CO
2
available from these sources is limited (Olsson et
al., 2020). Biogas, pulp and paper and waste-to-energy
plants could also provide additional amounts of CO
2
.
Other technologies to capture CO
2
from large power
plants producing electricity by burning biomass are under
development as well. However, given the amounts of CO
2
required in the long run, CO
2
capture from the atmosphere
will also have to be implemented.

RENEWABLE METHANOL 55
So-called direct air capture (DAC) technologies are
being developed by a number of companies, including
Climeworks, Carbon Engineering and Global Thermostat
(Sanz-Pérez et al., 2016; Goeppert et al., 2012). CO
2
capture from air is conducted at ambient temperature
using various CO
2
sorbents. The captured CO
2
is then
released in its concentrated form (up to 100%) by
heating the sorbent to a temperature high enough to
liberate the CO
2
, which can then be used for methanol
synthesis. Although DAC technologies are still relatively
new (TRL ~4-7 depending on the technology), they are
improving rapidly. DAC oers a number of advantages
compared to point-source capture. Air oers an almost
inexhaustible source of sustainable CO
2
that is available
anywhere on earth. The DAC plants are thus independent
of emission point sources and could be placed anywhere
to allow the capture of CO
2
.
Looking at Figure 30, which displays a proposed estimate
and distribution of global CO
2
availability, it would
seem that the amounts of renewable CO
2
potentially
available should allow the production of millions of
tonnes of e-methanol per year. However, these are
estimates of CO
2
availability for all uses, including CCS
and CCU, and for all products such as e-fuels including
e-methanol, e-kerosene and e-gasoline. E-methanol
production is therefore likely to require the use of CO
2
from all available renewable sources, in an “all of the
above” type approach, and not just the cheapest ones
(bioethanol and biogas) for which there will be more
competition and limited capacity. Ultimately, DAC oers
greater potential. The situation with CO
2
resources and
e-methanol is similar to the one for biomass and bio-
methanol, for which there will likely be competition for
the cheapest biomass feedstock.
Table 9. Selection of renewable and non-renewable sources of CO
2
1
Source or technology
CO
2
concentration in
exhaust or gas stream (%)
CO
2
concentration
after treatment (%)
Biomass to ethanol Up to 100
Up to 100 Renewable CO
2
Biomass combustion 3-8
Biomass gasification 20-90
Biogas 40-50
BECCS/BECCU Close to 100
DAC* 0.042
Coal power plant 12-14
Up to 100 Non-renewable CO
2
Coal power plant with
oxy-combustion
Close to 100
Natural gas power plant 3-5
Iron and steel plant 20-30
Cement plant 15-30
Natural gas purification 2-65
Ammonia synthesis Up to 100
* DAC produces renewable CO
2
only if powered by renewable energy.

Figure 30. Example of estimates for global renewable CO
2
availability from dierent sources by the
middle of the 21st century
Source: Based on Olsson et al. (2020).
INNOVATION OUTLOOK: 56
3
The capital investment for a methanol synthesis unit
using CO
2
and H
2
is estimated to be about the same
as that for a conventional syngas-based plant. The
technology to produce methanol is thus already mature
and very similar to the one used in traditional fossil fuel-
based plants. Overall, the plant will produce e-methanol
with >99% yield and selectivity. The reaction of CO
2
with hydrogen is exothermic (releases energy) and the
heat of the reaction can be used to provide other plant
services such as distillation. When relying on fluctuating
renewable energy to produce the necessary H
2
, some
load-following capability for the methanol production
unit would be advantageous, and also provide an
important energy storage service for the power grid
(CRI, 2020).
Compared to a conventional natural gas- or coal-based
methanol plant, the very energy-demanding and costly
reforming or gasification step is also eliminated, as is the
generation of waste products from this step (sulphur,
ash, NOx, PM, heavy metals, tars, etc.). In addition,
the lower by-product content of methanol produced
from CO
2
may simplify the methanol distillation step
(Pontzen et al., 2011). The overall eciency of methanol
production from electricity and CO
2
is about 50-60%.
This is largely due to the need to produce hydrogen
through water electrolysis.
An economical option that could be used to gradually
green the production of methanol would be to co-feed
CO
2
and renewable H
2
into a traditional methanol fossil
fuel-based plant. This would increase the know-how in
CO
2
capture and renewable H
2
technologies and allow
for a faster scale-up. Such an approach could also help
to absorb some of the fluctuation and intermittency of
renewable electricity.
The combination of bio- and e-methanol production
in one site also oers clear synergetic advantages by
proving a source of CO
2
for e-methanol production, and
a hydrogen source for the complete conversion of the
carbon contained in the biomass.
Estimated capture cost (USD/t CO
2
)
Estimated capacity (Mt CO
2/
yr)

RENEWABLE METHANOL 57
3.2. Renewable methanol vs
alternatives
Methanol has a number of advantages compared to some
other proposed renewable energy carriers, including
hydrogen, CNG/LNG, ammonia and batteries (Table 10
and Figure 31). Hydrogen gas has been proposed as an
energy storage medium and produces, besides energy,
only water when combusted. In practice, however,
because of its low volumetric density hydrogen requires
either compression to high pressures (350-700 bar) or
liquefaction at very low temperature (-253°C), making
its storage problematic and energy-intensive. It is also
highly flammable and explosive and can diuse through
many commonly used metals and materials.
The infrastructure needed to transport, store and
dispense hydrogen safely would therefore be very
expensive. LNG too requires cryogenic temperatures
for its storage (-162°C). If the space for the containment
is included in the comparison, the energy density of
methanol is comparable to that of LNG. Liquid ammonia
has either to be cooled down to -34°C or kept under
moderate pressure. Methanol, on the other hand, does
not need any refrigeration or pressurisation because it
is a liquid under ambient conditions.
The volumetric energy density of methanol is only about
half that of gasoline and diesel, but about three times
higher than compressed H
2
(700 bar) and two times
higher than liquid H
2
. One litre of methanol actually
contains more hydrogen than one litre of liquefied H
2
. An
often-proposed purely hydrogen-based economy would
require massive investment, and the construction of a
costly and specialised infrastructure that does not exist
presently. As a liquid fuel, methanol is relatively easy to
handle and does not need highly specialised equipment
for its transport, storage and distribution. With minor and
inexpensive modifications, the current infrastructure can
be adapted to methanol, enabling a smooth transition
to the use of renewable methanol. Renewables-based
gasoline and diesel equivalents can also be produced,
but the process is more complicated and the energetic
cost higher than for renewable methanol (Kramer, 2018).
Methanol itself can be converted to gasoline through the
well-developed methanol-to-gasoline process (MTG)
(IRENA, 2016a). However, the problems associated
with gasoline and diesel fuels such as PM, NOx and
hydrocarbon emissions would also remain.
While methanol can already be widely used today in
conventional ICEs, it can also act as a fuel for advanced
hybrid (methanol/electric) and FCVs. In that case
Table 10. Comparison of various fuel properties
1
Fuel type LHV (MJ/kg)
Volumetric energy
density (GJ/m
3
)
Storage pressure
(bar)
Storage
temperature (°C)
Methanol 19.9 15.8 1 20
DME 28.9 19.2 5 20
LNG 48.6 20.8 1 -162
CNG 48.6 9 250 20
Liquid ammonia 18.6 11.5 1–10
-34 (at 1 bar)–20
(at 10 bar)
Liquid hydrogen 120 8.5 1 -253
Compressed hydrogen 120 4.7 700 20
Gasoline 43.4 32 1 20
Marine gas oil 42.8 36.6 1 20
Lithium ion battery 0.4-1 0.9-2.4 1 20
Notes: LHV = lower heating value; GJ = gigajoule; MJ = megajoule.

Figure 31. Volumetric energy content of various fuels
INNOVATION OUTLOOK: 58
methanol is reformed to hydrogen on board a vehicle;
it is then fed to a fuel cell to charge batteries in an EV, or
to provide direct propulsion in an FCV. In this case too,
the use of liquid methanol avoids the need for on-board
systems able to store hydrogen safely gas under high
pressure (350-700 bar) in FCVs. To date, methanol is the
only liquid fuel that has been demonstrated on a practical
scale in fuel cell-based transport applications. An added
benefit of using methanol is that the same fuel can power
both conventional ICE vehicles and FCVs, leading to a
seamless transition to these more advanced powertrains.
Batteries are already being used for applications in
the transport sector. Most of the current progress on
battery vehicles is for passenger cars and light-duty
vehicles. As battery technology continues to develop
with improved performance and energy density, car
manufacturers are already introducing into the market
battery-powered buses and heavy-duty trucks. In the
shipping sector, some applications of electric ferries are
available, and in the aviation sector small electric aircraft
for short-haul flights. However, electrification of long-
distance maritime shipping and aviation with existing
battery technology seems to be more challenging. For
those applications, bio- and electro-fuels could play an
important role (Moser et al., 2018; IRENA, 2018).
The Research Association for Combustion Engines
(FVV) has conducted a study of e-fuels’ potential
in Germany. It determined that the e-fuels oering
the lowest mobility cost for cars and trucks were
e-methanol, e-DME and e-methane (Kramer, 2018).
FT fuels, H
2
and even battery electric mobility costs
were all higher. The cost calculations included the
production of the fuel, distribution infrastructure,
vehicle cost, etc. However, the result depends on the
availability of cheap feedstock such as biomass, green
hydrogen and renewable CO
2
. Another study identified
that e-fuels would be suitable only for sectors such
as aviation and shipping where no alternatives are
available due to the lower overall eciency when used
in a car or trucks (Calvo Ambel, 2017; Malins, 2017).
Methanol, as with any other alternative fuel or chemical,
also has drawbacks. Like gasoline, ethanol and hydrogen,
methanol is highly flammable and can lead to explosions
Energy content (GJ/m
3
)
Compressed hydrogen gas

RENEWABLE METHANOL 59
if stored or handled improperly. Methanol is also toxic
and can be lethal if ingested. It can absorb moisture from
the atmosphere, which can lead so phase separation in
methanol/gasoline blends. Methanol is corrosive to some
metals and is incompatible with some plastics, resins and
rubber. Thus, compatible metals, plastics and elastomer
materials should always be selected (details about the
pros and cons of methanol can be found in Annex 1).
3.3. Emissions and sustainability
Emissions
A main advantage of biomass- and CO
2
-based
methanol production is the reduction in overall CO
2
GHG emissions. For a complete life-cycle analysis
(LCA), also called cradle-to-grave analysis, all steps
of methanol production, distribution and use have to
be taken into account, addressing the environmental
impacts of each of these steps, including GHG emissions,
other pollutant emissions (NOx, CO, particulates, SOx,
etc.) and water use. These depend on a large number
of parameters including the nature of the feedstocks,
by-product generation, processes applied, how the
product is used, and so on. This makes the determination
of an actual set of numbers to compare with the overall
environmental impact of other fuels and feedstock
somewhat challenging. Nevertheless, such analyses
will be increasingly needed to assess the environmental
impact of various fuels/materials and processes.
The industrial sector, currently accounting for about a
third of global CO
2
emissions, has been identified as
one of the areas that will be challenging to decarbonise/
defossilise (IRENA, 2020b). In the chemical/
petrochemical subsectors relevant to methanol and its
derived products, improvements in energy eciency,
electrification and replacing fossil energy input with
renewable energy can greatly reduce the carbon intensity
of their processes. In that context, electric reforming of
natural gas to produce LCM is one option. However, to go
further, the chemicals and materials produced need to be
themselves progressively defossilised through the use of
renewable feedstocks (green hydrogen, renewable CO
2
,
biomass, etc.). This should allow related CO
2
emissions
to decrease over time to eventually reach net-zero
emissions by the end of the century. By following this
greening path, methanol and all the chemicals and
materials derived from it (including formaldehyde,
DME, MTBE, acetic acid, plastic, solvents) would thus
become carbon neutral. Of course, levels of CO
2
and
other emissions would have to be verified by rigorous
LCAs for all of these processes.
In the transport sector numerous studies have been
conducted to determine the level of emissions of
various fuels. So called well-to-wheel (WTW) analyses,
in particular for the use of methanol, DME and other
fuels, have been performed and generally focus on
GHG emissions and overall energy eciency of the fuel
pathways. The WTW analysis itself can be divided in two
individual steps: well-to-tank (WTT) and tank-to-wheel
(TTW) analysis. The WTT focuses on the extraction of the
raw materials, production of the fuel and its distribution to
the vehicle. The TTW accounts for the utilisation of the fuel
in the vehicle, i.e. the conversion of the chemical energy
contained in the fuel to kinetic energy in the power train.
Relative to conventional fuels on a WTT basis, producers
estimate that renewable methanol offers carbon
reduction benefits ranging from 65% to 95% (Law et al.,
2013). These GHG benefits were among the highest for
alternative fuels that can displace gasoline and diesel.
For the TTW portion of the full fuel cycle, methanol as
a transport fuel can also oer advantages. Methanol
has a higher octane number than gasoline (RON+MON
average of 100),
1
allowing higher compression ratios that
result in more ecient use of energy in an appropriate
engine, translating into lower tailpipe emissions of CO
2
for the same power output. Methanol/gasoline blends
also have considerably higher octane numbers than
gasoline alone and were found to reduce CO
2
emissions
as well (Sileghem et al., 2014; Turner and Pearson, 2011).
Furthermore, methanol is cleaner burning than regular
gasoline, reducing the emission of other pollutants
(PM, NOx, SOx). Methanol can also be used in diesel
engines equipped with glow plugs and newly developed
“methanol engines”, and even more advanced vehicles
propelled by fuel cells, reducing further the tailpipe
emissions (Olah et al., 2018, Schröder et al., 2020).
When used as a marine fuel, the SOx, PM and NOx
emissions decreased by more than 99%, 95% and
1 RON = research octane number; MON = motor octane number.

INNOVATION OUTLOOK: 60
60-80%, respectively, compared to fuel oil (Dolan, 2020;
MI, 2020b; Andersson and Márquez Salazar, 2015; DNV
GL, 2016). Comparing various biomass sources for the
production of methanol, it was determined that the
WTW CO
2
equivalent emissions of black liquor were 3-12
gCO
2
-eq/MJ, wood waste were 5.3-22.6 gCO
2
-eq/MJ,
and farmed wood (wood obtained from tree plantations)
were 4.6-16.5 gCO
2
-eq/MJ. The results depended on
the studies as shown in Table 11 and Figure 32 (see also
Schröder et al., 2020), and do not include land use
change or indirect land use change GHG emissions.
Methanol from crude glycerine and biogas had
somewhat higher emissions, at 30.6 gCO
2
-eq/MJ and
30-34.4 gCO
2
-eq/MJ, respectively. The WTW CO
2
emissions of methanol from CO
2
recycling and H
2
from
renewable sources was estimated at 1.74-33.1 gCO
2
-eq/
MJ, depending on various assumptions. Compared to
a reference fossil fuel emission of 83.8 gCO
2
-eq/MJ
for gasoline (EU, 2009), this is a substantial decrease.
Vulcanol, produced from geothermal CO
2
and green
hydrogen and sold by CRI, lowers GHG emissions by
up to 90% compared to gasoline (CRI, 2020). Methanol
from black liquor and farmed wood reduced WTW CO
2
emissions by up to 96% and 95%, respectively. Using
the GREET model, a 93% decrease in CO
2
-eq emissions
was also determined for methanol produced from
biomass (Wang and Lee, 2017). The WTW CO
2
emissions
reduction of methanol from CO
2
capture and recycling
was estimated at up to 98% compared to gasoline and
diesel. As such, these routes to methanol already fulfil
the emission-saving requirements for biofuels in the
European Union, which require all biofuels to achieve
a GHG emission reduction initially set to at least 35%
compared to the emissions of 83.8 gCO
2
-eq/MJ
from a fossil fuel reference. These emission reduction
requirements were gradually increased to 50% in 2017
and 60% in 2018.
Volvo also found that the WTW GHG emissions were
reduced by about 90% for methanol and 95% for DME
when these fuels were produced from black liquor. Similar
results were reported by the European Commission Joint
Research Centre, Institute of Energy-EUCAR-CONCAWE
collaboration (JEC), which published a series of studies
on GHG emissions from a large number of conventional
and alternative fuels, production routes and powertrains
(Edwards et al., 2011; Edwards et al., 2014). The reports
showed that, for example, for a diesel motor
2
the WTW
emissions were reduced from 145 gCO
2
-eq/km for
regular diesel to 5 gCO
2
-eq/MJ for DME from black
liquor, a reduction of 97% (Edwards et al., 2011).
For DME from waste wood and farmed wood, the
reduction was 94% and 92%, respectively. This is well
below the 95 gCO
2
-eq/km needed to comply with the
proposed EU regulation on GHG emissions from new
passenger cars for 2020 (EU, 2012a). It is also well below
the emissions of DME from coal and natural gas, the
latter being on a par with gasoline and diesel emissions
on a WTW basis. Methanol was not part of this study, but
from a production eciency point of view, DME (which
is dehydrated methanol) and methanol are very close to
each other. The energy eciency for the conversion to
methanol is actually slightly higher than that for DME.
As regards energy consumption, the most energy-
ecient biomass-to-DME route according to Edwards
(2011) is the one based on black liquor gasification. This
route has a value slightly below 200MJ/100km. Black
liquor is the large internal energy stream generated
during wood pulp production, which is normally
combusted in a so-called recovery boiler to generate
power and heat and recover the cooking chemicals. The
energy requirement in a mill with DME production is
met by the installation of an ecient biomass-fed boiler
producing heat and power. This boiler has much higher
energy eciency than the recovery boiler, which is the
main reason for the overall high energy eciency of the
concept. Energy eciency is calculated as DME energy
produced divided by added extra biomass energy
needed to bring the new mill with DME production to
the same overall net energy balance as before adding
DME production (Ekbom, 2003). The direct biomass
gasification route is around 250MJ/100km. This can
be compared with cellulosic ethanol, which has energy
consumption of around 300-500MJ/100km and emits
30-40 gCO
2
-eq/km.
Notably, various biogas routes have strongly negative GHG
emissions (in this case meaning highly favourable). This is
due to the high global warming potential of methane and
the fact it would be emitted to the atmosphere if not used
as fuel. However, they are large users of energy – more
2 DICI 2010 no DPF: 2010 direct injection compression ignition engine with no diesel particulate filter.

RENEWABLE METHANOL 61
than twice as much as the most ecient biomass to DME/
methanol cases described. For heavy-duty buses using
DME in compression engines and methanol combined
with fuel cells, WTW reductions in GHG emissions of
94% and 96% were calculated, respectively. In this case
methanol and DME were obtained from poplar trees
(Pont, 2007). In the case of ships, GHG emissions were
also considerably reduced when bio-methanol was used
instead of heavy fuel oil. Depending on the biomass
source and process, reductions of 80% to over 95% were
determined (Brynolf et al., 2014; Balcombe et al., 2019).
As we move forward, the increased use of biomass and
recycled CO
2
with H
2
from renewable energy will make
carbon fuels increasingly carbon neutral and renewable.
Eventually, CO
2
contained in the atmosphere – either
recycled directly or through biomass – will be our
predominant source of carbon, solving the problem of
excess emissions of this GHG.
Table 11. GHG emissions of methanol from various sources, ordered by feedstock type
Resource type Feedstock
Original
system
boundaries
Raw material
to final use
GHG emitted in
gCO
2
eq/MJ*
Source
Biomass-based
Farmed wood (A) 12
Majer and Gröngröft,
2010
Farmed wood (A) 16.5
RED II, Annex V, 2018
(EU, 2018)
Farmed wood
(current to near term)
(A) 7.3 Chaplin, 2013
Farmed wood
(novel medium term)
(A) 4.6 Chaplin, 2013
Waste wood (A) 10
Majer and Gröngröft,
2010
Waste wood (A) 13.5
RED II, Annex V, 2018
(EU, 2018)
Waste wood (A) 16.1 Rönsch et al., 2014
Waste wood (A) 22.6 BLE, 2017
Waste wood (A) 5.3 Chaplin, 2013
Waste wood (A) 18.3
Ellis and Svanberg,
2018
Wood (D) 25 Kajaste et al., 2018
Wood chips (B) 20.91 Ecoinvent, 2019
Black liquor (A) 10.4
RED II, Annex V, 2018
(EU, 2018)
Black liquor (B) 12 Lundgren et al., 2017
Black liquor (A) 3 Chaplin, 2013
Black liquor (A) 5.7
Ellis and Svanberg,
2018
Crude glycerine (A) 30.6 Chaplin, 2013
Biogas (A) 34.4 Chaplin, 2013
Biogas (manure, crops) (A) 30
Majer and Gröngröft,
2010

INNOVATION OUTLOOK: 62
Power-based
Renewable electricity,
flue gas from biomass
plant
(B) 3.23
Buddenberg et al.,
2016
Renewable electricity,
CO₂ from ethanol plant
(A) 13
Matzen and Demirel,
2016
Renewable electricity,
CO₂ from biogas
process
(B) 0.5 Hoppe et al., 2018
Renewable electricity,
CO₂ from ethanol plant
(D) 21.3 Kajaste et al., 2018
Renewable electricity,
CO₂ captured from coal
power plant
(D) 33.1 Kajaste et al., 2018
Renewable electricity,
flue gas (geothermal
energy plant)
(A) 12.1 CRI, 2020
Renewable electricity,
flue gas from biomass
plant
(A) 1.74 Chaplin, 2013
Fossil-based
Natural gas (B) 101.6 Ecoinvent, 2019
Natural gas (C) 94 Kajaste et al., 2018
Natural gas (A) 91
Ellis and Svanberg,
2018
Natural gas (A) 94.4 Chaplin, 2013
Hard coal (B) 262 Ecoinvent, 2019
Hard coal (C) 219 Kajaste et al., 2018
Lignite (A) 170.8 Rönsch et al., 2014
1
* Raw material to final use GHGs in g CO
2
-eq/MJ calculated from the original system boundary:
(A) From raw material extraction until use phase; no correction needed.
(B) From raw material extraction until methanol production gate; add the RED II default value of 2.0 g CO
2
-eq/MJ for transport and distribution
of MeOH.
(C) From raw material extraction until methanol production gate; add the RED II default value of 2.0 g CO
2
-eq/MJ for transport and distribution
and the combustion emission of MeOH of 69 g CO
2
-eq/MJ.
(D) From raw material extraction until methanol production gate; corrected for CO2 emitted during methanol use 69 g CO
2
-eq/MJ ; add the
RED II default value of 2.0 g CO
2
-eq/MJ for transport and distribution of MeOH.

Figure 32. GHG emissions of methanol produced from various feedstocks (from feedstock extraction to
final use, values from Table 11)
RENEWABLE METHANOL 63
Sustainability and carbon neutrality
The production of methanol from natural and
anthropogenic sources, including biomass and the
recycling of CO
2
from flue gases of various industries,
could be the first step towards an anthropogenic carbon
cycle. The removal of even a fraction of the CO
2
from
industrial emissions would result in the availability of
huge amounts of CO
2
. Using the CO
2
captured from fossil
fuel sources one more time to produce methanol instead
of simply releasing the CO
2
to the atmosphere could
potentially halve the emissions. This type of methanol
could be considered a low-carbon fuel.
This approach, however, does not provide a permanent
and sustainable solution. As fossil fuels become less
abundant and their use regulated by stricter emission
standards, related CO
2
emissions will eventually
diminish. And even though the carbon in CO
2
is used
one more time, it remains fossil carbon. Biomass can
help defossilise society. The amounts of biomass that
can be generated in a sustainable way are substantial,
but nevertheless limited, and are unlikely to be able
to cover all our needs (see Chapter 5). A combination
of bio- and e- methanol production could allow for
full utilisation of renewable carbon in the feedstock,
leading to a substantial increase in methanol production
from a given amount of biomass. The increase is more
than double compared to the conventional approach
without the addition of external renewable hydrogen.
CO
2
obtained from various other BECCS/BECCU units,
especially the ones that burn biomass for electricity
generation, could also be used in combination with
green hydrogen to generate e-methanol. However,
the limitations attached to biomass availability imply
that methanol and its derived products should also
be increasingly produced from CO
2
captured from
the air, which oers an inexhaustible carbon source
for humankind. The required energy will have to be
provided by renewable energy sources. This would
constitute an artificial version of nature’s CO
2
recycling
via photosynthesis, that is, a sustainable anthropogenic
carbon-neutral cycle (Figure 33). This is one of the
key concepts of the so-called methanol economy
(Goeppert et al., 2014; Olah et al., 2018) and also the
liquid sunshine concept (Shih et al., 2018).
0
300
250
100
200
50
150
GHG emissions (gCO
2
-eq/MJ))
Farmed
wood
Waste wood
and other
wood
Black
liquor
Crude
glyerine
Biogas Power-based
CO
2
+ H
2
Natural
gas
Coal and
lignite

Figure 33. Anthropogenic carbon cycle for a circular economy
Source: Olah et al. (2018).
INNOVATION OUTLOOK: 64
Methanol from non-renewable sources such as natural
gas and coal is already competitive from a cost
perspective with gasoline and diesel fuel (Figure 4).
It is also an essential feedstock for numerous chemicals,
materials and plastics. Hybrid systems using both
renewable and fossil fuels with fewer or no CO
2
emissions
to produce LCM could be used during the transition
period to a sustainable future. LCM could thus be part
of a bridge towards renewable methanol.
Once the infrastructure for the distribution and use of
methanol and LCM is in place, it could be seamlessly
shifted to sustainable renewable methanol in the future.
Fossil methanol and renewable methanol are the same
from a chemical point of view. Renewable methanol can
be a sustainable feedstock for many of the chemicals and
products currently obtained from petroleum, including
aromatic compounds (BTX) and plastics (polyethylene,
polypropylene) (Bazzanella and Ausfelder, 2017).
Catalytic or
electrochemical
CO
2
reduction and
transformations
CO
2
+ H
2
O
g
Atmospheric CO
2
Fuel uses
CO
2
+ 2H
2
O
CH
3
OH+3/2 O
2
g
Gasification
to syngas
H
2
+CO+CO
2
and other
transformations
O
2
+
Biomass
Photosynthesis
CO
2
+ H
2
O
h
O
2
+ Biomass
Methanol and DME
Synthetic
hydrocarbons
and their products
H
2
generation
from H
2
O
Electricity
e
-
CO
2
Capture
CCS
CO
2
CH
3
OH
DME
g
CO
2
g
Carbon capture
and recycling
CCR
CO
2
from point sources:
power plants, industries, etc.
Sun energy
CO
2
Storage
Solar
Wind
Hydro
Geothermal
Oceans
Atomic
energy
Renewable energy

RENEWABLE METHANOL 65
The cost of bio- and e-methanol produced from renewable
sources depends on a variety of factors, including
feedstock, choice of technology, energy demand,
production capacity, operating conditions, desired purity
of the product and availability of tax incentives.
4.1. Bio-methanol costs
Methanol production from biomass and MSW
via gasification
For bio-methanol the methodology applied in this report
to determine the cost of production is similar to that
used and accepted by a large number of stakeholders.
This was confirmed during the process of assembling
and arranging information used as the basis for the
“Cost of Biofuels” report by the Sub Group on Advanced
Biofuels (SGAB) (Maniatis et al., 2018), a group under the
Sustainable Transport Forum (EU STF, 2019).
3
The cited report was used as a basis for a project
presented in a report named “Advanced Biofuels
– Potential for Cost Reduction” (Brown et al., 2020).
The information on projects utilising thermal conversion
of biomass in these two reports was updated and
adjusted for this report and the same approach to
estimate production costs for various biofuels applied.
The method identifies the CAPEX contribution, the OPEX
contribution (excluding feedstock) and the feedstock
contribution. The CAPEX was calculated using data from
projects that were under construction where such data
were available. Costs were sometimes based on the cost
estimates for projects similar to that being investigated.
In this report, the investment intensity is presented as
USD/t/y as one product is in focus. In some cases where
methanol is not the product, USD/kW of product has
been added in order to be able to compare against a
common base – energy. When comparing investment
intensity, the size of various plants is an important
consideration. CAPEX is seen as equal to the overnight
investment cost for building the plant and no costs
for interest during construction or working capital are
added. The capital recovery charge is composed of an
annual cost estimated as a levelised annual capital cost
(based on an annuity loan using a real interest rate of 10%
for 15 years, i.e. a factor of 13.2%, expressed as CAPEX
per year or CAPEX/y). Elements of a fully elaborated
project economic model, such as level of grant support,
debt-to-equity ratio, loan repayment grace period and
amortisation periods, are not included.
OPEX, less feedstock, is expressed as an annual
percentage of CAPEX or as a percentage of the
production cost. The percentage includes co-feeds,
labour, feedstock-associated costs on the site,
maintenance and by-product disposal. When available,
relevant data from project estimates were the basis for
the percentage or other figures used.
The feedstock cost contribution is estimated from the
performance data and feedstock cost.
The production cost is estimated as the sum of the capital
recovery charge, OPEX and feedstock procurement costs
on an annual basis divided by the production output.
During Q2-3 of 2020 a number of project developers
and plant owners were contacted for information,
as specified in Table 4. Table 12 and Table 13 present
relevant information received, from which the CAPEX
element in the production cost can be specified. Table 12
lists projects that have specified methanol as an end
product, and Table 13 other gasification-based projects.
4. CURRENT COSTS AND COST
PROJECTIONS
3 The STF was formed in 2015 as a vehicle to implement the so-called Alternative Fuel Infrastructure Directive 2014/94/EU (EU,
2014). The forum has members from all EU member states plus about 40 specialists and is headed by DG MOVE.

INNOVATION OUTLOOK: 66
Projects listed in Table 13 do not produce methanol.
The feedstocks and production pathway via syngas
and then a synthesis plant to a product are, however,
similar to a plant configuration producing methanol.
Syngas generation, conditioning and cleaning comprise
the major part of the overall investment regardless of
the final product. Therefore, if investment per unit of
production capacity (USD/kW) is compared between the
two tables, a relevant comparison can be made. There is,
however, a need to include the potential eect of larger
or smaller investment in the synthesis unit (e.g. methanol
versus FT products), as well as the overall conversion
efficiency from feedstock to product. This is further
discussed below.
Table 12. Capital cost for bio-methanol plants
1
# Project/ study Status
Capacity
(t/y)
Investment
(million
USD)
Investment
(USD/t/y)
Investment
(USD/kW)
Source
1
Trans World
Energy (TWE),
Florida (US)
FEED done,
start-up Q2
2023
875 000 430 490 710 TWE
2
ENI Refinery,
Livorno (IT)
Basic
engineering
ready Q3
2020
115 000 330 2900 4280 NextChem
3
LowLand
Methanol (NL)
Start-up early
2023
120 000 130 1110 1620
LowLand
Methanol
4 Södra (SE) Operational 5000 11 2220 3230 Södra
5
Enerkem,
Rotterdam (NL)
Engineering 215 000 580 2690 3840 Enerkem
6
Enerkem,
Tarragona (ES)
Engineering 215 000 580 2690 3840 Enerkem
7 VTT
Detailed
study
265 000 385 1450 2070 VTT
8
Chemrec,
Domsjö (SE)
Preliminary
engineering
147 000 390 2640 3400 Chemrec
9
Chemrec, nth
plant
Concept 290 000 540/270* 1880/930* 2740/1370* Chemrec
10
New Hope
Energy, Texas
(US)
Investment
decision Q4
2020
715 000 500 700 1020
New Hope
Energy
* This investment is credited for the avoided investment in a new recovery boiler.

RENEWABLE METHANOL 67
Capital cost element of total production cost
CAPEX was converted into an investment intensity,
expressed as an average value with an interval of
+/- 20% and was expressed as USD/kW of product
capacity to allow comparison of CAPEX for various
projects with dierent products. The capital cost range
was then compared and adjusted in a conservative way
with other studies such as Brown et al. (2020) and
Maniatis et al. (2018). The range of investment cost
for a biomass-fed plant is assumed to be 1560-2220
USD/t/y and for MSW-based projects to be 2000-
2780 USD/t/y. The relative investment for MSW-based
projects was higher, but these plants are normally at a
smaller scale, in the range of 100000 t/y of methanol
compared to 200 000-250 000 t/y for biomass-based
projects, explaining why a higher relative investment
should be expected.
The new and updated data presented in Table 12
and Table 13 was obtained from various information
providers. There are, however, exceptions such as the
large Trans World Energy and New Hope Energy projects
in Table 12. These should clearly have relative investment
at the lower end of the proposed interval due to the
eect of economies of scale, but the investment numbers
provided are even below that. Low relative investment
can also be observed for the LowLand Methanol project
in the same table. This has partly to do with the fact that
a large proportion of the methanol production comes
from imported hydrogen (the investment-intensive
gasification part of the project is thus correspondingly
smaller). It also has a number of advantages in the form
of easily accessible utilities.
As explained between the two tables, projects can be
compared on a “cost per kW of product capacity” basis,
but taking into account various specific circumstances
for each referenced project.
Comparing the projects in Table 12 and Table 13 on such a
USD/kW basis (noting that tonnes of aviation fuel cannot
be compared with tonnes of methanol) shows that:
•
The Enerkem Edmonton plant (Table 13), which
produces methanol from MSW for further conversion
Table 13. Capital cost for gasification-based plants for other products
1
Project/product Status
Capacity
per year
Investment
(million
USD)
Investment
(USD/kW)
Source
1
Enerkem, Edmonton
(CA)/ethanol
Operational 30000 t 87 3110 Enerkem
2
Enerkem, Quebec
(CA)/ethanol
Announced/
construction
35000 t 78 2800
Public
domain
3
Fulcrum (US)/FT
liquids (jet fuels)
Start-up Q4
2020
40000 m
3
200 4560
Public
domain
4
Red Rock Biofuels/
FT liquids (jet fuels)
Under
construction,
start-up 2021
58000 m
3
355 5560
Public
domain
5 E.On/SNG Planned 1600 GW 470 2280 E.On
Note: SNG = synthetic natural gas.

INNOVATION OUTLOOK: 68
to ethanol, has a relatively low relative investment
in USD/kW of ethanol despite being of small size. If
feedstock preparation were included, this investment
level would increase to about USD4220/kW.
•
For the E.On. project (Table 13) bioSNG production
corresponds to about 250000t/y of methanol
production and falls in the middle of the interval
presented for this size of methanol plant.
•
The Fulcrum and Red Rock Biofuels projects (Table
13), both aiming to produce FT products, are
comparably small plants with a production capacity
corresponding to less than 100000t/y of methanol
equivalents. They also have a lower conversion
efficiency, which affects the relative investment
negatively, as do the additional upgrading units
needed in order to produce saleable products for
the market. Their data points at USD4440-5560/
kW are therefore not surprisingly high.
•
In the first referenced Chemrec project (Table 12) the
investment does not include a credit for avoiding the
investment in a replacement for the current recovery
boiler. It carries the cost of the boiler replacement.
This was agreed because that project would be the
first of its kind and the pulp mill thus acts as a test
mill for a new technology.
•
The second referenced Chemrec black liquor project
(Table 12) will in its commercial application (n
th
plant
case) be credited for avoiding the investment in a
replacement for the current recovery boiler. This
implies that the net investment has decreased by
approximately half.
•
With a specific investment for plants utilising biomass
feedstock of 1560-2220USD/t/y and based on a
capital cost per year corresponding to 15 years and
10% (annuity percentage of 13.2%), the result is a cost
of capital element in the product cost of USD206-
293/t or USD37-53/MWh of methanol (Table 14).
•
For MSW-based projects with investment in the range
of 2000-2780USD/t/y, the cost of capital in the
product cost is USD264-367/t or USD48-66/MWh.
Feedstock cost element of total
production cost
The energy conversion efficiency for biomass to
methanol is in the order of 60% (based on the feedstock
LHV at the plant gate). In the special case where black
liquor in a pulp mill is gasified and converted to methanol
and the black liquor energy is compensated by biomass
fed to a utility boiler on the site, the overall eciency
may reach around 70% (ratio of added biomass to
produced methanol). For MSW projects the conversion
eciency is generally somewhat lower, around 50-60%.
Cost of feedstock varies considerably depending on
the location of the facility and type of feedstock. Figure
34 shows a global supply curve for primary biomass
(IRENA, 2014). Domestic biomass feedstock cost ranges
from approximately USD3/GJ for processing residues
in Africa to USD17/GJ for energy crops. The lowest
feedstock cost of below USD5/GJ can be found with
MSW and processing residues. The medium cost group
between USD5 and USD8/GJ consists of harvesting
residues. And higher costs are mostly found in energy
crops and forestry products.
Table 14. Capital cost element in production cost
CAPEX/y
From biomass From MSW
Low High Low High
USD/t MeOH 206 293 264 367
USD/MWhMeOH 37 53 48 66
USD/GJMeOH 10.4 14.7 13.3 18.4

Figure 34. Global supply curve for primary biomass, 2030
Source: IRENA (2014).
RENEWABLE METHANOL 69
In Europe and the UnitedStates a typical price of woody
biomass at the plant gate is EUR50-100 per dry tonne
(USD3-6/GJ) according to Brown et al. (2020). In the
southern UnitedStates, parts of Canada and in Brazil the
price can be even lower, in the order of EUR25-50 per
dry tonne (USD1.5-3/GJ).
The price of EUR20/MWh (USD6/GJ) is indicated
in Figure 34 to illustrate the above-referenced price
levels. It is also used as a threshold when describing the
total production cost of bio-methanol, illustrating the
feedstock part of the total production cost. About 40%
of the feedstock potential would be available below this
price level.
Table 15 shows the cost of the feedstock element in
the total production cost as a function of the energy
conversion eciency. In some cases, the feedstock may
even come with a credit at that point. This potential
credit is not included in the production cost estimates.
Table 15. Feedstock cost element in production cost
Feedstock
cost
USD/GJ
feedstock
Conversion eciency, feedstock to methanol, %
50 60 70
USD/GJ MeOH USD/t MeOH USD/GJ MeOH USD/t MeOH USD/GJ MeOH USD/t MeOH
15 30.0 597 25.0 498 21.4 426
10 20.0 398 16.7 332 14.3 284
6 12.0 239 10.0 199 8.6 171
3 6.0 119 5.0 100 4.3 85
1.5 3.0 60 2.5 50 2.1 43
EUR 20/MWh
Supply cost (USD/GJ)
Potential (EJ)
Energy Crop
Harvesting residue
Processing residue
Biogas
Fuel wood
Logging residue
Wood waste
0
5
10
15
20
0 10 20 30 40 50 60 70 80 90 100 110 120 130 140
Domestic supply
Export potential

INNOVATION OUTLOOK: 70
Table 16. OPEX (excluding feedstock) cost element in production cost
Biomass as feedstock MSW as feedstock
Low High Low High
CAPEX, USD/t MeOH/y 1560 2220 2000 2780
OPEX Low 5%
USD/t
MeOH
78 111 100 139
OPEX High 10%
USD/t
MeOH
156 222 200 278
OPEX (excluding feedstock) element
of total production cost
Operating costs other than feedstock (such as utilities,
catalysts, chemicals, operations and maintenance)
are often not specified for projects at various stages
of planning, and when plants are operational OPEX
information is not provided for commercial reasons.
Available information is often aggregated and expressed
as an annual percentage of total investment cost (CAPEX).
Based on various sources contacted during the course
of developing this report, low numbers are in the 5-6%
range and high are about twice as much, 9-10%. For waste
gasification, the specific investment cost is higher, and
therefore the lower percentage still seems reasonable
as the operating cost contribution, expressed per tonne
per year, is higher than that for biomass feedstock. This
reflects, for example, the added cost for treatment of
higher levels of contaminants in the feedstock, together
with disposal of ash and other secondary wastes. The
variation in OPEX costs are summarised in Table 16.
Total methanol production cost from
biomass and MSW
Adding the three cost elements from Table 14, Table
15 and Table 16 together provides the total production
cost for methanol from biomass and MSW for various
cases, including low and high costs for investment,
feedstock and OPEX. These are put together in Table
17. As can be expected the interval between the most
cost-eective cases and the most expensive is quite
large. Low production cost cases are around USD300/t,
increasing to about USD600/t for high CAPEX, high
OPEX and feedstock at USD6/GJ. It increases further
to about USD1000/t at a feedstock cost of USD15/GJ
combined with high CAPEX and OPEX.
Potential production cost reduction for
methanol from biomass and MSW
With respect to the potential for cost reduction, the
CAPEX part of the equation, first and foremost, can be
influenced to a noticeable degree. Low-priced feedstock
is already part of the cost interval above and it is not
likely that other OPEX costs can decrease much below
the 5% of CAPEX per year, which is the low number used
in the calculations.
The interval given for overall energy efficiency also
includes future developments and the cost of the
feedstock element in the total production cost would
therefore not be expected to decrease due to this.
CAPEX can, however, expect to be influenced over time
by the well-known learning curve mechanisms such
as process improvements, improved and more (cost-)
eective plant configurations and plant size (economies
of scale). In the Brown et al. (2020) report regarding
potential cost reductions, this long-term potential is
quantified as 20-30%.
The capital burden in the production cost is based on an
internal rate of return (IRR) of 13.2%, corresponding to
15 years and 10% annuity financing of the total capital. In
the long term, when the technology is well-known and
risks have been mitigated through extensive learning
experiences, the cost of capital may come down.
RENEWABLE METHANOL 71
Table 17. Total production cost for bio-methanol from biomass and MSW
1
Biomass as feedstock MSW as feedstock
Low High Low High
CAPEX/y, USD/t MeOH 206 293 264 367
Overall conversion eciency, % 60 70 60 70 50 60 50 60
Feedstock cost
element for
methanol at
various level,
USD/t MeOH
At USD 15/GJ 498 426 498 426 - - - -
At USD 10/GJ 332 284 332 284 - - - -
At USD 6/GJ 199 171 199 171 - - - -
At USD 3/GJ 100 85 100 85 119 100 119 100
At USD 1.5/GJ 50 43 50 43 60 50 60 50
At USD 0/GJ
(a)
- - - - 0 0 0 0
OPEX at 5%, USD/t MeOH 78 111 100 139
OPEX at 10%, USD/t MeOH 156 222 200 278
Cost of
methanol
(USD/t
MeOH)
Feedstock cost below
USD6/GJ
327-561 447-714 414-583 556-764
Feedstock cost at
USD6-15/GJ
455-860 575-1013 - -
Carbon
credit
(USD/t
MeOH)
At USD 50/t CO
2
(b)
-82 -82 -82 -82
At USD100/t CO
2
(b)
-164 -164 -164 -164
(a)USD0/GJ for the feedstock being fed to the MSW gasifier is indicative and not used in the cost estimates.
(b) The carbon credit per tonne of bio-methanol is based on the dierence between the average CO
2
-eq emissions from methanol production
from natural gas (95.2 g CO
2
-eq/MJ) and the average CO
2
-eq emissions from bio-methanol production from renewable CO
2
and H
2
(12.7 g
CO
2
-eq/MJ) given in Table 11. Considering an LHV of 19.9 MJ/kg for methanol, this corresponds to 1.64 t CO
2
-eq of emissions avoided per tonne
of bio-methanol, compared to the traditional natural-gas based methanol.
If the capital part of the production cost were based
on an IRR of 10.2%, corresponding to 20 years and 8%
annuity financing, the cost of capital would be 23% lower
than presented in Table 14.
The potential for learning curve and capital risk mitigation
cost reductions, if combined, lowers the capital cost
element (CAPEX/y) of the total production cost by
40-45% (40% is used in the table below), distributed
approximately evenly between the two identified cost
reduction elements. OPEX is related to investment and
is assumed to be reduced proportionally with the cost
of capital. The results of the cost reduction assumptions
are shown in Table 18 and in Figure 35.

INNOVATION OUTLOOK: 72
Table 18. Total production cost for bio-methanol after potential cost reduction
Biomass as feedstock MSW as feedstock
Low High Low High
Before cost reduction
USD/t MeOH
(from Table 17)
Feedstock
below USD6/GJ
327-561 447-714 414-583 556-764
Feedstock at
USD6-15/GJ
455-860 575-1013 - -
CAPEX/y reduction, USD/t MeOH -82 -118 -106 -147
OPEX reduction, USD/t MeOH -18 to -36 -26 to -51 -23 to -46 -32 to -64
Cost of methanol
(USD/t MeOH) at
feedstock cost
below USD6/GJ
With no carbon
credit
227-443 303-545 285-431 377-553
With a credit of
USD50/t CO
2
*
145-361 221-463 203-349 295-471
With a credit of
USD100/t CO
2
*
63-279 139-381 121-267 213-389
Cost of methanol
(USD/t MeOH) at
feedstock cost at
USD6-15/GJ
With no carbon
credit
355-742 431-844 - -
With a credit of
USD50/t CO
2
*
273-660 349-762 - -
With a credit of
USD100/t CO
2
*
191-578 267-680 - -
Figure 35. Estimated costs of bio-methanol up to 2050
* Please see the note in Table 17.
Bio-methanol cost (USD/t)
Biomass MSW
Feedstock at USD 6-15/GJ
Feedstock below USD 6/GJ
OPEX
CAPEX
1000
600
800
400
200
0
1200

RENEWABLE METHANOL 73
Cost reduction activities are closely related to operating
experiences and how gained knowledge is preserved and
used over time when new facilities are brought online
using the same (improved) process. It typically takes
at least fouryears from a preliminary project idea until
a plant is up and running. Thereafter at least a year of
operation is necessary before any real conclusions can
be made from gained experiences. Therefore, a scenario
describing the potential production cost savings, as
per above, against time is very much dependent on
the number of plants built over time. The word “plant”
used in this cost reduction section should rather be
understood as “plant generation”. After changes from
one generation to the next, decisions could be taken to
build multiple units to meet market demand and ensure
economic production.
Figure 36 illustrates a production cost reduction scenario
where 4 plant generations producing bio-methanol are
put into operation over about 15 years (2020-2035). The
upper limit for the cost of feedstock in this illustration is
USD6/GJ. Four or five dierent development pathways
are expected to be commercialised and reach maturity in
parallel eorts. Following this development, the potential
cost reduction according to data presented in Table 18 is
expected to be achieved.
Analogous with bio-methanol, Figure 37 presents the
corresponding cost reduction potential for MSW-based
plant generations and plant installations
The scenarios presented should be regarded as fast
tracks. They are built on the assumption that plants now
at an advanced stage of planning and under construction
(in one case operational) are the first generation of
plants, which will be followed by threeplant generations
of similar but improved design in the coming period up
till 2035-2040. In another, slower scenario where long-
term stable legislation for the introduction of advanced
fuels and chemicals does not materialise, the timeline
can easily become much longer.
Methanol production from biogas
Biogas is mostly used for power and heat generation.
Small quantities are upgraded to gas pipeline quality
(biomethane) and blended into the natural gas
network or mixed with natural gas in order to create
an automotive fuel with a low-blend renewable
component. In some countries, which for example do
not have a gas network, smaller volumes are handled
separately in tankers and used as 100% renewable
automotive fuel.
Figure 36. Potential production cost reduction for
bio-methanol from biomass within a
15 to 20 year timeframe
Figure 37. Potential production cost reduction for
bio-methanol from MSW within a
15 to 20 year timeframe
YEAR
NUMBER OF PLANT
GENERATIONS
NUMBER OF PLANTS
USD/
tonne
700
600
500
400
300
200
100
0
2020-2025 2035-2040
1 2 3 4
4 8 14 20+
H
H
L
L
USD/
tonne
700
600
500
400
300
200
100
0
2020
-2025 2035-2040
1 2 3 4
4 8 14 20+
800
H
H
L
L

Figure 38. Production cost for biomethane via gasification and via anaerobic digestion
Source: EBA (2020).
INNOVATION OUTLOOK: 74
At two locations in Europe biomethane is co-fed with
natural gas into existing methanol plants. In this way, the
product is a mix of fossil and bioderived methanol. The
renewable part is formally certified and can be traded as
a renewable commodity. For more details see Section 2.2.
A process plant converting methane to methanol
will function identically regardless of whether the
methane is of fossil or renewable origin. This means
that if an existing methanol plant replaces part of its
methane feedstock from fossil to renewable origin, only
the dierence in feedstock price will aect the final
production cost of methanol.
In 2019 non-household natural gas in Europe had an
average price of about EUR35/MWh (USD10.8/GJ)
(Eurostat, 2020). According to data provided to the
SGAB report (Maniatis et al., 2018), typical biomethane
production costs are in the range of EUR70-80/
MWh (USD21.6-24.7/GJ) when based on anaerobic
digestion. Large and modern gasification-based plants
are expected to reach similar production cost levels as
shown in Figure 38.
The feedstock price eect on overall production is shown
in Table 8. The impact on methanol production costs
when moving from natural gas to biomethane feedstock
is clearly substantial. In the example shown in the table,
it corresponds to an increase of USD377/t of methanol.
Corresponding calculations for the UnitedStates would
show an even larger difference because natural gas
prices there are generally lower than in Europe.
Production economics for a new installation are not
covered in this report. To install a small to medium-sized
plant in, for example, Europe, which would only be fed
with biomethane, would lead to a very high production
cost. The feedstock cost alone would be in the order of
USD700/t of bio-methanol, to which CAPEX and OPEX
need to be added.
An alternative to the above route via biomethane which
is currently being investigated by, for example, Haldor
Topsoe in Denmark is direct conversion of biogas in
an electrically heated biogas reformer to generate
syngas for further conversion to methanol. They call
their development eSMR Methanol
TM
(HT, 2019b).
EUR/MWh
OPEX
CAPEX
Biomass
1G Biomethane from
anaerobic digestion
2G Biomethane from gasication
3 MW
Digestion
plant 2015
2.0 Technos
without
optimised
integration 2016
2.0 Integrated
technologies
(Different types
of biomass)
2020
1.0 Technos
without
optimised
integration 2006
EUR 90/MWh
FT - Biomethane
UK - NL 2015
EUR 30/MWh
Natural gas price
France 2015
200
150
100
50

RENEWABLE METHANOL 75
A 10 kg per hour methanol demonstration plant is
planned to be operational in 2022. Haldor Topsoe claims
that its compact and modular design should result in
plants that can be built on a commercially attractive basis
at a scale 100 times smaller than today’s typical plant
sizes, and produce methanol at the same production cost
level as large fossil gas-fed plants.
Methanol as by-product from wood pulping
Methanol extraction from pulp mills is a niche pathway with
limited global capacity. Worldwide capacity is estimated to
be less than 1.5 Mt in more than 300 pulp mills.
As described in Section 2.2, there are very few references
for this conversion pathway. Only two have been
identified, one in Sweden and one in Canada. Methanol
is currently used in pulp mills as a green fuel, for example
in the lime kiln or in an on-site utility boiler. This means
that if such methanol had to be withdrawn from the mill
and sold as chemical-grade methanol it would have to
be substituted by another fuel. In most cases this fuel
would be an inexpensive biomass, but in other locations
a more costly lime kiln fuel could be needed.
Södra has provided some ocial data (Södra, 2020b).
The investment is estimated at about EUR10million
(USD11 million) and capacity is calculated to be 5250 t/y
of chemical-grade bio-methanol. If the same CAPEX
factor (IRR=13.3%) is used for this investment as earlier
in this chapter, the CAPEX element in the production
cost corresponds to EUR250/t (USD280/t). One tonne
of methanol provides about 5.5 MWh of combustion
energy and if this is substituted with biomass at
EUR10-20/MWh (USD3-6/GJ) it would add another
EUR55-110/t (USD60-120/t) to the OPEX. The process
of making pure methanol has a number of extraction
and distillation steps, which will lead to additional OPEX-
related costs. An approximate estimate for bio-methanol
production from the pulping cycle is shown in Table
20. It gives a production cost of about EUR490-720/t
(USD540-800/t).
Table 19: Impact of feedstock price in production of methanol from methane/biomethane
Biomethane
price
Feedstock cost in production cost of
methanol (conversion eciency 65%)
Impact on
production
cost
USD/GJ
biomethane
USD/GJ MeOH USD/t MeOH USD/t MeOH
Natural gas in
western Europe
10.8 16.6 329
+ 377
Biomethane 23.1 35.5 706
Table 20. Approximate production cost for bio-methanol from wood pulping
Cost element USD/t MeOH
CAPEX 280
Feedstock replacement 60-120
OPEX 200-400
Total 540-800

INNOVATION OUTLOOK: 76
4.2. E-methanol costs
In the short term, the production of methanol from
biomass and waste products seems to be the most
economic route in most locations. However, the available
amounts of biomass and derived materials, despite being
enormous, are also limited and will not be able to cover
global energy needs by themselves. The largest potential
for the production of renewable methanol remains with
the hydrogenation of CO
2
to methanol. Production from
CO
2
does not suffer the same feedstock availability
limitations as biomass or waste products.
To produce e-methanol sustainably from the CO
2
in
the waste gas stream and flue gases of industry and
electricity generation, or from atmospheric CO
2
, the
most mature and scalable method is the combination
of water electrolysis to produce H
2
and subsequent
catalytic methanol synthesis with CO
2
. The cost of
e-methanol produced by this route is highly dependent
on the cost of the raw materials: CO
2
and hydrogen. The
cost of hydrogen itself is closely linked to the cost of the
electrical power needed to produce it. To produce one
tonne of e-methanol, about 10-11 MWh of electricity are
needed, most of it for the electrolyser (~9-10 MWh), and
not including CO
2
capture.
As in the case of natural gas plants, some economies of
scale should be achievable, resulting in a lower cost per
tonne of methanol produced at larger plants. In principle,
there is no reason why renewable methanol plants
should not be the same size as conventional plants, as
the technology is the same regardless of the raw material
source. As with other large thermocatalytic processes
akin to fossil fuel methanol facilities, the methanol
synthesis unit and distillation unit can exploit the lower
production costs associated with economies of scale.
The electrochemical process of water electrolysis can
also benefit from cost reductions with increased module
size, and innovation to increase stack manufacturing may
have significant impacts on cost.
As a comparison, methanol from natural gas has a
production cost of between about USD100/t where
natural gas is the cheapest (Middle East, North America)
and USD300/t or more in Europe. The production cost of
methanol from coal, almost exclusively located in China,
is roughly between USD150 and USD250/t (McCaskill,
2019; Blug et al., 2014).
E-methanol production costs – A literature
review
A number of studies have been conducted on the cost of
producing methanol from CO
2
and H
2
. In 2007 a review
evaluated the cost of production for CO
2
-based methanol
as being between USD550 and UDS670/t (EUR500-
600/t) (Galindo Cifre and Badr, 2007). In the previous
version of this IRENA report, the production cost using
CO
2
captured either from flue gases or the atmosphere
was estimated at USD570-1000/t (EUR510-900/t)
(Clausen et al., 2010; Galindo Cifre and Badr, 2007; Kim
et al., 2011; Specht et al., 1998; IRENA and IEA-ETSAP,
2013). Similar estimates were also obtained in a more
recent paper that reviewed past studies, as well as other
publications on the subject (Hank et al., 2018).
An overview of these estimated production costs is
presented in Table 21. Overall the costs are roughly
between USD300 and USD1000/t of e-methanol,
with plant sizes ranging from 4000t/y to 1.8milliont/y
capacity. The lower estimates tend to have very low
electricity production costs or/and cross-subsidise the
price of methanol from the sale of oxygen co-produced
during the electrolysis (from USD45 to USD180/t of
O
2
sold). For each tonne of methanol produced, 1.5t
of oxygen are generated from the electrolysis of water.
The sale of this oxygen could thus oset some of the
costs of e-methanol production in the short term.
However, as availability of large amounts of oxygen
from the electrolysis increases as a by-product of e-fuels
production, the supply will probably outpace demand,
leading to lower prices. If the sale of oxygen is not taken
into account, the overall cost of producing e-methanol is
in a range of approximatively USD400 and USD1000/t,
depending mostly on the cost of electricity. The cost of
CO
2
in most studies is between USD0 and USD55/t.
In the case of DAC, the cost to capture CO
2
would be
higher (Bos et al., 2020; Specht et al., 1998; Specht and
Bandi, 1999).

RENEWABLE METHANOL 77
Table 21. Production costs and production capacity of e-methanol reported in the literature
Carbon source
Electricity source for
electrolysis
Cost of electricity (US¢/
kWh)
Cost of CO
2
(USD/t)
capacity (t/y)
Capital cost (USD million)
Capital cost (USD/t/y)
OPEX (USD million/y)
OPEX
(USD/t)
Methanol cost (USD/t)
Source
Biogas/
ammonia
Grid/
wind
3.5-16.2 0-3.3
4000-
10000
16-30
1 680-
4 700
2.6-12.3
510-
1270
680-
1610
Hank et
al., 2018
DAC Wind --- --- 65000 222 3330 --- ---
830-
890
(a)
Bos et al.,
2020
Purchased
Grid 2.4-7.3 59 100000 134 1340 --- ---
365-
826
(b)
Zhang et
al., 2019
Flue gas
Hydro
power
--- --- 100000 333-555
3330-
3890
--- ---
890-
1000
~555
(g)
Swiss
Liquid
Future,
2020b
Flue gas/
DAC
Hydro
power
2 --- 70000 --- --- --- ---
390-
590
Specht
and
Bandi,
1999
CPP flue
gas/DAC
Hydro
power
3.9 --- 70000 --- --- --- ---
805-
1090
Specht et
al., 1998
CPP flue
gas
RES 1.7-2.4 ---
60000-
120000
95-322
1640-
3010
16.8-
36.9
230-300
620-
950
Mignard
et al.,
2003
CPP flue
gas
Grid/RES 4.4 15 300000 344 1150 161 540
620-
710
(h)
Clausen
et al.,
2010
CPP flue
gas
Grid/CPP 3.2-5.5 49 110000 --- --- --- ---
970-
1010
Atsonios
et al.,
2016
Ethanol
plant
Wind --- --- 32000 30 944 --- ---
405-
1070
Matzen et
al., 2015
CPP flue
gas
CPP 10.5-13.4 0 440000 552
(i)
1260 325 740 805
(f)
Pérez-
Fortes et
al., 2016
Purchased
RES 10.3 56 35000 51
(i)
1480 --- --- 1090
(f)
Tremel,
2015)
CPP flue
gas
RES 2.9-3.7 22
30000-
45000
56
1 240-
1 900
--- ---
500-
530
Varone
and
Ferrari,
2015
--- --- 5.5 3.3-11 16300 16 980 13.7 840 990
Rivera-
Tinoco et
al., 2016

INNOVATION OUTLOOK: 78
E-methanol production costs based on
feedstock costs
The cost of e-methanol can also be estimated from the
cost of hydrogen and CO
2
, which in large e-methanol
plants will represent most of the production cost. Once
CO
2
and green hydrogen are provided, the production
of methanol in a single step and its distillation are quite
straightforward and a mature technology (TRL 8-9).
It will only represent about USD30 to USD50/t of the
total cost of methanol production (Boulamanti and Moya,
2017). To produce 1t of methanol, 0.188 t of H
2
and 1.373 t
of CO
2
are needed.
Cost of hydrogen: Electrolysis of water is an energy-
intensive process. Producing 1 t of hydrogen with a 100%
theoretical eciency requires 39.4 MWh of electricity
(HHV of H
2
; 33.3 MWh/t for the LHV of H
2
). In practice,
however, it is closer to 50 MWh/t (Simbeck and Chang,
2002; IRENA, 2018). The cost of hydrogen is thus closely
linked to the cost of the electricity needed to produce
it. Renewable electricity prices continue to decrease. In
many places around the world, electricity from solar PV
and onshore wind is now cheaper than from fossil fuel
sources and expected to continue falling to reach levels
of about 4¢/kWh and below in the coming years (IRENA,
2019c). At electricity prices of 4¢/kWh, the production
Flue gas RES 1.1-5.5 44
1800000
2310
1385-
2770
--- ---
430-910
Räuchle
et al.,
2016
Flue gas --- 1.1-6 --- 50000 95 1900 11-38.3
220-770
210-
720
(c)
455-
970
(b)
Bellotti et
al., 2019
--- Wind --- (-22)-39 175000 370 2110 --- ---
390-
480
(d)
González-
Aparicio
et al.,
2017
Flue gas Grid --- ---
4000-
50000
11-83
1670-
2780
--- ---
555-
780
(d)
Bellotti et
al., 2017
Flue gas --- --- 28
1800000
424
(i)
235
755-
1670
(e)
420-922
420-
940
(e, f)
Nyári et
al., 2020
Flue gas RES 3 (-278)-0 100000 62 620 79 880
810-
1190
(j)
Szima
and
Cormos,
2018
CPP flue
gas
Grid/RES 4.4 43 110000 --- --- --- --- 645
Kourk-
oumpas
et al.,
2016
(a) Includes capital cost for a 100 MW wind
farm.
(b) Without sale of oxygen.
(c) With sale of oxygen.
(d) Costs with and without sale of oxygen.
(e)Cost depends on price of hydrogen
purchased and with or without oxygen sale.
(f) Hydrogen purchased.
(g) Estimated cost for methanol produced in
the wind and solar belts of the world.
(h) With and without district heating income.
(i) Cost of methanol plant does not include
hydrogen production.
(j) With and without a negative value of
USD278/t for CO
2
.
Notes: Methanol cost in 2018-2019 USD/t.
Exchange rate of USD1 = EUR0.9. CPP = coal
power plant. RES = renewable energy source.
US¢ = US cents.

RENEWABLE METHANOL 79
of hydrogen through electrolysis is about USD2.5-3/kg.
To make 1 t of methanol, 0.188 t of hydrogen are needed.
At a cost of USD3/kg, this represents USD560 of
hydrogen to make 1 t of methanol. According to IRENA
and depending on the energy scenario, this cost should be
USD1.8-5.0/kg of green hydrogen by 2030 and USD0.9-
3.3/kg of green hydrogen by 2050 (IRENA, 2020a) (see
Table 22). At USD1/kg, making 1t of methanol would only
require about USD190 of green hydrogen.
Cost of CO
2
: The cost of CO
2
depends greatly on its origin
and the amount of eort required to purify and compress
it to the pressure needed for the synthesis of methanol. CO
2
fulfilling these requirements at the lowest cost, from around
USD20-30/t, can be obtained from facilities that already
produce concentrated streams of CO
2
, such as natural gas
purification, fertiliser and bio-ethanol plants (Irlam, 2017).
However, these sources have relatively limited capacity.
A higher cost of between about USD50 and USD100/t
of captured CO
2
(depending on technology and location)
is incurred at power, steel and cement plants due to the
need to add a carbon capture unit. The technologies for
large-scale carbon capture at these facilities are relatively
mature, but have yet to be applied on the enormous scale
needed for the Power-to-X sector.
Most of these CO
2
sources are also not renewable or
sustainable as they still rely on fossil fuels. Biomass
can provide some of the required renewable CO
2
though BECCS/BECCU technologies. Costs can vary
greatly between roughly USD20 and USD400/t CO
2
,
depending on the BECCS technology used, the nature
of the feedstock, size of the plant, etc. (Fuss et al.,
2018). Bio-ethanol production, biomass gasification
and gasification of black liquor from paper mills oer
some of the lowest-cost CO
2
at ~USD20 to USD100/t
CO
2
. Combustion BECCS that produces electricity had a
somewhat higher cost, >USD90/t CO
2
.
Another source of CO
2
is the air. DAC technologies
are being developed by a number of companies
including Climeworks, Carbon Engineering and
Global Thermostat. Costs are still high, in the order
of USD300 to USD600/t CO
2
, but are expected to
decrease substantially to about USD50-150/t CO
2
in
the future as the technology is improved and scaled
up (Fasihi et al., 2019; Sanz-Pérez et al., 2016, Keith et
al., 2018). The cost of DAC is in great part related to the
relatively low concentration of CO
2
in the air, presently
around 420 parts per million. As pointed out in Section
2.2, the combination of bio-methanol and e-methanol
production could also oer considerable synergies.
Using green hydrogen to convert the CO
2
generated
during bio-methanol production could avoid the need
for CO
2
separation, reducing the cost of e-methanol
production.
Table 22. Cost of green hydrogen today and in the futures
1
Historical
progress
Where we are heading Where we should be
2015-2018 2030 2050 2030 2050
Cost (USD/kg H
2
) 4-8 2.5-5.0 1.6-3.3 1.8-3.2 0.9-2.0
Source: IRENA (2020b).

INNOVATION OUTLOOK: 80
Table 23. Cost of CO
2
from various sources
1
Source or technology
CO
2
concentration
in exhaust (%)
Estimated cost of CO
2
(USD/t CO
2
)
Source
Today 2050
Fossil carbon
Coal power plant 12-14 43-97 46-55
Irlam, 2017;
IEA, 2012;
Rubin et al., 2015
Coal power plant with
oxy-combustion
Close to 100 52-75 52
Irlam, 2017;
IEA, 2012
Natural gas power plant 3-5 80-89 43
Irlam, 2017;
IEA, 2012
Iron and steel 20-30 55-77 40-65
Irlam, 2017;
Leeson et al.,
2017
Cement 15-30 35-125 20-103
Irlam, 2017;
Leeson et al.,
2017
Natural gas purification 2-65 15-25 20
Irlam, 2017;
Leeson et al.,
2017
Ammonia synthesis Up to 100 20-25 24
Irlam, 2017;
Leeson et al.,
2017
Renewable carbon
Biomass to ethanol plant Up to 100 12-22 20
Irlam, 2017;
Leeson et al.,
2017
Biogas 40-50 ~30 ~30
Olsson et al.,
2020
DAC
0.042 in air
concentrated to
close to 100
300-600 50-150
Fasihi et al., 2019;
Keith et al., 2018;
Sanz-Pérez et al.,
2016
BECCS/BECCU Close to 100 20-400 --- Fuss et al., 2018
Biomass gasification or
biomethane reforming
and conversion to
methanol
Combined e- and
bio-methanol
production.
No or limited CO
2
separation needed.
Integrated
(a)
Integrated
(a)
Described in
Section 2.2:
Combination
of bio- and
e-methanol
production
Source: IRENA (2020b).
(a) The CO
2
is not separated in the process. H
2
from water electrolysis is added to use all or part of the CO
2
generated during biomass gasification.

Figure 39. Cost of methanol as a function of hydrogen and CO
2
cost
Notes: Assuming USD50/t synthesis cost for e-methanol once the raw material H
2
and CO
2
are provided.
Estimated cost of e-methanol today and in 2050 can be found in Table 24.
RENEWABLE METHANOL 81
Regardless of the origin of the hydrogen and CO
2
, the cost
of e-methanol production can be approximated by adding
the cost of the hydrogen, the cost of the CO
2
and the cost
to produce them in a large-scale methanol synthesis unit
(estimated at USD50/t e-methanol). As the results in
Figure 6 show, these estimates are in the same range as
those published in the literature and are highly dependent
on the cost of the feedstock: H
2
and CO
2
.
The cost of renewable methanol in the future can also
be estimated from the projected cost of hydrogen and
CO
2
, as can be seen in Table 24 and Figure 40. The cost
of hydrogen over time was taken from Table 11. The cost
of renewable CO
2
depends on its source, as can be seen
in Table 23 and Figure 30. At first relatively inexpensive
CO
2
sources including bioethanol and biogas production
will be used. These CO
2
sources, however, have limited
availability. Therefore, as the production of CO2-derived
fuels and materials such as e-methanol increases, costlier
options will have to be progressively used. These include
pulp and paper, waste-to-energy plants, biomass
combustion and DAC, which oers the greatest potential.
Availability and cost will also depend on competition
with other CCU technologies as well as CCS.
Table 24 also shows that carbon credits can have a large
impact on the cost of the renewable methanol produced.
A carbon credit of USD100/t CO
2
can reduce the cost
of methanol by USD172/t compared to no credit at all
(based on avoided CO
2
-eq emissions for e-methanol
compared to methanol production from natural gas
[Table 11]). As carbon credits are expected to become
more prevalent in the future, this could play a significant
role in making renewable methanol more competitive.
Current fossil methanol price
Estimated cost of e-methanol today
Estimated cost of e-methanol in 2050
Cost of CO
2
(USD/t)
Cost of hydrogen (USD/t)

INNOVATION OUTLOOK: 82
Table 24. Estimated costs of renewable methanol up to 2050
1
Estimated costs in
2015-2018 2030 2050
Cost of green hydrogen (USD/t H
2
)
(a)
4000-8000 1800-3200 900-2000
Methanol through CO
2
from combined renewable sources
Cost of CO
2
(USD/t CO
2
)
(c)
10-50 15-70 20-150
Cost of methanol
(USD/tMeOH)
(b)
With no carbon credit 820-1620 410-750 250-630
With a credit of
USD50/t CO
2
(d)
730-1540 320-660 160-550
With a credit of
USD100/t CO
2
(d)
640-1450 240-580 70-460
Methanol through CO
2
from DAC only
Cost of CO
2
from DAC (USD/t CO
2
) 300-600 150-300 50-150
Cost of methanol
(USD/t MeOH)
(b)
With no carbon credit 1 220-2 380 600-1 070 290-630
With a credit of
USD50/t CO
2
(d)
1 130-2 300 510-980 200-550
With a credit of
USD100/t CO
2
(d)
1 040-2 210 420-890 120-460
(a) Source: IRENA (2020b) using the “where we should be” assumptions in Figure S.6. Values reported in Table 11.
(b) Assuming USD50/t synthesis cost for e-methanol once the raw material H
2
and CO
2
are provided.
(c) Origin of the CO
2
will change over time as volumes increase (see text for details).
(d) The carbon credit per tonne of e-methanol is based on the dierence between the average CO
2
-eq emissions from methanol production
using natural gas (95.2 gCO
2
-eq/MJ) and average CO2-eq emissions from e-methanol produced from renewable CO
2
and H2 (8.645 gCO
2
-eq/
MJ) given in Table 11. Considering an LHV of 19.9 MJ/kg for methanol, this corresponds to 1.72 tCO
2
-eq of emissions avoided per tonne of
e-methanol, compared to traditional natural gas-based methanol.
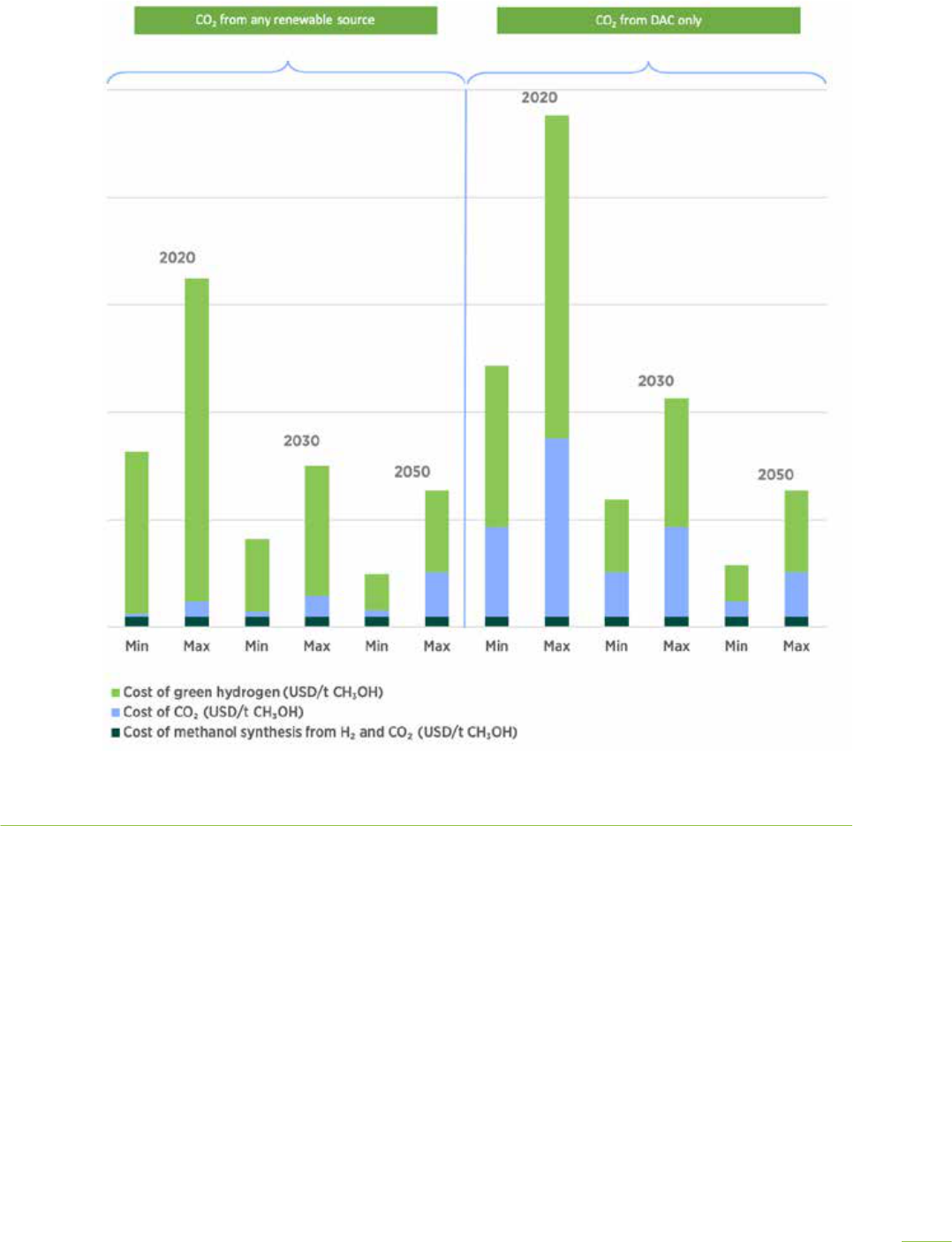
Note: CAPEX and OPEX for the production of hydrogen and CO
2
are already included in the respective cost of hydrogen and CO
2
.
RENEWABLE METHANOL 83
Capital cost of e-methanol plants for current
and proposed projects
Currently, only one commercial plant produces
e-methanol; it is operated by CRI on a 4000 t/y scale.
The information available on capital cost is therefore
very limited and mostly only for e-methanol projects and
technology. This information is summarised in Table 25.
The capital cost per unit of capacity is somewhat higher
for the e-methanol plants, but is close to those reported
in the literature in Table 21. They are, however, relatively
high compared to the cost of natural gas-based methanol
plants. It should be noted that most of the e-methanol
plants considered to date are relatively small, with a
production capacity of 12-300 t/d compared to world-
scale natural gas- and coal-based plants, which usually
have a methanol production capacity in the order of
2500-5000 t/d (mega-methanol plants). Small-scale
natural gas-based methanol plants, too, have a higher cost
per tonne of methanol produced (Sorensen, 2015). The
cost per unit of capacity for e-methanol is thus expected
to come down somewhat as the plants scale up and reach
capacities similar to traditional methanol plants.
Figure 40. Estimated costs of renewable e-methanol up to 2050 depending on the
renewable CO
2
source
USD/t CH
3
OH
2 500
2 000
1 500
1 000
500
0

INNOVATION OUTLOOK: 84
Reductions in electrolyser costs are especially important
as they represent a large share of the investment. The
cost of other parts of the plant including the methanol
synthesis and distillation units, conversely, can exploit
the reduced production costs associated with economies
of scale. A “typical” or “standard” e-methanol plant is
likely to be smaller than a current large-scale natural
gas- or coal-based methanol plant.
Interestingly, CRI’s estimated cost per t/y for the project
in Norway is not much higher than for a coal-based
methanol plant. It should also be noted that the capital
cost includes the electrolysers, which represent a large
share of the e-methanol plant cost.
It should also be pointed out that the capital cost
generally only represents a minor proportion of the cost
of e-methanol. The operating costs usually represent the
largest share, mainly due to the cost of the electricity
needed to produce the green hydrogen.
4.3. Summary of renewable methanol
costs today and in the future
Compared to today’s natural gas- and coal-based
methanol production (with production costs as low
as USD100-200/t and USD150-250/t, respectively),
renewable methanol production costs in most cases are
estimated to be higher. Methanol prices, on the other
hand, have been fluctuating roughly between USD200
and USD400/t (when adjusted for inflation, average
contract price for methanol in Europe, see Figure 8) (MI,
2020a; MMSA, 2020). Thus it should be noted here that
fossil-based methanol is already competitive today with
most petroleum oil-based fuels (gasoline, diesel, heating
oil, etc.) on an energy content basis: USD10-20/GJ for
methanol compared to about USD17/GJ for gasoline,
diesel, jet fuel and heating oil (Figure 9). Production
costs for bio-methanol and e-methanol are as follows:
Table 25. Capital cost for CO
2
-to-methanol plants
Feedstock
Capacity
(t/d)
Capacity
(t/y)
CAPEX
(million
USD)
CAPEX
(USD/
t/y)
Source
Thyssenkrupp CO
2
and H
2
12 4000 39 9720
Thyssenkrupp,
2020b
FlexMethanol
(bse engineering/
BASF)
CO
2
and H
2
~44 16400 ~50 3100
bse
engineering,
2019, bse
Engineering,
2020
CRI (Norway) CO
2
and H
2
300 100000 200 2000
Stefánsson,
2019
Swiss Liquid Future
(Norway)
CO
2
and H
2
220 80000
330-390
4170-
4780
Swiss Liquid
Future, 2020b
Typical plant based
on natural gas
Natural gas 2800 1000000
720-
1440
720-
1440
Bromberg
and Cheng,
2010
Typical plant based
on coal (US)
Coal 10000 3600000 6220 1720
US DOE NETL,
2014

RENEWABLE METHANOL 85
Figure 41. Current and future production costs of bio- and e-methanol
Note: Exchange rate used in this figure USD 1 = EUR 0.9.
•
The cost to produce bio-methanol from biomass and
MSW is estimated at between USD327 and USD764/t
(Figure 41) with a feedstock price up to USD6/GJ, which
corresponds to the upper limit of biomass and MSW
commodities currently used commercially in Europe
and the UnitedStates. At a feedstock price range of
USD6-15/GJ, the production cost may increase to
about USD1000/t. With process improvements, a cost
of around USD227/t to USD553/t should be achievable
for the lower feedstock price range up to USD6/GJ
and correspondingly higher for the higher price range.
Production of bio-methanol from the waste streams
of other industrial processes (e.g. black liquor from
paper mills and MSW) in particular offer opportunities
to simplify the feedstock logistics and improve overall
plant economics. Co-production of heat, electricity or
other chemicals has been suggested to further improve
bio-methanol’s economic performance. Another option
is to co-feed biomass into a coal-based gasifier, or
biogas into a natural gas-based methanol plant to allow
for the gradual introduction of biomass as a feedstock
and make methanol production more sustainable at a
potentially lower cost.
•
Current production of e-methanol based on
hydrogen and CO
2
is estimated to be more expensive,
approximatively USD800-1600/t (and possibly
higher if CO
2
is obtained by DAC only). The cost of
e-methanol depends to a large extent on the cost
of hydrogen and CO
2
. The cost of CO
2
will depend
on the source from which it is captured (biogenic,
DAC, industrial, etc.). The cost of hydrogen is strongly
correlated with the cost of the electricity used to
produce the hydrogen and the utilisation rate of
the electrolyser units and electrolyser cost. With
anticipated decreases in renewable power prices, the
USD/tonne
2 400
1 400
2 200
1 200
2 000
1 000
400
1 800
800
200
1 600
600
0
Current fossil
methanol price
Current fossil
methanol cost
E-methanol - CO
2
from combined
renewable source
E-methanol - CO
2
from DAC only
Bio-methanol < USD 6/GJ
feedstock cost
Bio-methanol USD 6-15/GJ
feedstock cost
Current production
cost levels
Mature production
cost levels
Current production
cost levels
Mature production
cost levels
1 013
884
455
355
764
327
553
227
1620
820
1120
2380
290
630
630
250
A carbon credit of USD 50/t CO2 would
lower renewable methanol production cost
by about USD80/t MeOH

Figure 42. Comparison of renewable methanol with other fuels on a price per unit of energy basis
Note: Exchange rate used in this figure USD 1 = EUR 0.9. Fuel costs and prices are averaged over 10years. See annex 3 for details.
INNOVATION OUTLOOK: 86
cost of e-methanol is expected to decrease as well to
reach levels of USD250-630/t without CO
2
credits by
2050. As in the case of bio-methanol, co-production
of brown/grey (fossil) and green e-methanol might
be a way to gradually introduce green e-methanol at
a reasonable cost.
In the case of both bio- and e-methanol, part of the
higher cost is also due to the smaller scale of the
plants. Nevertheless, the cost projections for renewable
methanol by 2050 are within the range of current fossil
methanol and petroleum-based fuels and products,
as can be seen in Figure 41 and Figure 42. By 2050,
a production cost of about USD11-43/GJ and USD12-32/
GJ is estimated for bio- and e-methanol, respectively.
The application of carbon credits could also lower
substantially the cost of both bio- and e-methanol.
Compared to the production of methanol from natural
gas, a decrease in CO
2
-eq emissions of about 1.6-1.7 t per
t of renewable methanol was estimated. This means that
for every USD1 credit per t of CO
2
-eq avoided, a decrease
in methanol cost of about USD1.6-1.7/t of methanol could
be expected. This means that for, example, with a credit
of USD100/t CO
2
-eq, a cost reduction for renewable
methanol of USD160-170/t could be reached.
USD/GJ
0
Current fossil
methanol price
Bio-methanol E-methanol
70
60
100
50
20
90
40
10
80
30
Current production
cost levels
Mature production
cost levels
Gasoline (US Gulf Coast)
Diesel (US Gulf Coast)
Heating Oil No. 2 (New York Harbor)
Jet Fuel (US Gulf Coast)
Gasoline (average US)
Diesel (average US)
Gasoline (average
EU)
Diesel (average EU)
Retail with tax
Before tax

RENEWABLE METHANOL 87
5.1. Demand
Methanol, whether from fossil fuels or renewable
sources, has the same chemical structure: CH
3
OH.
As such, renewable methanol could in principle replace
fossil methanol in any of its current uses, for example
as a feedstock for the production of various chemicals,
materials, plastics and products and as a fuel for
transport, shipping, cooking, heating and electricity
production. In addition, renewable green methanol
could replace most fossil fuel-based hydrocarbons and
petrochemicals either directly or through methanol
derivatives for a potential market in the hundreds of
millions of tonnes and possibly billions of tonnes of
methanol per year. Annual global methanol production
is expected to grow from its current 100Mt to more than
120Mt by 2025 (MMSA, 2020; Berggren, 2019) and 500
Mt by 2050 (Saygin and Gielen, forthcoming).
Most of the growth to 2028 is expected to occur in China,
and more specifically the demand to be for MTO and a
smaller share for gasoline blending, formaldehyde, acetic
acid and MTBE. The chemical sector will thus continue
to play an important role in methanol demand growth.
Looking ahead, however, the increase in methanol
production is expected to see a progressive shift to
renewable methanol, with an estimated annual production
of 250 Mt of e-methanol and 135 Mt of bio-methanol by
2050 (please see insert below and Figure 47).
The introduction of green methanol would allow for a
transition to a sustainable circular green economy in the
chemical industry, the transport sector and other energy-
related sectors. Of course, in practice the expansion of
renewable methanol is currently held back by its higher
production cost when compared to natural gas-based
methanol. Renewable methanol, however, is still one
of the easiest to implement sustainable fuels and a
promising feedstock in the chemical sector, and costs are
expected to continue falling as discussed in Chapter 4
of this report.
As with any other alternative fuel and chemical feedstock,
for renewable methanol to take off demand has to
be stimulated by adequate policies, regulations and
mandates. In the EuropeanUnion the RED II directive,
for example, mandates that 14% of the energy used
in transport should come from renewable sources by
2030. Other nations are also increasingly requiring part
of transport fuels to come from renewable sources. The
markets for renewable methanol to date are therefore
mainly concentrated in the transport sector where
regulations mandate the use of greener alternatives to
reduce emissions.
Vulcanol, produced in Iceland from CO
2
and H
2
by CRI, and
bio-methanol produced by BioMCN in the Netherlands,
are used as a fuel additive in Europe. In 2018, 57 million
litres of bio-methanol were blended with gasoline in the
UnitedKingdom (Dolan, 2019). Renewable methanol
can also be used for the production of biodiesel. In the
near term, assuming that the M3 standard (3% methanol
by volume in gasoline, EN 228) is implemented across
the EuropeanUnion (approximately 82 Mt gasoline in
2019), about 2.5 Mt of renewable methanol would be
required (CRI, 2019; Fuels Europe, 2020). In the longer
term, renewable methanol could address part of the
fuel needs for all cars, trucks, ships and so on (gasoline,
diesel, marine fuels, etc.). This is a market of 350Mt of
oil equivalent (~700 Mt of methanol on an energy basis)
in Europe and about 2 billion t in the world.
While other options are increasingly available for light
passenger cars (e.g. batteries, hydrogen), alternatives
for heavy trucks and shipping are limited. These
hard-to-electrify sectors are well-suited to the use of
renewable methanol (van Kranenburg et al., 2020).
Renewable methanol either pure or mixed with gasoline
is an excellent fuel for ICEs (Figure 43 and Figure 44).
Methanol can also be used as a marine fuel, in modified
diesel trucks and in hybrid and fuel cell-powered vehicles
5. POTENTIAL AND BARRIERS

INNOVATION OUTLOOK: 88
and ships (Figure 11 and Figure 46). DME, an easily
obtainable methanol derivative, is itself a superior fuel
for compression ignition engines (diesel-type engines,
Figure 45). The currently observed expansion of fossil
methanol as a fuel in many applications could ease
the gradual transition to renewable methanol as the
distribution and transport infrastructure would remain
unchanged. At the same time, demand for renewable
methanol in the chemical industry should also be
stimulated with various policies including incentives,
mandates and carbon taxes, in order to make this hard-
to-electrify sector greener and eventually carbon neutral.
Figure 43. Fleet of Geely Emgrand 7 cars operating in Iceland and powered by 100%
renewable methanol, in front of the CRI CO
2
-to-methanol production plant
Source: CRI (2020).Source: Courtesy of Maria Fäldt.Source: Stadt Essen, Peter Prengel.
Source: Rikard Gebart,
LTU Green Fuels.
Figure 44. Swedish car powered by an M56 mix (56%
methanol in gasoline) with bio-methanol from the LTU
Green Fuels plant (in the background)
Figure 45. Chemrec bioDME pilot plant and
Volvo DME-fuelled truck
Figure 46. Passenger ship MS innogy on Lake Baldeney (Germany)
powered by a hybrid fuel cell system fuelled by renewable methanol

RENEWABLE METHANOL 89
RENEWABLE METHANOL PRODUCTION FORECAST
IRENA is projecting that global
methanol production would increase
from 100 Mt currently to 500 Mt in
2050 (Saygin and Gielen, forthcoming)
based on the Transforming Energy
Scenario. This increase would also
need to be accompanied by a shift
to renewable methanol (Figure 47).
In 2050, 250 Mt of e-methanol and
135 Mt of bio-methanol are estimated
to be produced annually; this is an
ambitious yet realistic transformation
pathway built on renewable energy
and steadily improving energy
eciency.
e
-
methaNol
: To produce 250 Mt of e-methanol will require about 350 Mt of CO
2
and 48 Mt of hydrogen.
To produce this quantity of hydrogen through water electrolysis and assuming consumption of 50MWh/t
of hydrogen produced, about 2400000 GWh of electricity would be needed, corresponding to 8.6 EJ.
This would require about 275 GW of continuous electricity production, as well as 280 GW of electrolyser
capacity. In terms of solar power, installed capacity of about 920 GW (at a capacity factor of 30%) would
thus be required. In the case of wind power, about 500 GW of installed capacity (at a capacity factor of
55% as encountered in some oshore wind farms) would be called for.
Various combinations of these and other renewable power sources could be applied. The required CO
2
will be recycled preferably from renewable biogenic sources or DAC. About 280 methanol plants with a
capacity of 2500t/d (900000 Mt/y) will need to be constructed to produce 250 Mt/y. The construction of
an e-methanol plant takes about 2-3 years to build, or less if modularised and standardised. Production of
e-methanol from CO
2
and H
2
is very similar to current production of methanol from fossil fuel-based syngas
and thus relatively mature and scalable. Scale-up of water electrolysis technology to the gigawatt scale is
under way and should be widely available in the near future for large-scale green hydrogen generation.
BIo-methaNol: To produce 135 Mt of bio-methanol through gasification will require 4.1 EJ of biomass
material, which equals about 230 Mt of dry biomass. The global biomass potential is estimated at 97-147EJ
by 2050 (IRENA, 2014). Due to the nature of biomass and cost of collecting and storing the feedstock,
a typical biomass-fed bio-methanol plant is assumed to produce 300 kt/y. As a result, there would be a
need of 450 plants of that size to produce 135 Mt/y of bio-methanol. It would require an investment of
about USD130 billion.
If renewable hydrogen is added to gasification-based plants in order to utilise all in situ available renewable
carbon, partly in the form of CO and partly CO
2
, bio-methanol production could reach 290Mt/y using
the same biomass source, 4.1 EJ. This would require approximately 26 Mt/y of hydrogen, which would
need production of 1300000 GWh of renewable electricity (4.7 EJ). The typical plant size would as a
consequence increase to about 650 kt/y. A gasification-based plant of the quoted size will take 20-24
months to build from letting of contract to the point when the plant is ready for start-up.
Figure 47. Current and future methanol
production by source
Fossil methanol
Bio-methanol
E-methanol
Methanol production (Mt)

INNOVATION OUTLOOK: 90
5.2. Sustainable feedstock
Biomass
In the United States, around 300 Mt/y of unused (dry)
biomass are available today, and potentially 750-
1000Mt/y could be available in 2040, two-thirds of this
from energy crops that are not cultivated currently (US
DOE, 2016). In Europe, an estimated 1050-1370 Mt/y of
sustainable (dry) biomass could be available by 2030, of
which 525-850 Mt/y would be available after meeting
other demands (S2Biom, 2016). These volumes constitute
the sum of different types of agricultural residues,
additional biomass from sustainable forestry, wastes (the
lignocellulosic fraction after recovery and recycling) and
industrial crops on released agricultural land.
Production of bio-methanol based on the above
summarised maximum feedstock resources in the
UnitedStates and Europe (in 2030-2040), and
converted using a conversion eciency of 65%, would
result in a production potential of 1100Mt/y. Utilising the
combined bio- and e-methanol concept described in this
report would increase this by a factor of about 2.15, to
2350 Mt/y of methanol or about 50 EJ/y.
Taking a global perspective the potential for sustainable
biomass in 2030 has been estimated to be 97-147EJ/y
(based on total minimum and total maximum scenarios)
(IRENA, 2014). However, there is a substantial potential to
sustainably expand the bioenergy supply (IRENA, 2016b).
Calculated as above and based on IRENA (2014), this
global potential (including the UnitedStates and Europe)
corresponds to 3200-4800 Mt/y of methanol with a
conversion efficiency of 0.65, and to 6900-10400
Mt/y of methanol (147-218 EJ/y) if combined bio- and
e-methanol processes were fully implemented. By
comparison, the world’s total oil consumption in 2019
was 188 EJ/y (BP, 2020). Naturally, these figures are only
illustrative of the potential for bio-methanol production.
Presumably, not all potentially available biomass would
be utilised solely for the production bio-methanol.
Waste and residues from forestry and agriculture have
greater availability and would represent the bulk of the
raw materials for advanced biofuels. So-called energy
crops can also be grown, preferentially on land that is
not used for food or other crops such as contaminated
and marginal land.
To be sustainable these crops would also have to comply
with a number of other criteria, including impact on
soil quality, soil erosion, need for water and fertilisers,
biodiversity concerns, land tenure and emission of
pollutants to air and water.
CO
2
and hydrogen
For the production of e-methanol, sustainable sources
of both H
2
and CO
2
are required. Hydrogen is already
produced on a large scale of about 120 Mt/y, of which
two-thirds are pure hydrogen and one-third is in mixture
with other gases (IRENA, 2019d; IRENA, 2018). A mixture
of hydrogen with other gases is used, for example, in
methanol and iron/steel production. Pure hydrogen
is essential for various industrial processes, mostly
petroleum refining and ammonia synthesis. However,
over 95% of it comes from fossil fuels and only about
4% is presently supplied via electrolysis (IRENA, 2018).
To be sustainable in the long term, most hydrogen will
have to be produced from renewable energy sources,
and will thus depend on the cost and availability of
these resources. While any renewable source can be
used, solar and wind are the renewables with the highest
potential for expansion to the size needed for large-scale
deployment of e-methanol
Technology for the electrolysis of water with alkaline
electrolysers is already available on the 100 MW scale
for the chloralkali process. A new generation of alkaline
electrolyser dedicated to green hydrogen production is
being developed, with slightly dierent features, although
the fundamentals remain similar. Both alkaline and PEM
electrolysers are already commercial at the megawatt
scale, with facilities coming on line combining multiple
stacks to reach tens of megawatts, quickly moving
towards hundreds of megawatts per single facility.
The potential for green hydrogen production will mainly
depend on the combination of further reductions in the
cost of renewable power generation and electrolysers,
and gains in eciency and durability. Carbon dioxide is
in a situation similar to hydrogen. A lot of CO
2
is emitted
by industrial sources and fossil power plants that are
overwhelmingly powered by fossil fuels. The recycling/
upcycling of these CO
2
emissions to blue methanol

RENEWABLE METHANOL 91
using green hydrogen does not result in an increase in
atmospheric CO
2
when methanol is used or combusted.
Blue methanol could thus already be considered as a
low-carbon fuel.
Using the CO
2
from fossil fuel sources one more time
to produce methanol instead of simply releasing CO
2
to the atmosphere could potentially halve the overall
emissions. However, while these sources of fossil CO
2
can be certainly used to produce methanol in the
transition phase, to be at the same time carbon neutral
and sustainable the CO
2
will have to be sourced from
renewable sources, i.e. from biomass and via DAC at a
reasonable cost. BECCS/BECCU can already be applied
today. DAC, although promising, is still at the early stages
of development (Goeppert et al., 2014; Sanz-Pérez et
al., 2016). The amount of CO
2
available in the air is for all
practical purposes unlimited and its economic extraction
will only depend on the availability of a suitable DAC
technology and inexpensive renewable electricity.
5.3. Impact of renewable methanol
on the energy sector
The progressive defossilisation of the energy,
industrial and chemical sectors and their concomitant
electrification through the use of renewable energy
sources will have profound eects. Hourly, daily and
seasonal fluctuations and intermittency in the generation
of electricity from variable renewable energy sources
will need to be increasingly dealt with to maintain a
stable and reliable grid. In this context the production of
electrofuels and electrochemicals can help stabilise the
grid by providing an outlet for renewable power when
supply is higher than demand. Dynamic e-methanol
plants able to “follow the load” in the electric grid and
adjust their methanol output accordingly are also being
specifically developed for that purpose.
The production of e-methanol oers a way to increase
the value of green power and store electricity in a
convenient liquid that can be easily kept for later use. For
larger e-methanol plants, dedicated renewable energy
generation capacity will probably have to be built. Demand
for such large production facilities should further lower
the cost of renewable power and the materials produced
with that power. Producing renewable methanol, as
well as downstream products including polyethylene,
polypropylene and various other polymers and materials,
could also be a way for renewable energy-rich regions
such as Australia, the Middle East and Northern Africa to
export this resource in a convenient form, while benefiting
their economies and the planet.
5.4. Drivers
The main driver for the production of renewable
methanol is the need to decouple society from its
dependence on fossil fuels, which are the major source
of GHG emissions and associated environmental issues.
Sustainable and durable solutions based on renewable
resources are thus needed. In this energy transition,
renewable methanol can act as a new energy carrier to
reduce and eventually eliminate the carbon footprint of
the chemical/petrochemical and energy sectors.
To drive the change towards a sustainable future, strong
policies and regulation will be needed to push the
production and use of renewable fuels and materials.
The European Union’s Energy Roadmap calls for GHG
emission reductions of 80-95% by 2050 (EU, 2012b).
This will require a complete overhaul and transformation
of the energy sector, where about two-thirds of energy
will have to come from renewable sources. A similar
transition will be needed in most of the world to ensure
a secure, competitive and sustainable energy system for
the long run (IRENA, 2019c). According to IRENA, 70%
of the world’s energy-related CO
2
emissions need to be
cut by 2050, and eventually to zero beyond that (IRENA,
2020b). This is a unique opportunity for the development
of renewable methanol as a part of the solution.
Compared to other bio-based materials and fuels, bio-
methanol and bio-DME have, together with BioSNG and
biomethane, the lowest production costs, considerably
lower than cellulosic ethanol and FT-type products
(Maniatis et al., 2018; Brown et al., 2020).
Brown or grey methanol from fossil fuels is already a large-
scale commodity chemical and fuel. Chemically identical

INNOVATION OUTLOOK: 92
LCM, blue methanol and green methanol could easily
replace brown or grey methanol in any of its applications.
All of these lower carbon footprint methanol types are
ideal raw materials for the chemical industry for the
production of materials and products, such as plastics,
clothing, bottles and paint. They are also superior fuels for
cars, trucks, ships and hard-to-electrify sectors.
The key benefits and drivers of renewable methanol
include:
•
versatile intermediate for the production of numerous
chemicals and materials
•
can be produced with low GHG emissions
•
easy to produce from a variety of sustainable
feedstocks such as biomass, MSW or CO
2
and H
2
•
a liquid that is easy to store, transport and distribute
•
compatible with existing distribution infrastructure
and can partly be blended with conventional fuels
•
leads to a reduction in other harmful emissions (SOx,
PM, NOx, etc.)
•
liquid hydrogen carrier.
The greening of the industrial sector, especially the
chemical and petrochemical industries, although
challenging, should be a main driver for renewable
methanol. Renewable methanol can be a feedstock for
existing products currently obtained from fossil methanol.
In addition, renewable green methanol could find new
uses and replace most petroleum-based hydrocarbons
and petrochemicals either directly or through methanol
derivatives for a potential market in excess of a billion
tonnes of methanol per year. Production of plastics and
aromatics (BTX) from renewable methanol could, for
example, be greatly expanded (Bazzanella and Ausfelder,
2017). For example, each car currently produced in Europe
requires about 300 kg of methanol for the production
of various parts (Seuser, 2020). If the methanol used
was renewable, it could already considerably reduce the
carbon footprint of the automotive industry.
The ground and sea transport sectors are also likely to be
a main driver of the expansion of renewable methanol,
due to mandates and legislation being increasingly put in
place by regulating authorities to reduce GHG emissions
and achieve sustainability goals. The International
Maritime Organization, for example, aims to halve the
CO
2
emissions from shipping by 2050 (IRENA, 2019b).
Other options exist for the greening of light-duty
passenger vehicles, including batteries and hydrogen.
However, the electrification of heavy-duty trucks, ships
and other heavy equipment is more challenging. For
these hard-to-electrify transport sectors, renewable
methanol and its derivatives can be good options.
In the case of e-methanol, and electrochemicals and
electrofuels in general, one of the inherent drivers is
also the availability of inexpensive renewable power.
As fossil fuel-based industry and power generation
are increasingly scrutinised, permitting and licences to
operate should become relatively easier to obtain and
maintain for projects that include a significant green
component. From an energy security point of view, the
possibility of producing renewable methanol locally with
any available biomass and renewable power is attractive
too. Island-type projects where renewable energy can
be produced relatively cheaply, but the import of fuels
is costly, could be good candidates for local production
of green methanol. Production of renewable methanol
would also stimulate global trade between renewable
energy-rich regions such as North Africa and the Middle
East (solar power) and energy-importing regions such
as Europe, North America and Asia.
5.5. Barriers
The main barrier to the adoption of renewable methanol
is the same as for some other renewable alternative
fuels and feedstocks, namely the cost of production.
In that sense, policies to stimulate and sustain the
production and use of renewable methanol on a large
scale are needed. These are discussed in more details
in the policy section.
Bio-methanol
Although the production cost of bio-methanol is lower
than for e-methanol, in most cases it remains higher
than the cost of grey methanol from natural gas and to
a lesser extent coal. This is basically the case for all fuel
and chemical commodities that could substitute for their

RENEWABLE METHANOL 93
fossil counterparts. Bio-methanol, however, has one of
the most attractive production costs compared to other
alternatives, as shown in two recent studies (Brown et
al., 2020; Maniatis et al., 2018).
Capital costs for most advanced renewable fuel plants
are relatively high and they remain dicult to finance.
Even projects that have successfully demonstrated
their technology, and have mitigated and allocated all
their risks (technology, commercial etc.) are having
diculty securing financing for commercial-scale rollout.
One of the problems is that policy makers usually
provide only short-term and/or quota-based schemes,
which cannot support the long-term price floor required
for successful implementation of advanced renewable
fuels. The successful roll-out of renewable electricity
was based on feed-in taris, contracts for dierence
or similar instruments that meaningfully address risk
barriers. Similar schemes should be made available for
bio-methanol and e-methanol.
In the long term, biomass will be able to cover a
substantial share of global energy needs, but it has
also a number of limitations (IRENA, 2016b; IRENA,
2017). These include (among others) land availability,
competition with other crops including food crops,
impacts on soil quality, soil erosion, need for water
and fertilisers, biodiversity concerns, land tenure and
emissions of pollutants to air and water.
The requirement to collect biomass over a large area to
supply bio-methanol plants could also mean that these
plants remain smaller than current world-scale methanol
plants. This means that bio-methanol plants would have
to be optimised for that scale. In addition, seasonality of
the biomass feedstock needs to be addressed either by
storage or feedstock diversification in order to minimise
plant idling or shut down.
E-methanol
The main barrier to e-methanol production from CO
2
and
H
2
is its cost and more specifically the cost of providing
the hydrogen through the energy-demanding water
electrolysis step. About 50 MWh of electrical power
is needed to produce each tonne of hydrogen. This
process is, in turn, directly correlated with the cost of
the electricity used to run the electrolysers. As for most
electrofuels, lowering the cost of electricity is thus the
number one driver for lowering the cost of e-methanol
from its current USD800-1 600 per tonne. As renewable
energy costs are expected to continue decreasing in the
future, the cost of hydrogen and therefore e-methanol
should follow the same trend and reach levels closer to
USD250-630/t without CO
2
credits, and below that with
credits. Besides electricity cost, electrolyser costs also
need to decrease further and large sources of reasonably
priced renewable CO
2
secured.
Production of methanol from CO
2
and H
2
is not limited
by technology. The almost identical, proven and fully
commercial technologies used to make methanol from
fossil fuel-based syngas (TRL 9) can also be used for
e-methanol production. Electrolysis of water and CO
2
capture technologies are also available at a sucient level
of maturity. From a technological viewpoint it is entirely
possible to have an e-methanol plant of the same size as
a conventional methanol plant, i.e. 1000-5000t/d, as the
technologies are comparable. The diculty would mainly
be in finding the required feedstock at a reasonable cost
and capital to build the plant. Technically the production
of e-methanol is not limited by these factors.
Intermittency and fluctuations in power output from
solar and wind energy need to be managed to allow for
the e-methanol plant to operate most of the time. For
this, a robust and reliable electrical grid will need to be
developed. Some combination of solar, wind, hydro and
geothermal, as well as storage of energy or hydrogen,
could be envisioned. The development of e-methanol
plants able to handle dynamic fluctuation in electricity
power generation from solar and wind resources could
be advantageous.
In the short term CO
2
can be obtained from various
industrial sources and fossil fuel-burning power plants
at costs around USD50-100/t. However, to be really
renewable and net carbon neutral, e-methanol will
increasingly have to be made from biogenic CO
2
sources
or CO
2
from the air through DAC. While almost pure
CO
2
can be obtained from ethanol plants, these sources
are limited. Other biogenic sources have to be further

INNOVATION OUTLOOK: 94
developed to supply CO
2
reliably and at an acceptable
cost. A hybrid bio- and e-methanol plant in which the
syngas obtained from biomass is complemented with
green hydrogen is a sensible solution to this problem.
The cost for CO
2
obtained through DAC will also have to
come down considerably to become an economic option
for e-methanol production.
A progressive greening of methanol production is
probably an appropriate pathway to introduce renewable
methanol. Some of the “blue” methanol technologies
being implemented today to produce what is called LCM
are very important, especially the production of green
hydrogen to supplement the production of methanol from
natural gas. This should allow the electrolysis technology
to scale up to the hundreds of megawatts. Once these
large electrolysers are standard and low cost, large-scale
production of green methanol would be much easier to
introduce. The production of H
2
is the number one cost
driver for e-methanol. All that is needed at that point is
suciently low renewable electricity prices.
This seems to imply that for renewable methanol to be
used in commerce at any appreciable rate, much higher
levels of regulatory support will be needed, for example
through an increased carbon price or subsidisation of
the product price. Neither of these are technical issues,
but instead require a level of political will that is still not
evident in most jurisdictions.
5.6. Policies and recommendations
Crafting the right policies and incentives is crucial to
meeting the goals of carbon emission reduction, energy
security, sustainability and improvement in quality of
life. Sucient investment in long-lived capital-intensive
renewable technologies will not happen without
confidence in strong, stable, predictable and sustained
government policy.
In the transport sector, much of the policy focus is on
electromobility and support for increasing the share of
EVs, especially for passenger cars. However, batteries
and hydrogen fuel cells may be challenged in meeting
the energy demands of long-haul trucking, shipping and
aviation. Further, the legacy fleet of combustion engines
will continue to power cars, trucks, buses, ships and
aircraft for years to come even as electromobility makes
market inroads and charging infrastructure expands.
Besides batteries that have a relatively low energy density,
energy-dense fuels that store their energy in the form of
chemical bonds – such as bio-methanol and e-methanol –
also oer low-carbon and net carbon-neutral alternatives
to traditional fossil fuels. Renewable methanol can today
be mixed with fossil fuels and used in existing combustion
engines and current refuelling networks, providing
immediate benefits for GHG emission reductions. The
increasing substitution of gasoline and diesel fuels with
renewable methanol over time would enable a transition
to low-carbon and net carbon-neutral transport.
Similarly, as a basic building block for hundreds of
chemicals that touch our daily lives, the transition
towards renewable methanol can contribute to the
circular economy and the adoption of green chemicals.
Renewable methanol can facilitate sector coupling.
Renewable electricity from the power sector or biomass
from the agriculture sector can be used for e-methanol
and bio-methanol production to fuel transport and
industrial-sector energy demands. Each sector may
find a dierent pathway to carbon neutrality, and public
policy should create a level playing field to expand and
not limit opportunities.
A technology-neutral approach in mobility would place
an emphasis on carbon intensity rather than whether
propulsion came, for example, from batteries or from
fuel cells fuelled with green hydrogen or renewable
methanol. Such an approach needs to be supported by
political will and translated into regulatory measures for
fuel standards and approval of new fuels accounting for
the carbon footprint of the targeted market.
Legislation and standards for methanol used as a fuel for
road transport are already in place or being put in place
in many countries. Some examples can be found below.
While these were initially intended for fossil fuel-based
methanol, they also apply to renewable methanol and
will ease the transition.

RENEWABLE METHANOL 95
Over the last 15 years, various provinces in China
introduced standards for methanol blends in transport,
going from 5% methanol in gasoline (M5) all the way
to 100% methanol (M100). China’s central government
has adopted a policy paper supporting the commercial
introduction of M100 cars, trucks and buses. Israel
established an M15 standard in 2016. Other countries
that are either introducing or evaluating the introduction
of methanol blending in gasoline include Egypt (M15),
India (M15), Italy (M15/E5), New Zealand and Trinidad
and Tobago (M5) (Klein, 2020; Dolan, 2019). Standards
for high methanol blends and pure methanol (M100)
need to be put in place in more countries. Many countries
have only implemented methanol blending standards
for low-level methanol blends (M3-M5), including the
European Union (EN 228 standard, 3% methanol) and
the UnitedStates (Kramer, 2018). Refuelling stations
dispensing methanol are identical to today’s fuelling
stations dispensing gasoline and diesel fuel. In most
cases, after proper cleaning, the same storage tanks can
be used. Some changes to the refuelling lines, gaskets
and so on might be needed to accommodate methanol,
but the changes are in general minimal, low cost and do
not require much time to complete.
To overcome the barriers linked to the introduction and
development of renewable methanol, robust policies
directed towards renewable fuels will be needed.
Government mandates for fuel blending quotas,
incentives for renewable fuels, and carbon taxes would
have an impact on the willingness of the market to pay
a premium for renewable methanol. Over 60 countries
have put renewable fuel targets or mandates in place. In
the EuropeanUnion, the policy driver is the Renewable
Energy Directive (RED), with a recent 2018 recast (RED II)
requiring 14% renewable energy to be used in transport
by 2030. First-generation biofuels will be phased out,
initially capped at 7%, then reduced to 3.8% by 2030,
and ultimately eliminated, opening opportunities for bio-
and e-methanol. A report by Siemens notes that about
a quarter of renewable energy in transport will come
from electromobility, and coupled with the limits on
first-generation biofuels, e-fuels will be needed to meet
European targets, and much of that will be imported
from outside of Europe (Schnettler et al., 2020). The
EU RED II and Fuel Quality Directive classify renewable
methanol from non-biological origin (e-methanol) as
a renewable fuel. Other EU policies that also influence
the uptake of renewable methanol are (among others)
the Alternative Fuel Infrastructure Directive and the Air
Quality Directive.
While e-methanol would qualify as a renewable fuel of non-
biological origin, RED II places barriers to the purchasing
of renewable electricity from the grid that must be
overcome. The specification of a direct correlation in time
and geography of synthetic fuel production and renewable
electricity generation is a barrier to both investment and
e-fuel uptake, as noted by the Working Group Power-
to-X Applications (VDMA, 2020). Guarantees of origin
and purchase power agreements should be adequate
proof that renewable electricity from a wind turbine
or solar farm in one location has been purchased by a
producer of e-methanol in another location connected
by the transmission grid. Concepts such as “virtual
power plants” can allow for real-time monitoring and
validation of both manufacturers and consumers to avoid
double counting of the renewable power feedstock.
This “mismatch” between the goals of RED II and its
implementation must be corrected.
As an e-fuel, e-methanol can be produced in regions with
ample resources of renewable electricity, using carbon
as a carrier in the form of an easily transportable liquid
molecule. Investing in e-methanol production capacity in
dierent countries around the world will diversify energy
supply and reduce political risks. To make this a reality,
international co-operation will be needed, including
import strategies to harness the world’s best feedstock
locations for wind and solar energy. A perfect example
is the collaboration between Europe and Morocco to
promote Power-to-X, including e-methanol production
in Morocco for export to Europe, with the additional
benefit of creating a new market for European-based
technology for synthetic fuel production (Engelhardt,
2020). Such international co-operation can create jobs
and new competitive industries in both the e-methanol
producing and consuming regions.
The UnitedKingdom introduced its Renewable
Transport Fuel Obligation scheme in 2008. Fuels that are
categorised as Renewable Fuels of Non-Biological Origin

INNOVATION OUTLOOK: 96
such as e-methanol, are incentivised by awarding double
credits per litre or kilogram supplied. These credits are
known as Renewable Transport Fuel Certificates and can
be traded between suppliers of fossil transport fuels or
eligible biofuels. In 2018, 57 million litres of bio-methanol
were blended with gasoline in the UnitedKingdom.
In the UnitedStates, the Renewable Fuel Standard,
established in 2005, mandates the use of biofuels in the
transport sector. Bio-methanol, if approved, could meet
the requirement for cellulose-based biofuel or advanced
biofuel. In California the Low Carbon Fuel Standard
(LCFS) was introduced in 2011 to promote the use and
production of cleaner low-carbon fuels. The LCFS is
expressed in term of the carbon intensity (CI) of the fuel
used and depends on an LCA of this fuel. Fuels below the
CI benchmark generate credits, while those above the
CI benchmark generate deficits. LCFS programmes are
being progressively expanded to Oregon, Washington
and the Canadian province of British Columbia. This
programme has been designed to be fuel and technology
neutral. Any pathway that allows for a reduction of the CI
is potentially allowable, including renewable methanol.
This avoids the pitfalls of some other programmes, which
mandate specific fuels or pathways, such as cellulosic
ethanol, whose production on a large scale has failed
to materialise.
The European Union has put forward its “Green Deal”
roadmap, aiming to become carbon neutral by 2050
(EU, 2020a). This implies that in 30 years’ time all
transport fuels should be 100% renewable. At the same
time the only currently acknowledged pathway is a
system based on quotas that are put in place for low
concentrations (low blends) of renewable fuels blended
into crude oil refineries. Today’s refineries can only blend
low percentages of oxygen-containing renewable fuel
intermediates into their processes for both process and
construction material reasons. The current situation for
pure renewable fuels is thus weak, with a lack of support
mechanisms in most markets. Consequently, quotas
for 100% renewable fuels should also be introduced. A
necessary change in this respect is needed regardless of
which GHG-neutral fuel system one sees as the strongest
candidate, or rather candidates, to reach the goal of a
fully renewable transport sector.
In terms of CO₂ emission regulations, a cap-and-trade
system, the EU Emissions Trading System, for the
trading of carbon emission credits was introduced in
in 2005. Other countries that have also implemented
cap-and-trade programmes or carbon taxes include,
among others, South Korea, Australia, New Zealand,
Japan, Canada, Mexico, Argentina, the Chinese province
of Guangdong and the US state of California. Placing a
value on carbon is an important step in climate policies
to reflect the externalities created by pollution. A value
on carbon creates a business case for investment in
CCU, increased use of biomass and a progressive move
towards a net carbon-neutral society.
Renewable fuels are typically more expensive than fossil
fuels, and require higher up-front investment. Even though
methanol is one of the most cost-eective renewable fuels
to produce, this is also true for this alternative.
Policy instruments providing a long-term guaranteed
price floor for renewable methanol (as well as for
other promising fuel alternatives) would be beneficial
to remove some of the investment risks. A meaningful
production support system that could motivate
investment is a contract for dierence (CFD) scheme
in which advanced renewable fuel production projects
bid for – and winners are awarded – CFDs in so-called
reverse auctions (lowest bid wins). As illustrated in Figure
48, a CFD pays out the dierence between an uncertain
or insucient market price and the price required to
finance the project (strike price). Auctions are held on
a recurring basis according to set categories, each for a
dierent type of route to renewable fuels, with a specific
maximum administrative strike price and specific terms.
These parameters can change according to policy needs,
technology and cost reduction, leaving the government
in control – the key feature is that they do not change
for a project once oered and awarded, providing the
required long-term stability needed for finance.
CFDs are instruments that are well-known by capital
markets and which have been very successful, for
example, in developing and securing finance for oshore
and onshore wind in the UnitedKingdom and in Denmark
(UK GOV, 2020).

Figure 48. A hypothetical CFD smoothing returns in a volatile market
Source : Max Jönsson
RENEWABLE METHANOL 97
As part of the “EU Green Deal implementation” it has
been proposed to introduce a “carbon CFD” pilot scheme,
similar to tendering systems for renewable power, which
could pay the dierence between a CO
2
strike price and
the actual CO
2
price in the EUETS to bridge the cost gap
between conventional and decarbonised hydrogen (EU,
2020b). Applied at an EU or national level, an appropriate
state aid framework could be developed (2021 revised
state aid guidelines for energy and environmental
protection). This indicates that it should be possible to
expand the CFD mechanism, which was so successful in
helping to bring down the cost of wind power, to support
the commercial introduction of renewable methanol.
Policy experience has shown that picking winners at the
onset is not usually the best approach. To obtain the
best results, it seems that policies should be technology
and fuel agnostic and focus on the actual outcome, e.g.
lower pollutant emissions including CO
2
, sustainability,
and increased energy security though local production.
For this, LCAs and other benchmarks will be needed to
weight the benefit of each process and fuel.
In the transition to fully renewable methanol production,
the co-production of green and conventional products
with proportionate credit should also be allowed. These
include LCM technologies where green hydrogen is
added in the process of methanol production from
natural gas. This would allow for a progressive greening
of the methanol produced while keeping costs low. Once
the technologies (electrolyser) are scaled up and the
cost of renewable power low enough, the share of green
methanol, and credits, could increase.
Policies and tax incentives on fuel should be based on
energy content, not volume (e.g. USD per kWh, not
USD per litre); otherwise, the incentives would penalise
some renewable fuels that have lower energy density.
CFD payout
Market price proxy
Strike price/break-even price
Total price
C Construction
O Operation
0.80
0.70
0.60
0.50
0.40
0.30
0.20
0.10
0.00
0.90
1.00
O16O15O14O13O12O11O10O9O8O7O6O5O4O3O2O1C3C2C1 O17 O18
O19
O20

In 1997, the US Congress adopted the Taxpayer Relief
Act, which set the federal excise tax paid for alternative
fuels at the pump on a British thermal unit equivalency
with gasoline. For methanol, the federal excise tax was
reduced to USD0.0915 per gallon compared with the
excise tax for gasoline of USD0.184 per gallon. In 2013,
in Australia, methanol was granted excise tax-free status
(~38AU¢/litre) for 10 years to encourage its use as a fuel.
Energy tax reductions based on energy content can be
provided for renewable fuels including methanol fuels,
both bio-methanol and e-methanol. Taxation policy can
“make or break” alternative fuels.
Policies could also include eco-labelling of bio-and
e-based chemicals and products, information campaigns
and subsidies for producers of materials that would be
progressively phased out as technology matures and
production costs decrease.
Transitioning the global economy to carbon-neutral
energy will take massive investment in technology
development, infrastructure and deployment. Economies
of scale for renewable methanol production and use will
lead to competitive fuel pricing for multiple sectors. As a
liquid with the highest hydrogen-to-carbon ratio of any
liquid fuel, methanol can be a key energy carrier. Since
methanol can be utilised in existing combustion engines,
as well as more advanced powertrains and chemical
production processes, conventional grey and blue
methanol can be used today, with greater substitution
of green methanol over time. Renewable methanol is
uniquely positioned to be a future-proof fuel.

RENEWABLE METHANOL 99
AAAS (2020), “Thousand-ton scale demonstration of
solar fuel synthesis starts operation in Lanzhou,
China”, 17 January, Chinese Academy of Sciences
(CAS), https://www.eurekalert.org/pub_
releases/2020-01/caos-tsd011620.php (accessed
August 2020).
ABEL Energy (2020), Australia, https://www.abelenergy.
com.au/ (accessed July 2020).
AChT (Advanced Chemical Technologies) (2020), http://
advancedchemicaltech.com/ (accessed July 2020).
aet (2019), “Towards a new carbon-neutral economy in
the Ghent area of North Sea Port: Exploratory study
for the development of carbon capture and utilisation
for the Ghent area of North Sea Port; Study Carried
out by advanced energy technologies (aet)”, https://
stad.gent/sites/default/files/media/documents/
20191106_PU_CCUhub_Rapport%20A4%20EN.pdf
(accessed August 2020).
ALIGN-CCUS (2020), ALIGN-CCUS project, http://www.
alignccus.eu/ (accessed June 2020).
Andersson, K. and C. Márquez Salazar (2015), Methanol
as a Marine Fuel Report, report prepared for the
Methanol Institute by FCBI energy.
Arcoumanis, C. et al. (2008), “The potential of Di-Methyl
Ether (DME) as an alternative fuel for compression-
ignition engines: A review”, Fuel, Vol. 87, p. 1014.
Atsonios, K. et al. (2016), “Investigation of technical and
economic aspects for methanol production through
CO
2
hydrogenation”, International Journal of
Hydrogen Energy, Vol. 41, pp. 2202-2214.
Balcombe, P. et al. (2019), “How to decarbonise
international shipping: Options for fuels, technologies
and policies”, Energy Conversion and Management,
Vol. 182, pp. 72-88.
BASF (2018), https://www.basf.com/global/en/media/
news-releases/2018/11/p-18-370.html (accessed July
2020).
Basu, A. and J. M. Wainwright (2001), “DME as a Power
Generation Fuel: Performance in Gas Turbines”, New
Delhi, India, January, presented at the
PETROTECH-2001 Conf.
Bazzanella, A. M. and F. Ausfelder (2017), “Low carbon
energy and feedstock for the European chemical
industry – Technology study”, DECHEMA, Frankfurt
am Main.
Bell, D. et al. (2010), Coal Gasification and Its
Applications, Elsevier.
Bellotti, D. et al. (2019), “Economic feasibility of
methanol synthesis as a method for CO
2
reduction
and energy storage”, Energy Procedia, Vol. 158,
pp. 4721-4728.
Bellotti, D. et al. (2017), “Feasibility study of methanol
production plant from hydrogen and captured carbon
dioxide”, Journal of CO
2
Utilization, Vol. 21,
pp. 132-138.
Berggren, M. (2019), “Global methanol – State of the
industry”, presentation at the 22nd IMPCA Asian
Methanol Conference, Singapore, November 5-7.
Bertau, M. et al. (eds.) (2014), Methanol: The Basic
Chemical and Energy Feedstock of the Future:
Asinger’s Vision Today, Springer.
BioMCN (2020), BioMCN, https://www.oci.nl/operations/
biomcn/ (accessed July 2020).
BioTfuel (2020), Information obtained from a contact at
BioTfuel project, July.
BLE (2017), Evaluations und Erfahrungsbericht für das
Jahr 2016. Biomassestrom-
Nachhaltigkeitsverordnung. Biokraftsto-
Nachhaltigkeitsverordnung, Bundesanstalt für
Landwirtschaft und Ernährung, https://www.ble.de/
SharedDocs/Downloads/DE/Klima-Energie/Nachhaltige-
Biomasseherstellung/Evaluationsbericht_2016.html.
REFERENCES AND FURTHER
INFORMATION

INNOVATION OUTLOOK: 100
Blug, M. et al. (2014), “Methanol generation economics”,
in: Bertau, M. et al. (eds.) Methanol: The Basic
Chemical and Energy Feedstock of the Future:
Asinger’s Vision Today, Springer.
Bos, M. J. et al. (2020), “Wind power to methanol:
Renewable methanol production using electricity,
electrolysis of water and CO
2
air capture”, Applied
Energy, Vol. 264, p. 114672.
Boulamanti, A. and J. A. Moya (2017), “Production costs
of the chemical industry in the EU and other countries:
Ammonia, methanol and light olefins”, Renewable
and Sustainable Energy Reviews, Vol. 68,
pp. 1205-1212.
BP (2020), BP Statistical Review of World Energy 2020.
Bromberg, L. and W. K. Cheng (2010), “Methanol as an
alternative transportation fuel in the US: Options for
sustainable and/or energy-secure transportation”,
final report, Massachusets Institute of Technology
report PSFC/RR-10-12.
Bromberg, L. and D. R. Cohn (2010), “Heavy duty
vehicles using clean, high eciency alcohol engines”,
PSFC/JA-10-43, MIT, Cambridge.
Bromberg, L. and D. R. Cohn (2009), “Alcohol fueled
heavy duty vehicles using clean, high eciency
engines”, PSFC/JA-09-31, MIT, Cambridge.
Brown, A. et al. (2020), Advanced Biofuels – Potential
for Cost Reduction, IEA Bioenergy, https://www.
ieabioenergy.com/wp-content/uploads/2020/02/
T41_CostReductionBiofuels-11_02_19-final.pdf.
Brudermüller, M. (2019), Carbon management at BASF
– R&D strategies to reduce CO
2
, p 29.
Brynolf, S. et al. (2014), “Environmental assessment of
marine fuels: liquefied natural gas, liquefied biogas,
methanol and bio-methanol”, Journal of Cleaner
Production, Vol. 74, pp. 86-95.
bse Engineering (2020), http://www.bse-engineering.
eu, bse Engineering Leipzig GmbH, Germany
(accessed July 2020).
bse engineering (2019), Power-to-Methanol at Small
Scale, FlexMethanol 10 & 20 MW Module, bse
engineering/BASF, http://www.bse-engineering.eu/
(accessed June 2020).
Buddenberg, T. et al. (2016), “Power to fuel as a
sustainable business model for cross-sectorial energy
storage in industry and power plants”, presented at
the 5th Conference of Carbon Dioxide as a Feedstock
for Fuels, Chemistry and Polymers, Cologne, Germany,
6–7 December.
C3 Mobility (Closed Carbon Cycle Mobility) (2020),
http://www.c3-mobility.de (accessed August 2020).
Calvo Ambel, C. (2017), “Electrofuels what role in EU
transport decarbonisation?”, Transport &
Environment, https://www.transportenvironment.org/
sites/te/files/publications/2017_11_Briefing_
electrofuels_final.pdf (accessed November 2020).
Carbon2Chem (2020), Carbon2Chem project,
Fraunhofer UMSICHT, Carbon2Chem project
coordinator, https://www.umsicht.fraunhofer.de/en/
strategic-lines-of-research/carbon-cycle.html
(accessed June 2020).
Chaplin, A. G. (2013), “Renewable methanol. An analysis
of technological potentials in light of the EU biofuels
policy objectives of greenhouse gas savings, security
of supply and employment”, Master’s thesis,
Sustainable Energy Planning and Management,
Aalborg University.
Chatterton, C. (2019), “Methanol as a vessel fuel and
energy carrier”, Methanol Institute, presentation to
the International Tanker Technical Forum, Singapore,
12 September, https://www.methanol.org/wp-
content/uploads/2019/09/Methanol-as-a-vessel-fuel-
and-energy-carrier.pdf (accessed July 2020).
Chemrec (2020), Information obtained from contacts at
Chemrec.
Clary, J. J. (ed.) (2013), The Toxicology of Methanol,
Wiley, Hoboken, NJ.
Clausen, L. R. et al. (2010), “Technoeconomic analysis of
a methanol plant based on gasification of biomass
and electrolysis of water”, Energy, Vol 35,
pp. 2338-2347.

RENEWABLE METHANOL 101
Compagne, P. (2017), “Bio-methanol production at
BioMCN”, presentation, 10 May, https://brintbranchen.
dk/wp-content/uploads/2017/10/Paul-Compagne_
BioMCN.pdf (accessed August 2020).
Consoli, C. (2019), “Bioenergy and carbon capture and
storage, 2019 perspective”, Global CCS Institute,
https://www.globalccsinstitute.com/resources/
publications-reports-research/bioenergy
-and-carbon-capture-and-storage/.
CRI (Carbon Recycling International) (2020), http://
carbonrecycling.is/, Iceland (accessed July 2020).
CRI (2019), Technology Profile, Carbon Recycling
International, http://carbonrecycling.is/ (accessed
July 2020).
Daimler (2020), “History of fuel cell development at
Mercedes-Benz”, https://media.daimler.com/
marsMediaSite/en/instance/ko/History-of-fuel-cell-
development-at-Mercedes-Benz.xhtml?oid=9274161
(accessed July 2020).
DNV GL (2016), “Use of methanol as a fuel. Methanol as
a marine fuel: Environmental benefits , technology
readiness, and economic feasibility”, Report No. 2015-
1197, Rev. 2, prepared for the International Maritime
Organization (IMO) by DNV GL.
DNV GL (2020), Alternative Fuels Insight, DNV GL,
https://afi.dnvgl.com/ (accessed November 2020).
Dolan, G. A. (2019), “Overview of Global Methanol Fuel
Blending”, presentation at the Trinidad and Tobago
Methanol Fuel Blending Forum, Trinidad and Tobago,
24 January, https://www.methanol.org/wp-content/
uploads/2019/01/Dolan-TT-Methanol-Fuel-Blending-
Workshop-24-Jan-2018.pdf (accessed July 2020).
Dolan, G. A. (2020), “Methanol: emerging global energy
markets”, Methanol Institute, presentation for 16th
Annual State of the Energy Industry Forum,
Washington, DC, 23 January.
DOR (2020), “DOR Group”, http://www.dor-group.com/
(accessed November 2020).
EBA (European Biogas Association) (2020), Belgium,
https://www.europeanbiogas.eu/ (accessed
November 2020).
Ecoinvent (2019), Ecoinvent vs. 3.6, Ecoinvent
Association, Swiss Federal Institute of Technology
(ETH) Zurich, EPF Lausanne, Paul Scherer Institute
(PSI), Swiss Federal Laboratories for Material Science
and Technology (Empa), Agroscope, Institute for
Sustainability Sciences, https://www.ecoinvent.org/.
Edwards, R. et al. (2014), “Well-to-wheels analysis of
future automotive fuels and powertrains in the
European context”, Well-to-Wheels Report version
4a, JEC Well-to-Wheels Analysis, European
Commission Joint Research Centre (JRC), Institute for
Energy and Transport, Concawe, European Council for
Automotive R&D (EUCAR), https://publications.jrc.
ec.europa.eu/repository/bitstream/JRC85329/wtw_
report_v4a%20march%202014_final.pdf.
Edwards, R. et al. (2011), “Well-to-wheels analysis of
future automotive fuels and powertrains in the
European context”, Well-to-Wheels Report version
3c, JEC Well-to-Wheels Analysis, European
Commission Joint Research Centre (JRC), Institute for
Energy and Transport, Concawe, European Council for
Automotive R&D (EUCAR).
Ekbom, T. et al. (2003), “Technical and commercial
feasibility study of black liquor gasification with
methanol/DME Production as motor fuels for
automotive uses”, BLGMF, Altener Program of the
European Union.
Ellis, P. et al. (2019), “Renewable methanol synthesis
using electrochemistry”, presentation at the 15th
Biennial International Methanol Technology Operators
Forum (IMTOF), London, 16-19 June.
Ellis, J. and M. Svanberg (2018), “Expected benefits,
strategies, and implementation of methanol as a
marine fuel for the smaller vessel fleet”, SUMMETH
(Sustainable Marine Methanol), Deliverable D5.1,
http://summeth.marinemethanol.com/.
Energy Supply (2020), Fra PtX-idé til virkelighed:
Danmarks første eMethanolanlæg producerer
flydende el, https://www.energy-supply.dk/article/
view/726710/fra_ptxide_til_virkelighed_danmarks_
forste_emethanolanlaeg_producerer_flydende_
el#:~:text=Fra%20PtX%2Did%C3%A9%20til%20
virkelighed%3A%20Danmarks%20f%C3%B8rste%20

INNOVATION OUTLOOK: 102
eMethanolanl%C3%A6g%20producerer%20flydende
%20el,-Foto%3A%20Hydrogen%20Valley&tex
t=Under%20ledelse%20af%20virksomhederne%20
REintegrate,basis%20af%20brint%20og%20CO2.
Enerkem (2020a), Information obtained from contacts
at Enerkem, July 2020, https://enerkem.com/.
Enerkem (2020b), http://www.enerkem.com (accessed
November 2020).
Engelhardt, M. (2020), “Morocco-to-X: Badr Ikken and
his team at IRESEN plan to transform the country into
a hub for e-fuels”, Siemens Energy, Inc., https://www.
siemens-energy.com/global/en/news/magazine/
2020/power-to-x-morocco.html.
EU (European Union) (2020a), A European Green Deal.
EU (2020b), A Hydrogen Strategy for a Climate-Neutral
Europe, communication from the Commission to the
European Parliament, the Council, the European
Economic and Social Committee and the Committee
of the Regions, COM(2020) 301 Final, European
Commission, https://ec.europa.eu/energy/sites/ener/
files/hydrogen_strategy.pdf.
EU (2018), Directive (EU) 2018/2001 of the European
Parliament and of the Council of 11 December 2018 on
the promotion of the use of energy from renewable
sources (recast): RED II, European Parliament and
Council of the European Union, https://eur-lex.europa.
eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018
L2001&from=EN.
EU (2014), Directive 2014/94/EU of the European
Parliament and of the Council of 22 October 2014 on
the deployment of alternative fuels infrastructure, text
with EEA relevance, Ocial Journal of the European
Union, L 307/1.
EU (2012a), Proposal for a Regulation of the European
Parliament and of the Council amending Regulation
(EC) No 443/2009 to Define the Modalities for
Reaching the 2020 Target to Reduce CO
2
Emissions
from New Passenger Cars, COM(2012) 393 Final,
European Commission, http://eur-lex.europa.eu/
LexUriServ/LexUriServ.do?uri=COM:2012:0393:FIN:en
:PDF.
EU (2012b), Energy Roadmap 2050, https://ec.europa.
eu/energy/sites/ener/files/documents/2012_energy_
roadmap_2050_en_0.pdf.
EU (2009), Directive 2009/30/EC of the European
Parliament and of the Council of 23 April 2009
amending Directive 98/70/EC as regards the
specification of petrol, diesel and gas-oil and
introducing a mechanism to monitor and reduce
greenhouse gas emissions and amending Council
Directive 1999/32/EC as regards the specification of
fuel used by inland waterway vessels and repealing
Directive 93/12/EEC, Ocial Journal of the European
Union, L 140/88.
EU STF (Sustainable Transport Forum) (2019), https://
ec.europa.eu/transport/themes/clean-transport-
urban-transport/clean-power-transport/sustainable-
transport-forum-stf_en (accessed July 2020).
Eurostat (2020) https://ec.europa.eu/eurostat/web/
products-eurostat-news/-/DDN-20190521-1.
Fasihi, M. et al. (2019), “Techno-economic assessment
of CO
2
direct air capture plants”, J. Clean. Prod.,
Vol. 224, pp. 957-980.
Fastwater (FAST track to clean and carbon-neutral
WATERborne transport) (2020), https://www.
fastwater.eu/ (accessed July 2020).
FReSMe (From Residual Steel Gases to Methanol)
(2020), FReSMe, Project ID: 727504, European project
under Horizon 2020 Programme, http://www.fresme.
eu (accessed June 2020).
Fuels Europe (2020), Refining Products for our Everyday
Life, Statistical Report 2020, https://www.fuelseurope.
eu/.
Fuss, S. et al. (2018), “Negative emissions—Part 2: Costs,
potentials and side eects”, Environmental Research
Letters, Vol. 13, p. 063002.
Galindo Cifre, P. and O. Badr (2007), “Renewable
hydrogen utilization for the production of methanol”,
Energ. Convers. Manage., Vol. 48, pp. 519-527.

RENEWABLE METHANOL 103
Geely (2020), The Geely World of Methanol, Media
Center, Zhejiang Geely Holding Group, http://zgh.
com/media-center/story/the-geely-world-of-
methanol/?lang=en (accessed November 2020).
Goeppert, A. et al. (2014), “Recycling of carbon dioxide
to methanol and derived products – closing the loop”,
Chem. Soc. Rev., Vol. 43, pp. 7995-8048.
Goeppert, A. et al. (2012), “Air as the renewable carbon
source of the future: an overview of CO
2
capture from
the atmosphere”, Energ. Environ. Sci, Vol. 5,
pp. 7833-7853.
González-Aparicio, I. et al. (2017), “Opportunities of
integrating CO
2
utilization with RES-E: A power-to-
methanol business model with wind power
generation”, Energy Procedia, Vol. 114,
pp. 6905-6918.
GTI (2019), Low-Carbon Renewable Natural Gas (RNG)
from Wood Wastes, Gas Technology Institute, p. 12.
Gumpert Aiways (2020), https://www.rolandgumpert.
com/en/gumpert-aiways/ (accessed November
2020).
Hank, C. et al. (2018), “Economics and carbon dioxide
avoidance cost of methanol production based on
renewable hydrogen and recycled carbon dioxide –
power-to-methanol”, Sustain. Energy Fuels, Vol. 2,
pp. 1244-1261.
Hannula, I. and E. Kurkela (2013), “Liquid transportation
fuels via large-scale fluidised-bed gasification of
lignocellulosic biomass”, VTT Technology 91, VTT
Technical Research Centre of Finland, https://www.
vttresearch.com/sites/default/files/pdf/technology/
2013/T91.pdf.
Haraldson, L. (2015), “Methanol as a marine fuel”, 2015
CIMAC, Oslo, 28 January.
Held, M. et al. (2019), “On the energetic eciency of
producing polyoxymethylene dimethyl ethers from
CO
2
using electrical energy”, Energy & Environmental
Science, Vol. 12, (2019), pp. 1019-1034.
Hill, S. J. (2020), “Chinese coal miner starts on world’s
largest solar-powered hydrogen project”, Renew
Economy, 5 May, https://reneweconomy.com.au/
chinese-coal-miner-starts-on-worlds-largest-solar-
powered-hydrogen-project-72785/ (accessed August
2020).
Hobson, C. and C. Márquez (2018), Renewable Methanol
Report, Methanol Institute, https://www.methanol.
org/wp-content/uploads/2019/01/MethanolReport.
pdf (accessed August 2020).
Hoppe, W. et al. (2018), “Life cycle assessment of carbon
dioxide–based production of methane and methanol
and derived polymers”, Journal of Industrial Ecology,
Vol. 22, pp. 327-340.
HT (Haldor Topsoe) (2019a), “Methanol for a more
sustainable future”, https://info.topsoe.com/
emethanol (accessed June 2020).
HT (2019b), “Topsoe to build demonstration plant to
produce cost-competitive CO
2
-neutral methanol from
biogas and green electricity”, https://blog.topsoe.
com/topsoe-to-build-demonstration-plant-to-
produce-cost-competitive-CO
2
-neutral-methanol-
from-biogas-and-green-electricity (accessed
November 2020).
IEA (2012), Energy Technology Perspectives 2012:
Pathways to a Clean Energy System, International
Energy Agency, Paris
INOVYN (2020), “INOVYN plays role in ambitious ‘Power
to Methanol’ project in Antwerp”, https://www.inovyn.
com/news/inovyn-plays-role-in-ambitious-power-to-
methanol-project-at-antwerp/ (accessed July 2020).
IDA (International DME Association) (2020), http://
aboutdme.org/news-media-o (Accessed November
2020).
IRENA (2020a), “Recycle: bioenergy. Circular carbon
economy”, Report 05, International Renewable
Energy Agency, Abu Dhabi.
IRENA (2020b), “Global Renewables Outlook: Energy
transformation 2050”, International Renewable
Energy Agency, Abu Dhabi.
IRENA (2020c), “Green hydrogen cost reduction: Scaling
up electrolysers to meet the 1.5°C climate goal”,
International Renewable Energy Agency, Abu Dhabi.

INNOVATION OUTLOOK: 104
IRENA (2019a), “Advanced biofuels. What holds them
back?”, International Renewable Energy Agency, Abu
Dhabi.
IRENA (2019b), “Navigating to a renewable future:
Solutions for decarbonising shipping”, Preliminary
findings, International Renewable Energy Agency,
Abu Dhabi.
IRENA (2019c), “Renewable power generation costs in
2019”, International Renewable Energy Agency, Abu
Dhabi.
IRENA (2019d), “Global Energy Transformation.
A Roadmap to 2050”, International Renewable
Energy Agency, Abu Dhabi.
IRENA (2018), “Hydrogen from renewable power”,
Technology Outlook for the Energy Transition,
International Renewable Energy Agency, Abu Dhabi.
IRENA (2017), “Bioenergy for Sustainable Development”,
International Renewable Energy Agency, IEA
Bioenergy, Food and Agricultural Organization of the
United Nations, Abu Dhabi.
IRENA (2016a), “Innovation Outlook: Advanced Liquid
Biofuels”, International Renewable Energy Agency,
Abu Dhabi.
IRENA (2016b), “Boosting Biofuels: Sustainable paths to
greater energy security”, International Renewable
Energy Agency, Abu Dhabi.
IRENA (2014), “Global bioenergy: Supply and demand
projections”, a working paper for REmap 2030,
International Renewable Energy Agency, Abu Dhabi.
IRENA and IEA-ETSAP (2013), “Production of Bio-
Methanol”, Technology Brief 108, IEA-ETSAP and
IRENA.
Irlam, L. (2017), Global Cost of Carbon Capture and
Storage. 2017 Update, Global CCS Institute, https://
www.globalccsinstitute.com/resources/publications
-reports-research/global-costs-of-carbon-capture
-and-storage/.
IRS (United States Internal Revenue Service) (2020),
“Yearly Average Currency Exchange Rates”, https://
www.irs.gov/individuals/international-taxpayers/
yearly-average-currency-exchange-rates (accessed
November 2020).
JCAP (Joint Center for Artificial Photosynthesis) (2020),
https://solarfuelshub.org/ (accessed July 2020).
Jensen, M. F. (2019), “Blue World Technologies”,
presentation at the Brintbranchens Årsdag 2019 –
e-fuels, methanol and fuel cells, Christiansborg, 10
April, https://brintbranchen.dk/wp-content/
uploads/2019/04/E-fuels-methanol-and-fuel-cells-
Mads-Friis-Jensen.pdf (accessed August 2020).
JM (Johnson Matthey) (2020), https://matthey.com/en
(accessed July 2020).
Joo, O.-S. et al. (2004), “Camere process for methanol
synthesis from CO
2
hydrogenation”, in: J.-S. C. Sang-
Eon Park and L. Kyu-Wan (eds.), Stud. Surf. Sci. Catal.,
Elsevier.
Kajaste, R. et al. (2018), “Methanol – managing
greenhouse gas emissions in the production chain by
optimizing the resource base”, AIMS Energy, Vol. 6,
pp. 1074-1102.
Keith, D. W. et al. (2018), “A process for capturing CO
2
from the atmosphere”, Joule, Vol. 2, pp. 1573-1594.
Kim, J. et al. (2011), “Methanol production from CO
2
using solar-thermal energy: process development and
techno-economic analysis”, Energ. Environ. Sci.,
Vol. 4, pp. 3122-3132.
KIT (2020), Information obtained from a contact at the
Bioliq project at KIT (Kalsruhe Institute of Technology),
July 2020 and earlier, https://www.kit.edu/.
Klein, T. (2020), Methanol: A Future-Proof Fuel, a primer
prepared for the Methanol Institute, https://www.
methanol.org/wp-content/uploads/2020/03/Future-
Fuel-Strategies-Methanol-Automotive-Fuel-Primer.
pdf.
Kourkoumpas, D. S. et al. (2016), “Implementation of the
Power to Methanol concept by using CO
2
from lignite
power plants: Techno-economic investigation”,
International Journal of Hydrogen Energy, Vol. 41,
pp. 16674-16687.

RENEWABLE METHANOL 105
Kramer, U. (2018), Defossilizing the Transportation
Sector: Options and Requirements for Germany,
Forschungsvereinigung Verbrennungskraftmaschinen
e.V. (FFV), Germany, https://www.fvv-net.de/.
Landälv, I. (2017), “Methanol as a renewable fuel – a
knowledge synthesis”, Report No 2015:08, f3 The
Swedish Knowledge Centre for Renewable
Transportation Fuels, Sweden, www.f3centre.se.
Law, K. et al. (2013), “Methanol as a renewable energy
resource”, white paper prepared for the Methanol
Institute by TIAX LLC.
Leeson, D. et al. (2017), “A techno-economic analysis
and systematic review of carbon capture and storage
(CCS) applied to the iron and steel, cement, oil refining
and pulp and paper industries, as well as other high
purity sources”, International Journal of Greenhouse
Gas Control, Vol. 61, pp. 71-84.
Linde (2020), “Innovative dry reforming process”,
https://www.linde-engineering.com/en/innovations/
innovate-dry-reforming/index.html (accessed July
2020).
Liquid Wind (2020), https://www.liquidwind.se/
(accessed July 2020).
LowLands Methanol (2020), Information obtained from
a contact at LowLands Methanol, July 2020, https://
www.renewablemethanol.com/technology/.
Lumpp, B. et al. (2011), “Oxymethylene ethers as diesel
fuel additives of the future”, MTZ Worldwide
eMagazine, Vol. 72, pp. 34-38.
Lundgren, J. et al. (2017), “Methanol production via
black liquor gasification with expanded raw material
base”, f3 Swedish Knowledge Centre for Renewable
Transportation Fuels.
Maersk (2020), “Leading Danish companies join forces
on an ambitious sustainable fuel project”, 26 May
2020, https://www.maersk.com/news/
articles/2020/05/26/leading-danish-companies-join-
forces-on-an-ambitious-sustainable-fuel-project
(accessed August 2020).
Majer, S. and A. Gröngröft (2010), “Ökologische und
ökonomische Bewertung der Produktion von
Biomethanol für die Biodieselherstellung”, Kurzstudie,
Deutsches Biomasse Forschungs Zentrum (DBFZ).
Malins, C. (2017), “What role is there for electrofuel
technologies in European transport’s low carbon
future?”, Transport & Environment/Cerulogy, https://
www.transportenvironment.org/sites/te/files/
publications/2017_11_Cerulogy_study_What_role_
electrofuels_final_0.pdf (accessed November 2020).
Maniatis, K. et al. (eds.) (2018), Building Up the Future
– Cost of Biofuel, Sub-Group on Advanced Biofuels,
Sustainable Transport Forum, https://op.europa.eu/
en/publication-detail/-/publication/13e27082-67a2-
11e8-ab9c-01aa75ed71a1.
Matzen, M. et al. (2015), “Chemical storage of wind
energy by renewable methanol production: Feasibility
analysis using a multi-criteria decision matrix”,
Energy, Vol. 93, pp. 343-353.
Matzen, M. and Y. Demirel (2016), “Methanol and
dimethyl ether from renewable hydrogen and carbon
dioxide: Alternative fuels production and life-cycle
assessment”, Journal of Cleaner Production, Vol. 139,
pp. 1068-1077.
McCaskill, D. (2019), “Global Methanol Overview -
Cautiously Bearish”, presentation at Argus Methanol
Forum, Houston, TX, 9-11 September.
McGrath, K. M. et al. (2004), “Direct methanol fuel cells”,
J. Ind. Eng. Chem., Vol. 10, p. 1063.
MefCO
2
(Methanol Fuel from CO
2
) (2020), MefCO
2
,
Project ID: 637016, a European project under the
Horizon 2020 Programme, http://www.mefCO
2
.eu/
(accessed June 2020).
Methanex (2018), 2018 Responsible Care and
Sustainability, https://www.methanex.com/sites/
default/files/responsible-care/Methanex-2018-RCSR-
Full_rev-2019-11-16.pdf (accessed August 2020).
MI (Methanol Institute) (2020a), https://www.methanol.
org/ (accessed August 2020).

INNOVATION OUTLOOK: 106
MI (2020b), Methanol as a Marine Fuel, Methanol Institute,
https://www.methanol.org/ (accessed June 2020).
MI (2020c), Methanol Safe Handling Manual 5th Edition,
Methanol Institute, https://www.methanol.org/
(accessed November 2020).
MI (2020d), Methanol Use in Gasoline: Blending, Storage
and Handling of Gasoline Containing Methanol,
Methanol Institute, https://www.methanol.org/
(accessed July 2020).
Mignard, D. et al. (2003), “Methanol synthesis from flue-
gas CO
2
and renewable electricity: a feasibility study”,
Int. J. Hydrog. Energ., Vol. 28, pp. 455-464.
Mitsui Chemicals (2010), CSR Report 2010, https://
jp.mitsuichemicals.com/en/sustainability/index.htm.
Mitsui Chemicals (2009), CSR Report 2009, https://
jp.mitsuichemicals.com/en/sustainability/index.htm.
MMSA (Methanol Market services Asia) (2020),
“Methanol price and supply/demand”, Methanol
Institute, https://www.methanol.org/methanol-price-
supply-demand/ (accessed June 2020).
Moser, P. et al. (2018), “Demonstrating the CCU-chain and
sector coupling as part of ALIGN-CCUS – Dimethyl ether
from CO
2
as chemical energy storage, fuel and feedstock
for industries”, presentation at the 14th International
Conference on Greenhouse Gas Control Technologies,
GHGT-14, Melbourne, Australia, 21-25 October.
NextChem (2020a), “Waste to Chemicals Technologies,
Bio-Methanol from Waste”, Methanol Institute
Webinar M8 228, 2020, Fig. 18 and 25 of 51.
NextChem (2020b), Information obtained from a contact
at NextChem, July 2020, https://nextchem.it/.
Nouryon (2020), “Nouryon-led consortium wins EU
backing for pioneering green hydrogen project”, 22
Jan, https://www.nouryon.com/news-and-events/
news-overview/2020/nouryon-led-consortium-wins-
eu-backing-for-pioneering-green-hydrogen-project/
(accessed July 2020).
Nyári, J. et al. (2020), “Techno-economic barriers of an
industrial-scale methanol CCU-plant”, J. CO
2
Util.,
Vol. 39, p. 101166.
Oberon Fuels (2020), Information obtained from a
contact at Oberon Fuels, July 2020, and http://
oberonfuels.com/2019/06/13/oberon-fuels-secures-
2-9-million-grant-from-state-of-california-for-first-
ever-production-of-renewable-dimethyl-ether-rdme-
in-united-states/.
OCI (2020), OCI N.V. Investor Presentation, January,
https://www.oci.nl/media/1637/oci-nv-investor-
presentation-january-2020.pdf (accessed November
2020).
Olah, G. A. et al. (2018), Beyond Oil and Gas: The
Methanol Economy, 3rd ed., Wiley-VCH, Weinheim,
Germany.
Olivier, J. G. J. and J. A. H. W. Peters (2019), “Trends in
global CO
2
and total greenhouse gas emissions: 2019
report”, PBL, Netherlands Environmental Assessment
Agency, The Hague.
Olsson, O. et al. (2020), “Deployment of BECCS/U –
technologies, supply chain setup and policy options”,
IEA Bioenergy Task 40 webinar, 16 June, https://www.
ieabioenergy.com/wp-content/uploads/2020/06/
BECCUS-Webinar-Slide-OO20200616-final.pdf
(accessed November 2020).
Palcan Energy Corp. (2020), http://www.palcan.com/
(accessed November 2020).
Pedersen, T. H. and R. H. Schultz (2012), “Technical and
economic assessment of methanol production from
biogas”, Master’s thesis, Department of Energy
Technology University of Aalborg, Denmark, p. 35 of
138.
Pérez-Fortes, M. et al. (2016), “Methanol synthesis using
captured CO
2
as raw material: Techno-economic and
environmental assessment”, Applied Energy, Vol. 161,
pp. 718-732.
Perstorp (2020), “Perstorp plan to reduce carbon
emission with half million tons by producing
sustainable methanol”, https://www.perstorp.com/en/
news_center/pressreleases/2020/perstorp_
producing_sustainable_methanol (accessed Jan
2021)

RENEWABLE METHANOL 107
Pont, J. (2007), Full Fuel Cycle Assessment: Well-to-
Wheel Energy Inputs, Emissions, and Water Impacts,
State Plan to Increase the Use of Non-Petroleum
Transportation Fuels AB 1007 (Pavley), Alternative
Transportation Fuels Proceeding, consultant report,
prepared for the California Energy Commission by
TIAX LLC.
Pontzen, F. et al. (2011), “CO
2
-based methanol and DME
– Ecient technologies for industrial scale
production”, Catal. Today, Vol. 171, pp. 242-250.
QAFAC (Qatar Fuel Additives Company) (2020), Carbon
Dioxide Recovery (CDR) Plant, https://www.qafac.
com.qa/carbon-recovery-plant (accessed August
2020).
Räuchle, K. et al. (2016), “Methanol for renewable energy
storage and utilization”, Energy Technology, Vol. 4,
pp. 193-200.
Red Rock Biofuels (2020), Information obtained from a
contact at Red Rock Biofuels and in public domain,
July, https://www.redrockbio.com/.
REDcert (2020), https://redcert.org/en/ (accessed July
2020).
REintegrate (2020), https://reintegrate.dk/ (accessed
August 2020).
Reschetilowski, W. (2013), “Alternative resources for the
methanol economy”, Russ. Chem. Rev., Vol. 82,
pp. 624-634.
RH
2
C (2020), Renewable Hydrogen Canada, http://www.
renewableH
2
canada.ca/ (accessed July 2020).
Rivera-Tinoco, R. et al. (2016), “Investigation of power-
to-methanol processes coupling electrolytic hydrogen
production and catalytic CO
2
reduction”, International
Journal of Hydrogen Energy, Vol. 41, pp. 4546-4559.
Rönsch, S. et al. (2014), “Treibhausgasvermeidungskosten
von synthetischem Methan und Methanol aus
Biomasse und Braunkohle”, Chemie Ingenieur Technik,
Vol. 86, pp. 1678-1689.
Rubin, E. S. et al. (2015), “The cost of CO
2
capture and
storage”, International Journal of Greenhouse Gas
Control, Vol. 40, pp. 378-400.
S2Biom (2016), “D8.1 Overview report on the current
status of biomass for bioenergy, biofuels and
biomaterials in Europe”, https://www.s2biom.eu/en/.
Sanz-Pérez, E. S. et al. (2016), “Direct capture of CO
2
from
ambient air”, Chem. Rev., Vol. 116, pp. 11840-11876.
Saygin, D. and D. Gielen (forthcoming), Submitted for
publication, Zero emission pathway for the global
chemical and petrochemical sector.
Schmidt J. (2020), “Large scale renewable methanol –
chances and challenges from an industrial producers
view”, presentation for the webinar Methanol: A
Sustainable, Scalable, Storable Energy Carrier,
organised by Lund University/Fastwater Consortium,
5 November, https://fastwater.eu/methanol_webinar/
results.html (accessed December 2020).
Schnettler, A. et al. (2020), “Power-to-X: The crucial
business on the way to a carbon-free world”, Siemens
Gas and Power GmbH & Co. KG and Siemens Energy,
Inc., https://new.siemens.com/global/en/products/
energy/topics/power-to-x.html.
Schröder, J. et al. (2020), “Methanol as motor fuel”,
summary report, Annex 56, a report from the
Advanced Motor Fuels Technology Collaboration
Programme, https://www.iea-amf.org/.
Semelsberger, T. A. et al. (2006), “Dimethyl ether (DME)
as an alternative fuel”, J. Power Sources, Vol. 156, 497.
Seuser, W. (2020), “European Methanol Outlook: COVID-
19 Challenges for the Continent”, presentation at the
2020 International Methanol Conference – Looking
Beyond the Pandemic, 21-22 October.
SGS (2020), Methanol: Properties and Uses, SGS INSPIRE
Team, https://3xxngg2wmai7100rss2cgkmj-
wpengine.netdna-ssl.com/wp-content/
uploads/2020/03/SGS-INSPIRE-Methanol-
Properties-and-Uses.pdf (accessed November 2020).
Sheldon, D. (2017), “Methanol production - A technical
history”, Johnson Matthey Technol. Rev., Vol. 61,
pp. 172-182.
Shih, C. F. et al. (2018), “Powering the future with Liquid
Sunshine”, Joule, Vol. 2, pp. 1925-1949.

INNOVATION OUTLOOK: 108
Sileghem, L. et al. (2014), “Performance and emissions
of iso-stoichiometric ternary GEM blends on a
production SI engine”, Fuel, Vol. 117, pp. 286-293.
Simbeck, D. R. and E. Chang (2002), Hydrogen Supply:
Cost Estimate for Hydrogen Pathways - Scoping
Analysis, NREL/SR-540-32525, National Renewable
Energy Laboratory, Golden, CO.
Södra (2020b), https://www.atl.nu/teknik/sodra-
storinvesterar-i-biodrivmedel/ (accessed October
2020).
Södra (2020a), Information obtained from contacts at
Södra, July, https://www.sodra.com/sv/se/.
Soloveichik, G. (2018), “Electrified future of aviation:
batteries or fuel cells?”, presentation for arpa-e,
13 March, https://arpa-e.energy.gov/sites/default/
files/Grigorii-Soloveichik-Fast-Pitch-2018.pdf
(accessed August 2020).
Sorensen, E. (2015), “Minimizing associated gas flaring
with smaller scale methanol production”, presentation
at the EFI Gas Flare Reduction and Monetization
Forum, Denver, CO, 15-17 June.
Specht, M. and A. Bandi (1999), The Methanol Cycle –
Sustainable Supply of Liquid Fuels, Center for Solar
Energy and Hydrogen Research (ZSW), Stuttgart,
Germany, https://d-nb.info/1137531355/34.
Specht, M. et al. (1999), “Synthesis of methanol from
biomass/CO
2
resources”, in: B. Eliasson et al. (eds.),
Greenhouse Gas Control Technologies, Pergamon,
Amsterdam.
Specht, M. et al. (1998), “Comparison of CO
2
sources for
the synthesis of renewable methanol”, Stud. Surf. Sci.
Catal., Vol. 114, p. 363.
Stefánsson, B. (2019), “Commercial scale CO
2
-to-
methanol plant in Norway”, Carbon Recycling
International, presentation at CO
2
Value EU workshop
on Innovation Fund, Brussels, Belgium, 19 September.
Stena Line (2020), http://www.stenaline.com. (Accessed
November 2020).
SunGas Renewables (2020), Information obtained from
contacts at SunGas Renewables, July, https://www.
sungasrenewables.com/.
Swiss Liquid Future (2020a), “Fuel from water power,
water and CO
2
”, Swiss Liquid Future AG, http://www.
swiss-liquid-future.ch/ (accessed June 2020).
Swiss Liquid Future (2020b), “Fast-track to carbon
capture in Norwegian industry”, press release, 1 July
2020, https://www.swiss-liquid-future.ch/medien/
(accessed July 2020), and communication with SLF.
Synhelion (2020), https://synhelion.com/ (accessed July
2020).
Szima, S. and C.-C. Cormos (2018), “Improving methanol
synthesis from carbon-free H
2
and captured CO
2
: A
techno-economic and environmental evaluation”,
Journal of CO
2
Utilization, Vol. 24, pp. 555-563.
Temchin, J. (2003), “Analysis of market characteristics
for conversion of liquid fueled turbines to methanol”,
prepared for The Methanol Foundation and Methanex
by Electrotek Concepts.
Thyssenkrupp (2020a), https://www.thyssenkrupp-
uhde-chlorine-engineers.com/en/products/water-
electrolysis-hydrogen-production/ (accessed June
2020).
Thyssenkrupp (2020b), https://www.thyssenkrupp-
industrial-solutions.com/ (accessed June 2020).
Total (2020), e-CO
2
Met Project, From CO
2
to Methanol,
Information obtained from a contact at Total, 2020,
http://www.total.com.
Trans World Energy (2020), Information obtained from
a contact at Trans World Energy, July 2020, https://
www.twenergy.net/.
Tremel, A. et al. (2015), “Techno-economic analysis for
the synthesis of liquid and gaseous fuels based on
hydrogen production via electrolysis”, International
Journal of Hydrogen Energy, Vol. 40,
pp. 11457-11464.
TRI (2020), Information obtained from a contact at TRI
(ThermoChem Recovery International), July, https://
tri-inc.net/, and from the Fulcrum project, https://
fulcrum-bioenergy.com/company/projects/.

RENEWABLE METHANOL 109
Turner, J. and R. Pearson (2011), “Ternary blends of
gasoline”, proActive Mag., Vol. 42, pp. 27-31.
UK GOV (2020), “Contracts for Dierence (CfD)”, policy
paper, Department for Business, Energy & Industrial
Strategy, UK Government (accessed July 2020).
US DOE (Department of Energy) (2016), 2016 Billion-Ton
Report: Advancing Domestic Resources for a Thriving
Bioeconomy, Volume: Economic Availability of
Feedstocks, M. H. Langholtz, B. J. Stokes and L. M.
Eaton (Leads), ORNL/TM-2016/160, Oak Ridge
National Laboratory, Oak Ridge, TN.
US DOE NETL (National Energy Technology Laboratory)
(2014), Baseline Analysis of Crude Methanol
Production from Coal and Natural Gas, DOE/NETL-
341/101514, Final Report, 15 October.
van Kranenburg, K. et al. (2020), “E-fuels: Towards a
more sustainable future for truck transport, shipping
and aviation”, report, 20 September, TNO, VoltaChem,
SmartPort, http://publications.tno.nl/
publication/34636875/KDhcac/vankranenburg-2020-
efuels.pdf.
Värmlandsmetanol (2017), Information obtained from a
contact at Värmlandsmetanol, http://www.
varmlandsmetanol.se/.
Varone, A. and M. Ferrari (2015), “Power to liquid and
power to gas: An option for the German
Energiewende”, Renewable and Sustainable Energy
Reviews, Vol. 45, pp. 207-218.
VDMA (2020), “Position paper on the FuelEU-Maritime
Initiative”, Working Group Power-to-X for
Applications, German Mechanical Engineering
Industry Association (VDMA), https://p2x4a.vdma.
org/documents/27093545/48392715/VDMA%20
P2X4A%20FuelEU-Maritime_final_1588055844958.
pdf/960e0067-5da3-64c2-2b36-c64b2b762a1f.
Wang, M. and U. Lee (2017), “Life-cycle energy use and
greenhouse gas emissions of methanol pathways
from GREET Model”, presentation at the workshop on
Opportunities and Challenges for Methanol as Liquid
Energy Carrier, Stanford University, CA, 31 July–1
August, https://ngi.stanford.edu/sites/g/files/
sbiybj14406/f/Methanol_LCA_presentation_as_
Stanford%20Workshop-201707.pdf.
Wang, W.-C. et al. (2016), “Review of biojet fuel
conversion technologies”, Technical Report NREL/
TP-5100-66291, NREL, https://www.nrel.gov/docs/
fy16osti/66291.pdf (accessed August 2020).
Wang, Z. et al. (2015), “Performance, combustion and
emission characteristics of a diesel engine fueled with
polyoxymethylene dimethyl ethers (PODE3-4)/diesel
blends”, Energy Procedia, Vol. 75, pp. 2337-2344.
Waterfront Shipping/MOL (2020), https://www.wfs-cl.
com/ (accessed November 2020).
Wellinger, A. (2019a), “Overview of biomass and
biomethane: Flexibility and integration”, presented at
the 7th European Biomass Conference & Exhibition
(EUBCE), Lisbon, 27-30 May.
Wellinger, A. et al. (2019b), “Results of the Biomethane
Group”, ART Fuels Forum.
Westküste 100 (2020), Westküste 100, https://www.
westkueste100.de/en/news/ (accessed July 2020).
Wismann, S. T. et al. (2019), “Electrified methane
reforming: A compact approach to greener industrial
hydrogen production”, Science, Vol. 364, p. 756.
Wormslev, E. C. and M. K. Broberg (2020), “Sustainable
jet fuel for aviation. Nordic Perspective on the use of
advanced sustainable jet fuel for aviation”, update
2019, Nordic Energy Research, https://www.
nordicenergy.org/wp-content/uploads/2020/02/
Sustainable-Jet-Fuel-Update-FinalNER.pdf (accessed
August 2020).
ZEF (Zero Emission Fuels) (2020), https://
zeroemissionfuels.com/ (accessed July 2020).
Zhang, H. et al. (2019), “Techno-economic optimization
of CO
2
-to-methanol with solid-oxide electrolyzer”,
Energies, Vol. 12, p. 3742.
Zhu, J. Y. et al. (2000), “Methanol formation during
alkaline wood pulping”, TAPPI J. , Vol. 83.

INNOVATION OUTLOOK: 110
Annex 1. Some of the pros and cons of methanol and renewable methanol
ANNEXES
Pros Cons
+
Commonly produced on an industrial scale
with high yields and efficiency from various
carbon-containing feedstock. Natural gas
and coal today; biomass, solid waste and
CO
2
+ H
2
tomorrow.
+
Already used to produce hundreds of
everyday industrial chemicals and products
as well as consumer items.
+
Could be used for the production of aromatic
compounds (BTX) and other chemicals
currently obtained from petroleum.
+
No inherent technical challenges in scaling
up the production of methanol to meet the
needs of the transport or chemical industry
sectors.
×
Production from coal has a large carbon
footprint.
×
Production of renewable methanol remains
more expensive than fossil methanol.
×
Production of renewable methanol needs to
be scaled up.
×
Competition for renewable feedstock
(biomass, CO
2
, renewable power) with other
renewable alternatives.
Pros Cons
+
Methanol is a liquid. This makes it easy
to store, transport and distribute by ship,
pipeline, truck and rail.
+
Requirements for methanol storage and
transport are similar to other flammable
liquids such as gasoline, jet fuel, and ethanol.
+
Methanol used as an automotive fuel can be
dispensed in regular filling stations, requiring
×
Can be corrosive to some metals such
as aluminium, copper, zinc, titanium and
some of their alloys. Methanol may also
attack some plastics, resins and rubbers.
Compatible metals, plastics and elastomer
materials have to be selected.
×
Methanol can absorb moisture from the
atmosphere. To prevent this, methanol
should be stored in a sealed container
productIoN aNd chemIcal applIcatIoNS
propertIeS
, traNSport aNd Storage
RENEWABLE METHANOL 111
Pros Cons
+
Growing market for methanol use as a fuel.
Currently about 31% of methanol demand.
×
Competition with established fuels (gasoline,
diesel) as well as alternatives including
electrification, hydrogen, biofuels, CNG,
LPG, etc.
×
Relatively low volumetric energy content
compared to some fuels. About half the
volumetric energy density of gasoline and
diesel fuel.
Pros Cons
•
High octane rating (RON of 109) and high
knocking resistance. Allows the engine to run
at high compression ratios for higher efficiency
•
Can be blended with gasoline in various
ratios from 3% to pure methanol (e.g. M3,
M15, M85, M100). Concentrations up to 15%
(M15) can be used in regular gasoline cars.
Higher concentrations (e.g. M85) can be used
in flex-fuel vehicles (similar to E85).
×
Methanol has low vapour pressure at low
temperatures. Cold start system or higher
vapour pressure additives might be needed
×
Poor lubrication properties.
×
For optimum efficiency at higher
concentrations of methanol, engines might
need some modifications.
only minimal and relatively inexpensive
modifications.
+
Bunkering of methanol for marine
applications is similar to marine fuels such
as heavy fuel oil. Only minor modifications
to existing infrastructure are needed at a
modest cost.
+
When properly stored methanol is stable
and its shelf life is indefinite.
where there should be an allowance for
thermal expansion (larger tank, floating
roof tank, pressure relief valve). Moisture
absorbed by neat methanol is fully
miscible, and is retained as a single phase
that does not affect combustion. Moisture
absorbed by gasoline-methanol blends,
however, can form immiscible phases. If
the amount of water is small it has little
effect on combustion, but larger amounts
of water phase material can interfere with
combustion.
uSe aS a fuel
aS
a gaSolINe addItIve aNd SuBStItute

INNOVATION OUTLOOK: 112
+
High oxygen content (avoids fuel-rich
combustion zones).
+
High heat of evaporation.
+
Low lean flammability limit.
+
High volatility.
+
Compatible with hybrid (fuel/electric)
systems and vehicles.
+
Methanol-to-gasoline (MTG) offers another
derivative route, and can be used in existing
engines.
Pros Cons
+
Methanol can be used in combustion ignition
(diesel) engines.
+
Dimethyl ether derived from methanol is
a substitute for diesel fuel (high cetane
number). Methanol is also a main component
of biodiesel (biodiesel is obtained by
transesterification of plant oil and animal
fats with an alcohol). Oxymethylene ethers
(OME) derived from methanol are also being
tested as diesel substitutes.
+
Trucks with modified engines running on
methanol and DME are available or under
development.
×
Neat methanol in itself is a poor diesel
substitute (very low cetane number). To be
used in diesel-type engines it needs glow
plugs, additives or co-injection of small
quantities of diesel (~5%) to ignite when
compressed.
aS a dIeSel SuBStItute

RENEWABLE METHANOL 113
Pros Cons
+
Use of methanol as a marine fuel fulfils
the more stringent emission standards in
Emission Control Areas (ECAs) and new
global emission standard set by the IMO that
took effect in 2020 (0.5% sulphur content in
marine fuel starting in 2020, compared to
3.5% before). Renewable methanol can also
provide pathways to meet the IMO’s GHG
emission reduction ambitions.
+
Bunkering of methanol already widely
available in many ports around the world.
+
There are currently more than 20 large ships
in operation and on order operating on
methanol (DNV GL, 2020). Powered by diesel
engines modified to run on both methanol
and diesel. Methanol-optimised engines in
development are expected to perform even
better
×
Competing technologies (e.g. selective
catalytic reduction, scrubber, filter, exhaust
recirculation systems).
×
Competing fuels (e.g. low-sulphur fuel oil,
low-sulphur distillate fuels, LNG, hydrogen,
ammonia).
Pros Cons
+
Can be used in a direct methanol fuel cell
(DMFC) to produce electricity.
+
Good liquid hydrogen carrier (one litre of
methanol contains more hydrogen than a
litre of liquid hydrogen). Methanol is easily
reformed to hydrogen for use in fuel cells
(reformed methanol fuel cells).
+
Fuel in methanol-fired turbine engines.
+
Fuel for cookstoves, industrial boilers, kilns
and home heating.
×
DMFCs remain costly and capacity-limited.
×
Methanol reforming to hydrogen should
be further improved (e.g. minimise carbon
monoxide concentration in reformer outlet
to avoid additional treatment).
aS a marINe fuel
other
fuel uSeS

INNOVATION OUTLOOK: 114
Pros Cons
+
Lower pollutant emissions when combusted:
+
No carbon-carbon bonds allow for soot-free
combustion (no PM).
+
No SOx.
+
Lower NOx.
+
Low-carbon and renewable methanol can
provide reduced overall CO2 emissions
compared to fossil fuels.
×
Incomplete combustion can lead to
formaldehyde and formic acid pollutants.
Pros Cons
+
Some fuel and vehicle standards already in
place:
United States: ASTM D4814 (M2.7)
Europe: EN 228:2012+A1:2017(M3)
Israel: SI 90 parts 2 and 4 (M3-M15)
India: IS 17076:2019 (M15)
United States: ASTM D5797-18 (M51-M85)
China: GB/T 23510-2009 (M100)
China: GB/T 23799-2009 (M85)
China: Provincial standards
×
Methanol fuel standards need to be
expanded to allow for wider use in more
countries and for more applications.
pollutaNt emISSIoNS
fuel
aNd vehIcle StaNdardS
Note: More details can be found, for example, in MI (2020c); DNV GL (2016); Schröder (2020); and SGS (2020).

RENEWABLE METHANOL 115
Pros Cons
+
Safer fuel in fires than gasoline. Methanol
generates less heat and transfers less of
the heat to the surroundings. Methanol fires
can be extinguished with water or alcohol-
resistant foams.
+
Methanol in small concentrations is present
naturally in the human body and food and
drinks such as fruits, vegetables, beer, wine,
etc.
×
Highly flammable. Burns with a low-
temperature non-luminous clear blue flame
that might be difficult to see in bright light.
Combustion is also smokeless.
×
Can form explosive mixture in air.
×
Toxic. Toxic exposure can occur by inhalation,
skin and eye contact and ingestion. Ingestion
of more than 20 mL can be lethal; lesser
amounts are known to cause irreversible
blindness. Metabolism and toxicity of
methanol are similar to those of ethylene
glycol. The degradation products of methanol,
formaldehyde and formate are responsible for
its toxicity. Adequate precautions should be
taken while handling and dispensing.
Pros Cons
+
Methanol is water soluble and readily
biodegradable. Methanol dissolves completely
in water. When released into water, it will
rapidly disperse to low concentrations,
allowing micro-organisms occurring naturally
to degrade it in a relatively short time.
+
Methanol is used in water treatment plants for
denitrification. Methanol is an energy source
for the organisms breaking down the nitrogen-
containing compounds present in wastewater
+
Methanol is a naturally occurring substance
which does not bio-accumulate.
+
Non-environmentally hazardous according
to the dangerous goods regulations.
×
Spillage to the environment. When released
into soil, methanol could enter groundwater.
However, because methanol is readily
biodegradable its accumulation in soil or
groundwater is unlikely.
health aNd Safety
eNvIroNmeNt

INNOVATION OUTLOOK: 116
Annex 2. Overview of major methanol production processes from various carbon sources.
Methane reforming
Crude syngas
Syngas
CO
2
Methanol, bio-methanol
or e-methanol
Gasification
Air
separation
Oxygen
Electrolysis
Hydrogen
Mixing CO
2
/ H
2
Natural
gas
Biogas
Coal Waste
Biomass
CO
2
Renewable
power
Syngas cleaning and
conditioning
•
Water gas shift reaction
•
CO
2
removal
•
Hydrogen addition
•
Other
carBoN SourceS

RENEWABLE METHANOL 117
Annex 3. Comparison of renewable methanol with other fuels on a price per unit of energy basis
Fuel type
Price
(USD/GJ)
Price
(EUR/GJ)
Source
Fossil methanol 10.1-20.1 9.0-18.1 This report
Bio-methanol (current)
< USD6/GJ
feedstock cost
16.4-38.4 14.8-34.6 This report
USD6-15/GJ
feedstock cost
22.9-50.9 20.6-45.8 This report
Bio-methanol (mature process
2030-2050) cost
< USD 6/GJ
feedstock cost
11.4-27.8 10.3-25.0 This report
USD6-15/GJ
feedstock cost
17.8-42.4 16.1-38.2 This report
E-methanol (current) cost
From combined
renewable
source
41.2-81.4 37.1-73.3 This report
From DAC only 67.8-119.6 61.1-107.6 This report
E-methanol (mature process
2030-2050) cost
From combined
renewable
source
12.6-31.7 11.3-28.5 This report
From DAC only 14.5-31.7 13.0-28.5 This report
Gasoline (US Gulf Coast) before tax 16.9 15.2 EIA
Diesel (US Gulf Coast) before tax 16.0 14.4 EIA
Heating Oil No. 2 (New York Harbor), before tax 15.8 14.3 EIA
Jet fuel (US Gulf Coast), before tax 16.1 14.5 EIA
Petroleum oil (US, WTI) 11.7 10.5 EIA
Petroleum oil (Europe, Brent) 12.7 11.5 EIA
Gasoline (retail, average US, with tax) 23.4 21.0 AFDC
Diesel (retail, average US, with tax) 23.4 21.1 AFDC
LNG (retail, average US, with tax) 20.8 18.7 AFDC
CNG (retail, average US, with tax) 17.1 15.4 AFDC
Gasoline (retail, average EU, with tax) 48.9 44.0 EEA
Diesel (retail, average EU, with tax) 44.3 39.9 EEA
Notes: Values calculated according to the LHV of the fuel. Conversion factor used USD 1 = EUR0.9. Average of
prices over the past 10 years.
Sources: EIA (US Energy Information Administration), https://www.eia.gov; AFDC (Alternative Fuels Data Center), US DOE,
https://afdc.energy.gov/fuels/prices.html; EEA (European Environment Agency), https://www.eea.europa.eu/data-and-maps/indicators/fuel-prices-and-taxes.

INNOVATION OUTLOOK: 118
Annex 4. Overview of existing or planned facilities and technology providers for e-methanol
and bio-methanol production
plaNtS (exIStINg aNd projected)
e-methaNol
Country Company Start-up year
Capacity
(t/y)
Feedstock Source
Iceland
Carbon Recycling
International (CRI)
2011 4000
Geothermal CO
2
and H
2
from water electrolysis
CRI, 2020
Product sold
under the name
“Vulcanol”
China
Dalian Institute of
Chemical Physics
2020 1000
CO
2
and H
2
from water
electrolysis (PV)
AAAS, 2020
Sweden Liquid Wind
2023 (plan
for 6 facilities
by 2030)
45000
Upcycled industrial
CO
2
and H
2
from water
electrolysis
Liquid Wind, 2020
Australia
(Tasmania)
ABEL 2023 60000
Biogenic CO
2
and H
2
from water electrolysis
ABEL Energy,
2020
China
Henan Shuncheng
Group / CRI
2022 110000
CO
2
from limekiln and
H
2
from coke oven gas
CRI, 2020
Norway
Swiss Liquid
Future /
Thyssenkrupp
n/k 80000
CO
2
from ferrosilicon
plant and H
2
from water
electrolysis (hydro)
Swiss Liquid
Future, 2020a,
Swiss Liquid
Future, 2020b
Norway Joint Venture/CRI 2024 100000
CO
2
and H
2
from water
electrolysis
Stefánsson, 2019
Canada
Renewable
Hydrogen Canada
(RH
2
C)
n/k 120000
CO
2
and H
2
from water
electrolysis (hydro)
RH
2
C, 2020
Belgium
Consortium at the
port of Antwerp
n/k 8000
CO
2
and H
2
from water
electrolysis
INOVYN, 2020
Belgium
Consortium at the
port of Ghent
n/k
46000-
180000
Industrial CO
2
and H
2
from water electrolysis
aet, 2019
The
Netherlands
Consortium
Nouryon/Gasunie/
BioMCN/3 others
n/k 15000
CO
2
and H
2
from water
electrolysis
Nouryon, 2020
Germany Dow n/k
~
200000
CO
2
and H
2
from water
electrolysis
Schmidt, 2020
Denmark
Consortium of
companies
2023-2030 n/k
CO
2
from MSW and
biomass. H
2
from water
electrolysis (oshore
wind). Up to 1.3 GW
electrolyser capacity by
2030
Maersk, 2020
Germany Consortium n/k n/k
CO
2
from cement plant
and H
2
from water
electrolysis (wind)
Westküste 100,
2020

* Syngas conversion to methanol, which is further converted to ethanol.
** Plant capacity: (Saygin and Gielen, forthcoming) bio-methanol share is around 15%.
*** Biomethanol part: (Compagne, 2017).
**** Plant capacity: (OCI, 2020) bio-methanol share not given.
RENEWABLE METHANOL 119
plaNtS (exIStINg aNd projected)
BIo-methaNol
Country Company Start-up year
Capacity
(t/y)
Feedstock Source
United
States
LowLand Methanol
Consortium of
companies
2023 120 000
MSW/waste
wood
LowLands
Methanol, 2020
Sweden Södra Operational 5 250
Extraction from
pulping process
Södra
Canada Alberta Pacific Operational 3 000
Extraction from
pulping process
Alberta Pacific
Sweden Värmlandsmetanol Planning 100 000 Biomass
Värmlandsmetanol,
2017
Sweden Domsjö
Preliminary
engineering
147 000 Black liquor Chemrec
United
States
New Hope Energy 2023/24 715 000 Biomass New Hope Energy
Canada Enerkem Operational
30000
(ethanol*)
MSW Enerkem
Canada Enerkem
Under
construction
35000
(ethanol*)
MSW Enerkem
Netherlands
Enerkem
Consortium of
companies
Engineering
phase
215 000 MSW Enerkem
Spain Enerkem
Engineering
phase
215 000 MSW Enerkem
Germany BASF Operational
480
000**
Natural gas/
biomethane
BASF
Netherlands
OCI/BioMCN Operational
60
000***
Natural gas/
biomethane
OCI/BioMCN
United
States
OCI Beaumont Operational
1 075 000
****
Natural gas/
biomethane
OCI
Sweden Perstorp Planning 200 000
Biomethanol/
Green hydrogen
Perstorp, 2020

INNOVATION OUTLOOK: 120
techNology demoNStratIoN plaNtS (paSt aNd curreNt)
e-methaNol
Country Company
Start-up
year
Capacity
(t/y)
Feedstock Source
Sweden FReSMe 2019 1 t/d
CO
2
and H
2
waste
stream from steel
manufacturing
and H
2
from water
electrolysis
FReSMe, 2020
Germany MefCO
2
2019 1 t/d
Power plant flue
gas CO
2
and H
2
from
water electrolysis
MefCO
2
, 2020
Denmark
Power2Met
Danish
Consortium
2019 800 L/d
CO
2
from biogas
and H
2
from water
electrolysis (wind
and solar)
REintegrate,
2020
Germany Carbon2Chem 2020 50 L/d
CO
2
/CO/H
2
from
steel mill gases
and H
2
from water
electrolysis
Carbon2Chem,
2020
Germany
ALIGN-CCUS
Project DME from
CO
2
2020
50 L
DME/d
CO
2
from power
plant flue gas and
H
2
from water
electrolysis
ALIGN-CCUS,
2020
Switzerland
Swiss Liquid
Future
2012 75 L/d
CO
2
and H
2
from
water electrolysis
Swiss Liquid
Future, 2020a
Germany
TOTAL / Sunfire
e-CO
2
Met project
2022 1.5 t/d
CO
2
from a Refinery
and H
2
from water
electrolysis
TOTAL, 2020
Germany
bse Engineering
/Institute for
Renewable
Energy Systems
(IRES)
2020 28 L/d
CO
2
and H
2
from
water electrolysis
(wind)
bse
Engineering,
2020
Japan Mitsui 2009 100 t/y
CO
2
and H
2
from
water electrolysis
Mitsui
Chemicals,
2009, 2010
Korea
Korean Institute
of Science and
Technology
(KIST) /CAMERE
process
2004 100 kg/d
CO
2
from power
plant flue gas and
H
2
from water
electrolysis
Joo, 2004

RENEWABLE METHANOL 121
techNology demoNStratIoN plaNtS (paSt aNd curreNt)
BIo-methaNol
(gasification technologies generating syngas for methanol and other products)
Country Company
Start-up
year
Capacity
(t/y)
Feedstock Source
France
BioTfueL Demo
Project 2019
(BioTfuel, 2020)
2019
15 MW
feedstock
Biomass (torrefied)
To FT products
BioTfuel, 2020
Sweden Chemrec 2005
3 MW
feedstock
Black liquor to
methanol and DME
BioDME demo
plant Chemrec,
2020
Germany
KIT, Karlsruhe
Institute of
Technology
2013
1 t/hr
feedstock
pyrolysis oil from
straw to gasoline
via DME
KTI demo
project
KIT, 2020
UnitedStates
GTI 2012 19 t/d
Various biomass
materials to
gasoline, SNG and
other
GTI demo
plant
GTI, 2020
UnitedStates
TRI, ThermoChem
Recovery
International, Inc
Biomass and MSW
to FT products
TRI demo
project
TRI, 2020
Canada Enerkem 2009
48 dry
t/d
feedstock
MSW and biomass
to methanol and
ethanol
Enerkem
demo plant
Enerkem,
2020

INNOVATION OUTLOOK: 122
BIo-methaNol
(gaSIfIcatIoN techNologIeS producINg SyNgaS for further coNverSIoN e.g. to methaNol)
Country Company Start-up year
Capacity
(t/y)
Feedstock Source
UnitedStates
TRI,
ThermoChem
Recovery
International,
Inc
Technology
provider
The
gasification
unit can
have
multiple
parallel
trains. One
gasifier
train varies
in size from
20-30 MW
to 100-150
MW
feedstock.
Various
biomasses and
MSW
TRI
Germany
KIT, Karlsruhe
Institute of
Technology
Technology
provider
Pyrolysis oil and
char from straw
KIT
Sweden Chemrec
Technology
provider
Black liquor and
similar
Chemrec
Germany ThyssenKrupp
Technology
provider
Biomass ThyssenKrupp
Canada Enerkem
Technology
provider
MSW and
biomass
Enerkem
UnitedStates
GTI/Sungas
Technology
provider
Biomass GTI/Sungas
Italy NextChem
Technology
provider
MSW NextChem
Some of the techNology provIderS
e-methaNol
Country Company Start-up year
Capacity
(t/y)
Feedstock Source
Iceland
Carbon
recycling
International
(CRI)
Technology
provider
50 000-
100 000
CO₂ and H₂ from
water electrolysis
CRI, 2020
Germany
Thyssenkrupp/
Uhde/Swiss
Liquid Future
Technology
provider
3 600-
72 000
CO₂ and H₂ from
water electrolysis
Thyssenkrupp,
2020a
Germany
bse Engineering
/BASF
Technology
provider
8 200-
16 400
CO₂ and H₂ from
water electrolysis
bse Engineering,
2020
Denmark Haldor Topsoe
Technology
provider
Variable
CO₂ and H₂ from
water electrolysis
HT, 2019a
United
Kingdom
Johnson
Matthey
Technology
provider
Variable
100 000-
1 700 000
CO₂ and H₂ from
water electrolysis
JM, 2020


www.facebook.com/irena.org
www.twitter.com/irena
www.instagram.com/irenaimages
© IRENA 2021
www.irena.org
