Mal J Med Health Sci 16(SUPP16): 50-56, Dec 2020
50
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346)
ORIGINAL ARTICLE
The Changes in pH Levels, Blood Lactic Acid and
Fatigue Index to Anaerobic Exercise on Athlete After
NaHCO
3
Administration
Afif Rusdiawan
1
, Anindya Mar’atus Sholikhah
2
, Septyani Prihatiningsih
3
1
Department of Physical Education, Health, and Recreation, IKIP Budi Utomo Malang, Simpang Arjuno No.14B, Kauman,
Klojen, Malang, East Java 65119, Indonesia
2
Department of Education, Health, and Recreation Faculty of Sport Science, Universitas Negeri Surabaya, Rektorat Unesa,
Lidah Wetan, Lakarsantri, Surabaya, East Java 60213, Indonesia
3
Occupational Health and Safety, Department of Health, Faculty of Vocational Studies, Universitas Airlangga, Dharmawangsa
Dalam Selatan No. 68, Airlangga, Gubeng,Surabaya, East Java, 60286, Indonesia
ABSTRACT
Introduction: Previous studies have revealed that sodium bicarbonate consumption prior to physical activity
has a performance-enhancing effect on anaerobic activity. Methods: It was an experimental research using
retest and posttest design. 36 healthy professional athletes were randomly assigned to either NaHCO
3
group (SBC=18) or placebo group (PLA=18). Respondents in SBC were given sodium bicarbonate solution
at a dose of 0.4 gram/kg of bodyweight dissolved in 500 ml of aquades, while PLA group were given
mineral water. Sixty minutes after NaHCO3 administration, both groups were assigned to anaerobic
activity which was 300 m sprint test. pH level and lactic acid were measured 5 minutes after anaerobic activity,
while fatigue index was measured 30 minutes after anaerobic activity. Results: Significant differences between
SBC and PLA were found in pH level (p=0,000), blood lactic acid (p=0,000), and fatigue index (p=0,003).
Conclusion: The administration of NaHCO3 was able to prevent a decrease in pH and an excessive increase
of lactic acid and fatigue index as the result of anaerobic activity.
Keywords: Anaerobic activity, Blood lactic acid, Fatigue index, pH, Sodium bicarbonate
Corresponding Author:
Anindya Mar’atus Sholikhah, M.Kes.
Email: [email protected]
Tel: (+62)-8384-9865-215
INTRODUCTION
Aerobic and anaerobic exercise are two different type
of exercise classified based on the interval, intensity,
and muscle fibers involved (1). Anaerobic is a type of
sports activities that increases the concentration of lactic
acid in muscle cells. The increase of lactic acid causes a
decrease of pH cells, a decrease in pH causes a decrease
in reaction rate of catalyst and finally it decreases the
ability of metabolism and ATP production (2). Anaerobic
glycolysis exercise leads to excessive accumulation of
lactic acid in the blood which results in disruption of
muscle contraction (3). Accumulation of lactic acid and
carbon dioxide can lower the force or power, speed,
and cause fatigue (4). Previous study reported that the
increase in lactic acid would be followed by an increase
of fatigue index during an anaerobic (5).
In high intensity and short duration exercises, the
fulfillment of energy requirement increases almost 100
times (6). Oxidative phosphorylation is not able to
produce large amounts of energy in a short period of
time, therefore, the fulfillment of energy requirement of
this type of sport depends on the phosphagen system
and anaerobic glycolysis (7). The phosphagen system
can only provide energy for activities with a span
of 10-15 seconds, thus, anaerobic glycolysis is the
major metabolic pathway in high intensity exercise
(7). However, this anaerobic glycolysis metabolic
pathway produces by-product in the form of lactic
acid (8). Increased reliance energy causes lactic acid
accumulation. According to some researchers, lactic
acid accumulation causes muscle fatigue that arises
during the intense exercise (9).
Recovery from exercise or competition is an important
component of the overall sports paradigm. And the
most important thing from recovery is to support a
better performance in the next exercise or competition
(10). Recovery is useful for body adaptation after
51
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346)
Mal J Med Health Sci 16(SUPP16): 50-56, Dec 2020
physical activity. Increased recovery time helps athletes
in maintaining health and performance in order to
increase opportunities to enter or undergo a competition
favorably (11).
Strategies for optimizing recovery from exercise or
physical activity depend on type of exercise, specific
exercise, duration, intensity, and time between exercise
sessions or competitions (12). Successful recovery
involves many physiological and metabolic processes
that act to prepare athletes for the next competition or
exercise (12). Adequate recovery has been proven to
result in the recovery of physiological and psychological
processes, so that athletes can compete or train again
at the appropriate level. Recovery from exercise and
competition is very complex and usually depends on
the nature of the exercise performed and other external
stressors (13).
There are a number of popular methods used by
athletes to improve recovery. Its use depends on the
type of activity carried out, and the period until the next
exercise session. Some popular recovery techniques for
athletes include stretching, active recovery, compression
sportswear, hydrotherapy, massage, good quality sleep,
and nutrition. Nutrition is one of the best ways for
athletes to do recovery, such as consuming balanced
diet and drinks containing sodium bicarbonate.
Consuming sodium bicarbonate has been proven to
increase exercise tolerance, but its effect on intermittent
sports with high intensity is obscure (14). Bicarbonate
is an alkaline neutralizer, naturally regulating acid-base
homeostasis in the body. Chemically, bicarbonate binds
with hydrogen lactate ions to form water and carbon
dioxide, and carbon dioxide dissolved in the blood will
be released through the respiratory system (15).
Anerobic glycolysis will generate lactic acid due to
incomplete breakdown of glucose (16). The higher
level of lactic acid in the blood will engage a decrease
in blood pH which then cause physical fatigue and
alter physical appearance. Moreover, prolonged
physical activity or high intensity exercise will trigger
the formation of lactic acid (9). When exercise occurs,
anaerobic fatigue may develop as a result of lactic acid
accumulation (17). Lactic acid in muscle cells causes
lactic acidosis so that the neuromuscular junction
stops the nerve stimulation to the muscle fibers,
consequently, the muscles are unable to contract (18).
Acidic status in the blood (low pH) can cause
interference with various cellular muscle mechanisms
such as decreased aerobic resistance due to inhibition
of aerobic enzymes, poor movement coordination due
to inhibition of creatin phosphate formation, decreased
fat oxidation rate due to increased urea levels (19). A
method that can be used to slow down the occurrence
of fatigue due to lactic acid that accumulates in the
body during physical activity is providing alkaline fluids
such as sodium bicarbonate drinks. Sodium bicarbonate
(NaHCO
3
) is an alkaline that has the ability to damage
and oxidize acids in food ingredients. This happens
because NaHCO
3
is the weakest sodium alkali and has
a pH of 8.3 in aqueous solution (19).
Some literatures also explain that drinks containing
sodium bicarbonate can neutralize low pH levels during
exercise or physical activity, thus it can alleviate the
lactic acid production during exercise (20). Sodium
bicarbonate is a strong base that quickly reacts with H
+
and NaOH quickly release acid molecules in solution.
Alkali is a molecule generated by the formation of one
or more alkali-sodium metals such as potassium and
lithium (19). The acid solution will be removed by this
alkaline molecule because it can react quickly with H
+
.
Normal pH level of the liquid in human body ranges
from 7,34 to 7,40 (21). If the pH is out of this range, the
mechanism of homeostasis will improve buffer process,
and consequently it will set a new pH level (19).
A pH level beyond 7.4 is categorized as alkaline, and
previous studies revealed that sodium bicarbonate drink
is one of the high alkaline-containing liquids that that
causes blood become more alkaline at a low pH or
acidic (3). Consuming sodium bicarbonate is an effort
to reduce the body acidity due to the physical activity,
especially in doing exercise, thus, the acidic condition
or decrease in pH can be reduced to postpone the
risk of fatigue as a result of lactate ions and H
+
ions
accumulation (3).
Therefore, the authors are interested to analyze the
effect of NaHCO
3
on pH levels, blood lactic acid, and
fatigue index after anaerobic activity. The aim of this
research is to investigate whether the administration
of NaHCO
3
can withstand the decrease in pH level and
fatigue index as well as an increase in blood lactic acid
due to anaerobic activity so that this treatment can delay
fatigue and improve athletic performance, especially
athletes in intermittent sports such as badminton (22)
MATERIALS AND METHODS
This research was an experimental research with
a pre and post control group design. A total of 36
PBSI Jombang (Badminton Association of Indonesia
in Jombang district) athletes were employed as the
research subject with inclusion criteria aged 16-20
years old, male, having normal weight and BMI, and
agreed to participate in this study and followed all
the protocols. All participants were informed about
the research purpose, procedures, and any potential
risks that may develop as side effects. The research
subjects were then randomly divided into two groups
(PLA and SBC) with a total of 18 people in each group
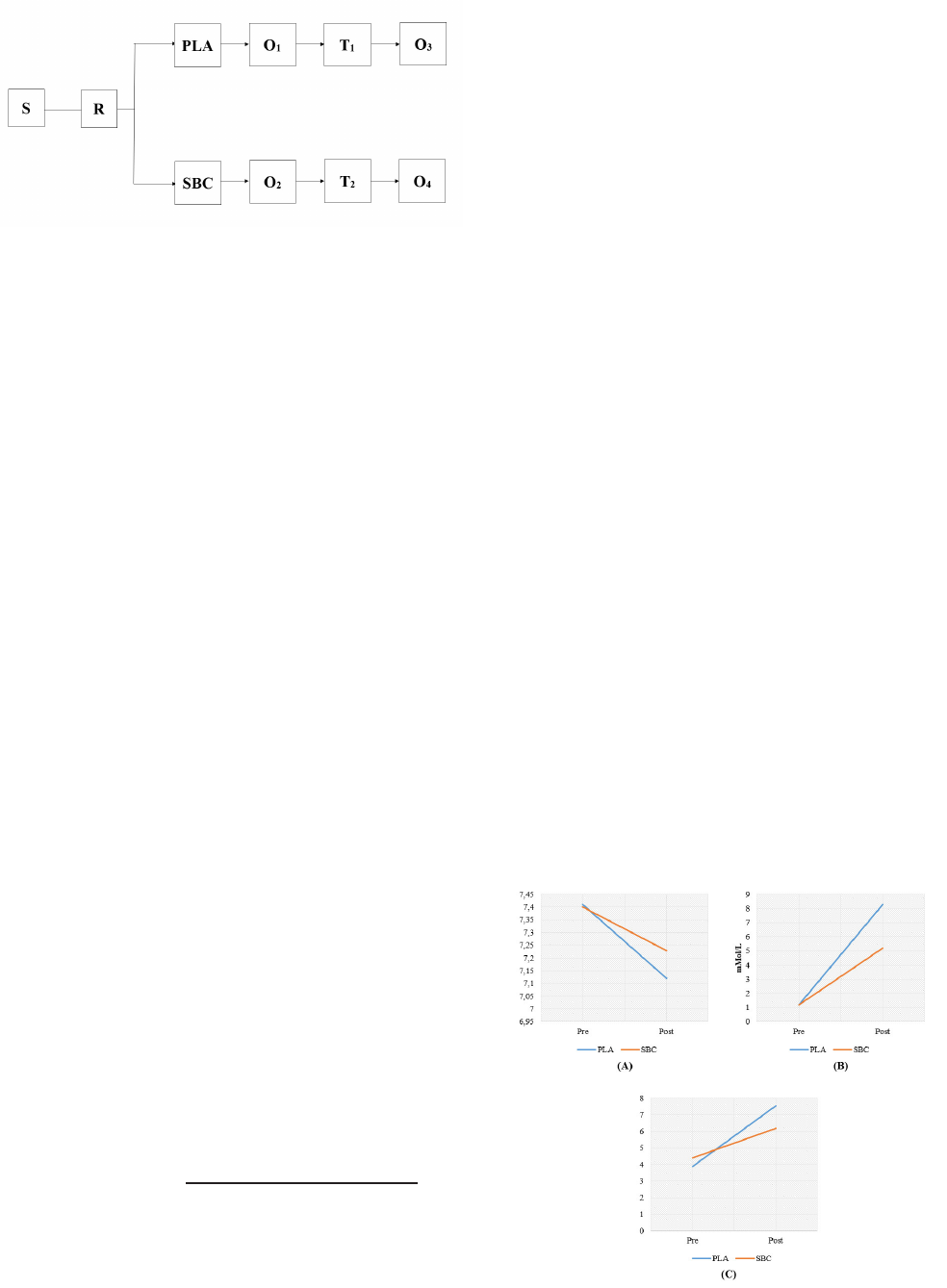
(See Fig. 1 below).
52
Mal J Med Health Sci 16(SUPP16): 50-56, Dec 2020
Remarks:
S : Sample
R : Randomization
PLA : Placebo group
SBC : Treatment group (with NaHCO3)
T1 : Mineral water
T2 : NaHCO3 with dose 0,4 gram/kg bodyweight in
500 ml mineral water
AA : Anaerobic activity
O1 : Pretest of PLA (pH levels, blood lactic acid, fatigue
index)
O2 : Pretest of SBC (pH levels, blood lactic acid,
fatigue index)
O3 : Post-test of PLA (pH levels, blood lactic acid,
fatigue index)
O4 : Post-test of SBC (pH levels, blood lactic acid,
fatigue index)
The research data were obtained from tests on blood
pH, blood lactic acid and anaerobic fatigue index
conducted 2 times (pretest and posttest). Blood pH
was measured with a pH meter 1-STAT tool and lactic
acid was measured using a lactate meter Roche Cobas
Accutrend Plus GCTL Meter. Blood pH and lactic acid
was employed by taking blood from the respondent’s
fingertips and then dripping on the instrument.
Whereas, the measurement of anaerobic fatigue index
used RAST-test (Running-based Anaerobic Sprint-test)
(23).
The procedure for carrying out the RAST-test was
done by asking the respondents to perform six 35-meter
sprint, with 10 seconds break for each sprint, then
the time was recorded. Next, speed (distance/time),
acceleration (speed/time), force (weight x acceleration)
and power (force x speed)were calculated (24). The
fatigue index is calculated using RAST calculator as
follows:
Fatigue index =
Before taking the pretest data, respondents were asked
to fast for 8 hours while still consuming mineral water
and checking their health condition both pulse and
blood pressure. Besides, respondents were also asked
questions about their medical history, eating habit and
physical activity carried out during the last 3 days.
30 minutes after the pretest, respondents consumed
sodium bicarbonate solution at a dose of 0.4 gram/
kg of bodyweight for the treatment group (SBC),
and drank mineral water for the placebo group (PLA)
(14). Sodium bicarbonate was dissolved in 500 ml of
aqua water (25). To avoid vomiting, sodium was given
in 3 stages: 60 minutes, 45 minutes and 30 minutes.
After 60 minutes of consuming sodium bicarbonate
or mineral water, the research subjects conducted
anaerobic activity by running 300 meters (26)(27).
Bloods were drawn after 5 minutes of conducting
anaerobic activity to re-measure pH and lactic acid
(posttest) (28). 30 minutes after anaerobic activity,
the subjects were measured their anaerobic fatigue
index by doing the RAST posttest.
Data were analyzed with statistical software and were
presented as mean values and standard deviation
(SD). Paired t-test was performed to compare pH level,
blood lactic acid, and fatigue index before and after
NaHCO
3
administration. Independent t-test was
performed to compare between groups. The tests were
all two-tailed and p<0,05 was considered statistically
significant.
RESULTS
Thirty-six healthy athletes were participated in this
study. The mean values and standard deviation of
physical characteristic (age, height, weight, and BMI) in
both groups are shown in Table I. For PLA group, the
mean value of age, height, weight, and BMI were 17,56
± 1,29, 168,89 ± 2,81 cm, 60,56 ± 3,85 kg, and 21,22
± 0,94, respectively. For SBC group, the mean value
of age, height, weight, and BMI were 18,44 ± 1,25,
167,00 ± 4,56 cm, 63,00 ± 3,85 kg, and 22,61 ± 1,40,
respectively.
Maximal Power - Minimal Power
Total time of 6 sprints
Fig. 1 : Research Design
Fig. 2 : The changes in pH levels (A), blood lactic acid (B); and fatigue
index (C) after anaerobic activity in control (PLA) and treatment
(SBC) group.

Mal J Med Health Sci 16(SUPP16): 50-56, Dec 2020
53
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346)
DISCUSSION
The Effects of anaerobic exercise on pH levels, blood
lactic acid and fatigue index
Based on the results of the statistical tests in this study,
anaerobic exercise was able to influence the pH levels,
serum lactate and fatigue index (see Table II). These results
are in line with research conducted by Rashidi, Salehian,
& Vaezi (2013) that blood lactate levels increased after
5 minutes of anaerobic activity. Increased blood lactate
was caused by the active anaerobic glycolysis system
in the muscles during anaerobic activity resulting the
production of lactic acid (29). The lactic acid from
metabolism result in the muscle then was secreted into
the blood. It caused the decrease of blood pH and the
blood become acidic. The decreased blood pH would
disrupt the activity of glycolytic enzymes and muscle
contraction, consequently, it would cause fatigue and
decreased performance (30).
Anaerobic activity is a high intensity exercise with a
short duration and fueled by energy sources that are
Fig. 2 shows the changes in pH levels, blood lactic
acid, and fatigue index after respondents in both groups
performed running exercise. Both groups experienced
a significant decrease in pH levels, but the PLA group
decreased more sharply. A significant increase was
found in blood lactic acid and fatigue index, with PLA
group experience a sharper increase than SBC.
Table I : Baseline characteristic of respondents in both group
Group
Mean ± SD
Age Height (cm) Weight (kg) BMI
PLA 17,56 ± 1,29 168,89 ± 2,81 60,56 ± 3,85 21,22 ± 0,94
SBC 18,44 ± 1,25 167,00 ± 4,56 63,00 ± 3,85 22,61 ± 1,40
PLA = mineral water + anaerobic exercise
SBC = group of NaHCO
3
+ anaerobic exercise intervention
Table II shows the changes in pH levels, blood lactic
acid and fatigue index in the PLA and SBC groups.
Paired sample t-test results showed that data before
(pre) and after (post) intervention was significantly
different p<0,05. It can be concluded that anaerobic
exercise affected pH levels, serum lactate and fatigue
index in both PLA and SBC groups.
Table II : Effect of anaerobic exercise on the changes of pH levels, blood lactic acid and fatigue index
Group
pH levels
Mean ± SD
sig.
Blood lactic acid
(mg/dl)
Mean ± SD
sig.
Fatigue index (watt/s)
Mean ± SD
sig.
PLA
pre 7,41 ± 0,02
0,000*
1,20 ± 0,57
0,000*
3,89 ± 1,20
0,000*
post 7,12 ± 0,01 8,26 ± 1,16 7,55 ± 1,77
SBC
pre 7,40 ± 0,02
0,000*
1,21 ± 0,35
0,000*
4,38 ± 1,29
0,000*
post 7,22 ± 0,02 5,17 ± 1,19 6,17 ± 0,66
*
significant difference at α=0,05
PLA= mineral water + anaerobic exercise
SBC= NaHCO
3
+ anaerobic exercise
To find out the difference in the effect of NaHCO
3
administration on pH levels, blood lactic acid and
fatigue index, the difference between the pre and post
data in the PLA group and the SBC group was calculated
using an independent sample t-test.
The results of the independent sample t-test are
presented in Table III. The results of the independent
sample t-test showed the value of p <0,05 on the pH
level variable (p = 0,000), blood lactic acid (p = 0,000),
and fatigue index (p = 0,003). These results indicated
that there were significant differences between the
PLA group and the SBC group at pH levels, blood lactic
acid and fatigue index after anaerobic exercise. Thus, it
can be concluded that there was an influence of giving
NaHCO
3
on pH levels, blood lactic acid and fatigue
index after anaerobic exercise.
metabolized without oxygen (31). Energy in anaerobic
exercise is obtained from the ATP-PC system and
anaerobic glycolysis (32). ATP-PC system and anaerobic
glycolysis causing lactic acid production (27). The
examples of anaerobic exercises are sprint, bicycle
racing, weight-lifting (33). 300 meters run is anaerobic
activity because it is a high intensity in a short duration
exercise (34).
Anaerobic activity metabolism causes the production
of lactic acid in the working muscles (8). The part
of produced lactic acid is released into the blood,
consequently it reduce blood pH and disturb the
acid-base balance (35). Lactic acid is a three-carbon
biomolecule with a carboxyl group and a hydroxyl
group. It is the final product of the anaerobic glycolysis
process produced by red blood cells and active muscle
Table III : Differences in pH levels, blood lactic acid, and fatigue index after anaerobic exercise in the PLA group and SBC group
Group
Δ pH levels
Mean ± SD
sig.
Δ Blood lactic acid (mg/dl)
Mean ± SD
sig.
Δ Fatigue index (watt/s)
Mean ± SD
sig.
PLA 0,291 ± 0,03
0,000*
-7,061 ± 1,31
0,000*
-3,658 ± 1,87
0,003*
SBC 0,183 ± 0,02 -3,961 ± 1,17 -1,791 ± 1,65
*
significant difference at α=0,05
PLA= mineral water + anaerobic exercise
SBC= NaHCO
3
+ anaerobic exercise
Mal J Med Health Sci 16(SUPP16): 50-56, Dec 2020
54
However, the administration of sodium bicarbonate
70-90 minutes before competing does not improve
the performance of rowing athletes (41). Sodium
bicarbonate is the monosodium salt of carbonic acid
with alkalinizing and electrolyte replacement properties
(45). After dissociation, sodium bicarbonate forms
sodium ions and bicarbonate. This ion formation will
increase plasma bicarbonate levels and act as buffer
in excess concentrations of hydrogen ion, thereby
increasing blood pH (45). Sodium bicarbonate that
enters the body will cause metabolic alkalosis which is
good for buffering lactic acid. However, consumption
of sodium bicarbonate can cause discomfort in the
stomach as it emits a lot of carbon dioxide (46). The side
effect of sodium bicarbonate consumption is a decrease
in cardiac output and impaired lactate clearance by the
liver (47).
When someone does exercise, the body will produce
lactic acid (48), therefore, the body needs additional
sodium bicarbonate to buffer lactic acid. Continuous
training can cause mineral deficiency and sodium
bicarbonate deficiency (electrolytes lost through sweat
or urination) which can cause latent tissue acidosis, pain,
edema, hyponatremia and death (15). The administration
of alkaline sodium bicarbonate is a good treatment and
it is commonly used in overcoming the problem of
acidosis (49). Sodium bicarbonate increases hydroxyl
ions or electron levels through increased alkalinity to
cells that protect metabolic acids (15). Nanang et al.
(2018) in his research revealed that giving pH 9 alkaline
water could prevent fatigue due to high lactic acid and
low pH after submaximal exercise. Sodium bicarbonate
that is absorbed by the body immediately binds with
pyruvate molecules to form oxaloacetate and malate,
strong binding occurs by bicarbonate against hydrogen
ions, so that it leads to functioning bicarbonate, and
LDH enzyme does not work optimally (50).
Lactate level in the SBC group was lower because
bicarbonate was a buffer against lactic acid.
Biochemically the role of bicarbonate is a binding of
H
+
ions in intracellular fluid and extracellular fluid to
form carbonic acid (H
2
CO
3
) Furthermore, carbonic acid
in the blood fluid will be brought to the respiratory
system into H2 O and CO2. Water and carbon dioxide
will then be excreted during the respiratory process.
Bicarbonate will combine with pyruvic acid in muscle
cells to form malate, then it will directly enter the Krebs’s
Cycle (50). The fatigue index of the SBC group was also
lower because the level of lactic acid was also low due
to the administration of sodium bicarbonate which was
buffered against lactic acid.
CONCLUSION
There was a decrease in pH level, as well as an increase
in lactic acid and fatigue index after anaerobic exercise.
The administration of NaHCO
3
was able to prevent a
cells (36). Lactic acid is a strong acid, and consequently
it will dissociate into lactic and H+. The increase of H
+
ions will decrease pH and cause acidosis (4).
The discussion of acidosis during the intense exercise
has been explained as a result of lactic acid production,
causing proton release and formation of sodium lactate.
This biochemical event is called as lactic acidosis (37).
Other theories reveal that acidosis is not the only way
to decreases the speed of observed contraction during
fatigue (38).
The pH change in the muscles that become acid will
inhibit the work of glycolysis enzymes, consequently,
it will disrupt the chemical reactions in the cell (39).
It causes the reduction of energy produced so that
the muscle contraction gets weaker and eventually,
and finally the muscles will experience fatigue. Low
pH levels will inhibit the performance of the enzyme
phosphofructokinase which plays an important role in
fulfilling muscle energy, so that it will cause muscle
fatigue (40).
From the above theories it can be concluded that
anaerobic exercise could increase the index of fatigue
due to the acid-base imbalance in the blood or it is called
as experiencing metabolic acidosis. Metabolic acidosis
was caused by an increase in lactic acid in the blood
that leads to decrease in blood pH and disruption the
performance of glycolytic enzymes to produce energy
used in muscle contraction.
The effect of NaHCO
3
administration on pH levels,
blood lactic acid and fatigue index
This study found that there was a significant difference
of pH levels as well as blood lactic acid with p-value
of zero and fatigue index (p = 0.003). The results of
this study indicated that the administration of NaHCO
3
affected the pH levels, serum lactate and fatigue index
after anaerobic exercise. In accordance with Hartono &
Sukadiono (2017) which stated that the administration
of sodium bicarbonate and sodium citrate at a dose of
300 mg/kg in 500 ml aqua was able to increase blood
pH and the time duration to fatigue after performing
anaerobic exercise (25). In line with research by Kupcis
et al. (2012) that there was an increase in blood pH and
a decrease in lactic acid after sodium bicarbonate was
given to the rowing athletes but there was no increase
in their performances (41). Krustrup in his study stated
that the administration of sodium bicarbonate at a dose
of 0,4 g•kg
−1
body weight could increase the results of
Yo-Yo IR2 performance by 14% higher than the placebo
group (14). These results indicated that administration
of sodium bicarbonate was able to increase fatigue
resistance.
Sodium bicarbonate has an ergogenic effect that can
improve performance in swimming, rowing, middle-
distance running, sprinting and boxing (42–44).
Mal J Med Health Sci 16(SUPP16): 50-56, Dec 2020
55
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346)
decrease in pH and an excessive increase of lactic
acid that led to a decrease in the fatigue index values.
However, the dosage given should be considered for
sodium bicarbonate has side effects such as headache,
stomachache, and diarrhea.
ACKNOWLEDGEMENT
This work was supported by PBSI (Badminton
Association of Indonesia) Jombang. The author is
thankful to the organizations and the athletes who
have supported and participated in this study. Without
their contribution, the data collection process in this
study would not have been possible.
REFERENCES
1. Patel H, Alkhawam H, Madanieh R, Shah N,
Kosmas CE, Vittorio TJ, et al. Aerobic vs anaerobic
exercise training effects on the cardiovascular
system. World J Cardiol. 2017;9(2):134–8.
2. Hopkins ETSSS. Physiology , Acid Base Balance.
StatPearls Publishing LLC; 2018. p. 2–7.
3. Wan J-J, Qin Z, Wang P-Y, Sun Y, Liu X. Muscle
fatigue: general understanding and treatment. Exp
Mol Med. 2017;49(10):384.
4. Westerblad H, Allen DG, Lännergren J. Muscle
fatigue: Lactic acid or inorganic phosphate the
major cause? News Physiol Sci. 2002;17(1):17–21.
5. Andersen LW, Mackenhauer J, Roberts JC, Berg
KM, Cocchi MN, Donnino MW. Etiology and
Therapeutic Approach to Elevated Lactate Levels.
Mayo Clin Proc [Internet]. 2013;88(10):1127–
40. Available from: http://dx.doi.org/10.1016/j.
mayocp.2013.06.012
6. Miladiyah I, Trunogati P, Lestariana W. Perbandingan
Efektivitas Teofilin (1,3-Dimethylxanthine) dan
Kafein (1,3,7-Trimethylxanthine) dalam Menunda
Kelelahan Otot pada Tikus. Mutiara Med J Kedokt
dan Kesehat. 2017;17(2).
7. Baker JS, Mccormick MC, Robergs RA. Interaction
among skeletal muscle metabolic energy system
during intense exercise. J Nutr Metab. 2010;2010.
8. Plowman SA, Smith DL. Anaerobic Metabolism
during Exercise Exercise physiology for health,
fitness, and performance. In: Sports-Specific
Rehabilitation. Elsevier Inc.; 2007. p. 39–63.
9. Cairns S. Lactic Acid and Exercise Performance,
Culprit or Friend? Sport Med. 2006;36(4):279–91.
10. Dalleck L. Post-Exercise Recovery. Am Counsil
Exerc. 2014;(161):10–4.
11. Mujika I. Recovery for Performance in Sport.
Hausswirth C, editor. France: Human Kinetics Inc.;
2013. 296 p.
12. Williams C. Recovery from exercise: role of
carbohydrate nutrition. Malaysian J Movement,
Heal Exerc. 2014;3:1–13.
13. Halson SL. Recovery Techniques for Athletes. Sport
Sci Exch. 2013;26(120):1–6.
14. Krustrup P, Ermidis G, Mohr M. Sodium bicarbonate
intake improves high-intensity intermittent exercise
performance in trained young men. J Int Soc Sports
Nutr. 2015;12(1):1–7.
15. Young RO. Using Sodium and Potassium
Bicarbonates in the Prevention and Treatment of
all Sickness and Disease. Int J Complement Altern
Med. 2017;9(6).
16. Brooks GA, Gladden LB. The Metabolic
Systems: Anaerobic Metabolism (Glycolytic and
Phosphagen). In: Exercise Physiology. Springer
New York; 2003. p. 322–60.
17. Takeda K, Machida M, Kohara A, Omi N, Takemasa
T. Effects of citrulline supplementation on fatigue
and exercise performance in mice. J Nutr Sci
Vitaminol (Tokyo). 2011;57(3):246–50.
18. Wada M, Mishima T, Yamada T. The role of lactic
acid in muscle contraction. Taiikugaku kenkyu
(Japan J Phys Educ Heal Sport Sci. 2006;51(3):229–
39.
19. Nanang M, Fuad N, Didik R, Topo S, Panuwun J.
Effect of Alkaline Fluids to Blood pH and Lactic
Acid Changes on Sub Maximal Physical Exercise
Effect of Alkaline Fluids to Blood pH and Lactic
Acid Changes on Sub Maximal Physical Exercise.
IOP Conf Ser Earth Environ Sci. 2018;197:12049.
20. Hadzic M, Eckstein ML, Schugardt M. The impact
of sodium bicarbonate on performance in response
to exercise duration in athletes: A systematic
review. J Sport Sci Med. 2019;18(2):271–81.
21. Sandhya TTAAB. Internal Ph in Health and Disease.
Int J Curr Res. 2016;8(7):34315–20.
22. Aydoğmuş M, Arslanoğlu E, Özmen T. Effect of
Badminton Specific Training Versus Badminton
Match on Aerobic Fitness. Online J Recreat Sport.
2015;4(2):12–5.
23. Mackenzie B. Running-based Anaerobic Sprint
Test - RAST. 1998.
24. Nasuka, Santosa I, Setiowati A, Indrawati F. The
Running-based Anaerobic Sprint Test of different
Type of Sports. J Phys Conf Ser. 2019;1387(1).
25. Hartono S, Sukadiono. The effects of sodium
bicarbonate and sodium citrate on blood pH,
HCO3-, lactate metabolism and time to exhaustion.
Sport Mont. 2017;15(1):13–6.
26. R. Callister, A. Miller, E. Aguiar, B. Dascombe C,
Smith, L. Clark TR. Blood lactate levels support
classification of the 300 m shuttle run as an
anaerobic capacity field test. J Sci Med Sport.
2010;13:e29–30.
27. Vilmi N, Ayramo S, Nummela AT, Pullinen T.
Oxygen uptake, acid-base balance and anaerobic
energy system contribution in maximal 300-400 m
running in child, adolescent and adult athletes. J
Athl Enhanc. 2016;5(3).
28. Pennington C, Kinesiology MS. The Exercise Effect
on the Anaerobic Threshold in Response to Graded
Exercise. Int J Heal Sci. 2015;3(1):225–34.
29. Rashidi M, Salehian O, Vaezi G. The effect of
Mal J Med Health Sci 16(SUPP16): 50-56, Dec 2020
56
high intensity anaerobic training on the blood
lactate levels after active recovery. Eur J Exp Biol.
2013;3(6):346–50.
30. Sesboüé B, Guincestre JY. La fatigue musculaire.
Ann Readapt Med Phys. 2006;49(6):257–64.
31. Patel H, Alkhawam H, Madanieh R, Shah N,
Kosmas CE, Vittorio TJ. Aerobic vs anaerobic
exercise training effects on the cardiovascular
system . World J Cardiol. 2017;9(2):134.
32. Mancha-Triguero D, García-Rubio J, Antúnez
A, Ibáñez SJ. Physical and Physiological Profiles
of Aerobic and Anaerobic Capacities in Young
Basketball Players. Int J Environ Res Public Health.
2020;17(4):1409.
33. Manansang GR, Rumampuk JF, Moningka MEW.
Perbandingan Tekanan Darah Sebelum dan
Sesudah Olahraga Angkat Berat. J e-Biomedik.
2018;6(2).
34. Hecksteden A, Heinze T, Faude O, Kindermann
W, Meyer T. Validity of Lactate Thresholds in
Inline Speed Skating. J Strength Cond Res. 2015
Sep;29(9):2497–502.
35. Chycki J, Kurylas A, Maszczyk A, Golas A, Zajac
A. Alkaline water improves exercise-induced
metabolic acidosis and enhances anaerobic
exercise performance in combat sport athletes.
PLoS One. 2018;13(11):1–10.
36. Marshall W, Lapsley M, Day A, Ayling R.
Biochemical aspects of anaemia. In: Clinical
Biochemistry: Metabolic and Clinical Aspects. 3rd
Editio. Churchill Livingstone; 2014. p. 515–32.
37. Shalayel M, Ahmed S. Lactic acid – the innocent
culprit of muscle fatigue. Sudan J Med Sci.
2010;5(2).
38. Theofilidis G, Bogdanis G, Koutedakis Y,
Karatzaferi C. Monitoring Exercise-Induced Muscle
Fatigue and Adaptations: Making Sense of Popular
or Emerging Indices and Biomarkers. Sports.
2018;6(4):153.
39. Šimčíková D, Heneberg P. Identification of alkaline
pH optimum of human glucokinase because
of ATP-mediated bias correction in outcomes
of enzyme assays. Sci Rep. 2019;9(November
2018):1–6.
40. Bruton JD, Lännergren J, Westerblad H. Effects
of CO2-induced acidification on the fatigue
resistance of single mouse muscle fibers at 28°C. J
Appl Physiol. 1998;85(2):478–83.
41. Kupcis PD, Slater GJ, Pruscino CL, Kemp JG.
Influence of Sodium Bicarbonate on Performance
and Hydration in Lightweight Influence of Sodium
Bicarbonate on Performance and Hydration in
Lightweight Rowing. Int J Sports Physiol Perform.
2012;7:11–8.
42. MacNaughton LR. The effect of sodium bicarbonate
on rowing ergometer performance in elite rowers.
Aust J Sci Med Sport . 1991;23(3).
43. Zajac A, Cholewa J, Poprzecki S, Waśkiewicz Z,
Langfort J. Effects of sodium bicarbonate ingestion
on swim performance in youth athletes. J Sport Sci
Med. 2009;8(1):45–50.
44. Siegler JC. Sodium bicarbonate ingestion and
boxing performance. J strength Cond Res.
2010;24(1).
45. Carr AJ, Slater GJ, Gore CJ, Dawson B, Burke LM.
Effect of sodium bicarbonate on [HCO3-], pH, and
gastrointestinal symptoms. Int J Sport Nutr Exerc
Metab. 2011;21(3):189–94.
46. Ferreira CR, van Karnebeek CDM. Inborn errors of
metabolism. In: Handbook of Clinical Neurology.
Elsevier B.V.; 2019. p. 449–81.
47. Levraut J, Labib Y, Chave S, Payan P, Raucoules-
Aime M, Grimaud D. Effect of sodium bicarbonate
on intracellular pH under different buffering
conditions. Vol. 49, Kidney International. 1996.
48. Ishii H, Nishida Y. Effect of lactate accumulation
during exercise-induced muscle fatigue on
the sensorimotor cortex. J Phys Ther Sci.
2013;25(12):1637–42.
49. Forsythe SM, Schmidt GA. Sodium bicarbonate
for the treatment of lactic acidosis. Chest.
2000;117(1):260–7.
50. Wang Y, Huang Y, Yang J, Zhou F, Zhao L,
Zhou H. Pyruvate is a prospective alkalizer to
correct hypoxic lactic acidosis. Mil Med Res.
2018;5(13):1–9.
