
Eastern Journal of Medicine 18 (2013) 26-31
Case Report
26
Fat embolism: A report of three cases
Elif Tanrıverdio, Aysegul Karalezli, Aysegul Senturk, Berna Botan Yildirim
*
, Hatice Canan
Hasanoglu
Department of Pulmonology, Ataturk Training and Research Hospital, Ankara, Turkey
Abstract. Fat embolism syndrome (FES) is a rare complication which usually follows long bone fracture. Clinical
manifestations present immediately or 24 to 72 hours after injury. Symptoms are dyspnea, tachypnea, tachycardia,
fever, mental status changes and petechial rashes. We reported three cases of FES. The first case had admitted to
the pulmonology department with confusion and respiratory distress within 24 hours after tibia fracture. He had
petechial rashes on the axillar area and subconjunctival hemorrhages. He was diagnosed as FES and treated. The
second case who had right femur fracture had stupor after 48 hours. He had axillary petechial rashes and
respiratory distress. He was diagnosed as FES and treated. The third case had admitted to the pulmonology
department with the complaints of axillary petechial rashes, subconjunctival hemorrhages after a pelvic fracture.
Both first and second patients who had respiratory failure intubated. The third patient died despite treatment. The
signs of this syndrome must be carefully examined and considered in the diagnosis of the patients which attend the
emergency service with confusion and petechial rashes after long-bone fractures.
Key words: Fat embolism, diagnosis, therapy
1. Introduction
Fat embolism syndrome (FES) is a rare
complication that usually seen in long-bone
fractures. The incidence and mortality rates are
unknown because of comorbid injury and other
problems (1). Clinical findings can be seen
immediately after the fracture causing embolism
or may also occur between 24 and 72 hours after
the accident (2). It is a multisystemic disease
which pulmonary, neurological, hematological,
and dermatological system involvements are
observed. Dyspnea, tachypnea, tachycardia, fever,
axillary petechial rash, and mental changes are
the common symptoms of the disease and early
diagnosis and treatment is important (2).
We presented 3 cases of fat embolism as a rare
complication of long-bone fractures and we
believe that it will contribute for early diagnosis
and treatment.
*
Correspondence: Dr. Berna Botan Yildirim
Department of Pulmonology, Ataturk Training and Research
Hospital, Ankara, Turkey
E-mail: [email protected]
Tel: +903122912525/ 4304
Received: 18.05.2011
Accepted: 01.02.2012
2. Case report
2.1. Case
The 29 years old male patient with tibia
fracture had been admitted to the orthopedic
clinic and was referred to our clinic because of
respiratory distress and confusion. The patient
was also referred to neurology clinic because he
developed mental confusion in the last 24 hours
and this is followed by two generally tonic-clonic
seizures each lasted 5 minutes. Treatment was
started with intravenous mannitol, steroids, and
antiepileptic agents.
In physical examination there was no respond
to verbal stimuli. He had tachypnea, and
tachycardia. Arterial blood pressure was 100/80
mmHg, and fever was 37.2 °C. In the patient's
follow-up, the fever reached to 38°C,
subconjuctival hemorrhage, petechial rashes
occur in axillary and neck region was observed
(Figures 1a, b). Fundus examination was normal.
Arterial blood gases (ABG) analysis was pH:
7:48, PO
2
: 49.7 mmHg, pCO
2
: 30.4 mmHg, SaO
2
:
85.7%, HCO
3
: 22.9 mmol/L, respectively.
hemoglobin (Hb) level was 12.6 g/dL (N: 13.5-
18) and platelets (PLT) 344 K/uL at admission
and Hb level was 9.8 g/dL and PLT level was 144
E. Tanrıverdio et al / Fat embolism
27
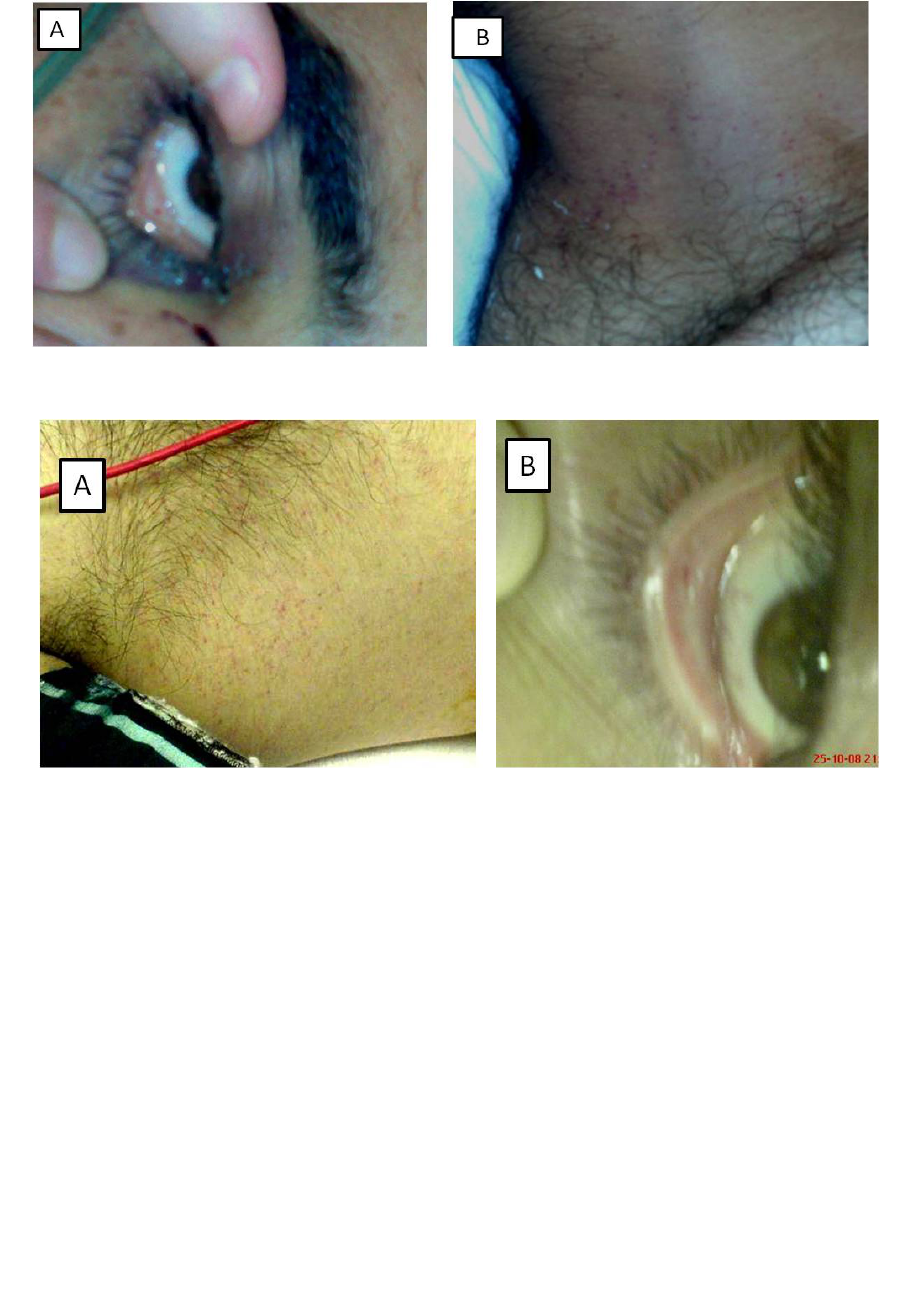
Fig. 1(a, b). Subconjuctival hemorrhage, and petechial rashes are seen in the neck (case 1).
Fig. 2 (a, b). Axillary petechial rash, and subconjuctival hemorrhage are seen (case 2).
K/uL at diagnosis. Other biochemical parameters
and urinary findings were normal. D-dimer was
2607 ng/mL (N: 000-500), and fibrinogen was
587 mg/dL (N: 150-400). Chest radiography was
normal. In Thoracic CT, suspicious filling defects
suggesting of pulmonary embolism were reported
in the middle segmental and subsegmental
branches of the left pulmonary artery. Bilateral
lower extremity venous doppler ultrasound was
normal. The current clinical findings suggested
FES and the patient underwent supporting
treatment, intravenous steroids, and subcutaneous
low-molecular-weight heparin. He regained
consciousness in the fifth day. The patient
clinically recovered and was discharged after
being operated.
2. 2. Case
23-year-old male patient was admitted to
orthopedics service with right femoral shaft
fracture and was consulted to our clinic because
of confusion and respiratory distress, 48 hours
after the accident. Considering that confusion
may be due to trauma, brain CT was taken within
12 hours interval and no pathology was detected.
He did not respond to verbal stimuli in physical
examination. Arterial blood pressure was 100/55
mmHg, and pulse rate was 106/min. Respiratory
system examination was normal. There was
petechial rash on right axillary (Figure 2a). The
patient had subconjuctival hemorrhage (Figure
2b) Ocular fundus evaluated by ophthalmology
service, and clinical examination was normal.
Fever rose up to 39.5 °C during follow-up. ABG
analysis results were as follows: pH: 7:51, pO2:
61.4 mmHg, pCO2: 26 mmHg, SO2: 90.4% and
HCO3: 20.7 mmol/L, respectively. Hematology
results were as follows: Leucocyte: 15.7 K/uL,
neutrophils 84.6%, lymphocytes 8.7%, Hb: 13.5
g/dL, Htc: 38.2%, and PLT: 299 K/uL. He had
higher levels of WBC but it returned to normal
after one day. Urine output, urea and creatinine

Eastern Journal of Medicine 18 (2013) 26-31
Case Report
28
levels were normal. There were no fat globules in
urine and serum lipase level was normal. Thorax
CT revealed pleuritis on both hemithorax and
alveolar consolidation areas (areas of ground
glass density) consistent with pleural contusion in
bilateral lower lobes (Figures 3a, 3b). The patient
was diagnosed as FES due to existing clinical
findings. Low molecular weight heparin and
steroid treatment was started. The patient
regained consciousness and oxygen saturation
improved. The patient was discharged from the
hospital after being operated.
Fig. 3 (a, b). Ground-glass density areas, and bilateral pleural effusion are seen on Thorax CT (case 2).
Fig. 4 (a, b). Case 3, subconjuctival hemorrhage, and axillary petechial rashes are seen (case 3).
2. 3. Case
The patient was 38-year-old male. The patient
was brought to hospital because of a traffic
accident and was diagnosed with pelvic fracture,
subtrochanteric fracture in right femur and shaft
fracture on left femur. The patient was referred to
our clinic due to the development of drowsiness
and respiratory failure within 48 hours. He was
tachypneic and blackout of consciousness.
Arterial blood pressure was 110/60 mmHg, heart
rate was 120/min, and fever was 37.5 °C.
Respiratory system examination was normal.
Petechial rash and subconjuctival hemorrhage
were detected on patient's axillary region of
anterior chest wall (Figures 4a, 4b). Fundus
examination was normal.
The results of ABG analysis without oxygen
were as follows: pH: 7:45, PO
2
: 48.2 mmHg,
pCO
2
: 31.2 mmHg, SaO
2
: 85.7%, HCO
3
: 21.7
mmol/L. Other assays were detected as fallows:
AST 90 U/L (N: 5-37), ALT 59 U/L (N: 10-49),
CK 3722 U/L (N: 21-215), CK-MB: 28.5 U/L (N:
0-15), LDH 400 U/L (N: 100-190), Hb: 8.2 g/dL
(N: 12-16), Htc: 23.7% (N: 36-45), D-dimer:
4154 ng/mL (0-500), and fibrinogen: 920 mg/dL
(N: 150-400). Kidney function tests, erythrocyte
sedimentation rate, platelet count and other
laboratory parameters were normal. Brain CT was
taken with 12 hours interval and no pathology
was detected. The patient was diagnosed with
FES with the current clinical findings. The
patient was transferred to intensive care unit
because of general poor health and intubated
because did not respond to continuous oxygen
therapy and non-invasive mechanical ventilation.
Low molecular weight heparin and steroid
treatment was started. Chest X-ray on admission

E. Tanrıverdio et al / Fat embolism
29
was normal (Figure 5a). CT scan of thorax taken
because of the formation of bilateral basal
reticular pattern on the tenth day of the follow-up
(Figure 5b) and revealed minimal pleural effusion
in both lungs and consolidations compatible with
atelectasis in lower lobes. There was fat density
in the atelectatic field on the right. The current
symptom was reported to be due to fat embolism
(Figures 6a, 6b). Despite the supporting treatment
patient died on the 13
th
day of admission.
Fig. 5 (a,b). Chest X-ray on admission( Fig. 5a) and on the tenth day (Fig. 5b).
Fig. 6 (a, b). Torax BT, on cross-section of mediastinum, minimal bilateral pleural effusion, areas of atelectasis
and fat density areas (arrow) in atelectasis are observed (case 3).
3. Discussion
Fat embolism syndrome was first identified by
Von Bergman, in 1873, in a patient with fracture
of the femur (3). FES usually occurs as a
complication of the lower extremity long bone
and pelvic fractures. The other etiological factors
include total knee and hip replacement, renal
transplantation, sickle cell anemia, osteomyelitis,
burns, severe infections, blood transfusions,
diabetes mellitus, alcohol-related hepatic failure,
high-dose corticosteroid therapy, chronic
pancreatitis, parenteral lipid infusion, and
liposuction (2-4). Incidence and mortality rates
are unknown because of the comorbid injury and
other problems (1). FES has a wide range of
incidence between 0% - 35% reported in patients
with bone fractures. Incidence depends on bone
involvement [isolated or multiple], age and
gender. Rarely occurs as a result of medical
conditions. Classical triad includes symptoms in
pulmonary (dyspnea), skin (petechiae) and central
nervous system (mental confusion) (5). History
and clinical symptoms are important for the
diagnosis. The presence of major and minor
clinical symptoms, characterized by Gurd and
Wilson (Table 1), should be investigated (1-6).
Diagnosis is made by the presence of at least two
major, or one major and four minor criteria (1).

Eastern Journal of Medicine 18 (2013) 26-31
Case Report
30
All of the major criteria and the minor criteria in
part were defined in all three cases we presented.
Two theories have been suggested for
pathogenesis of the disease. The mechanical
theory explains particularly fat embolisms
occurred after long bone fractures; fat droplets
released from the bone marrow after fracture
cause blockage of the pulmonary and systemic
vessels. Biochemical theory explains rather
pathogenesis of non-traumatic fat embolisms.
Hormonal changes after the trauma or sepsis
induce systemic release of free fatty acids. Fatty
acids are toxic on the capillary endothelium and
pneumocytes. As a result, vasculitis in lung, brain
and skin vessels, hemorrhage, edema and tissue
damage occurs (1-3-7). The second effect may be
more important because it may cause leakage
from cerebral, pulmonary and other vascular
veins and diffuse vasculitis (7).
Table 1. Criteria for Fat Embolism Syndrome by Gurd and Wilson
Major Criteria Minor Criteria
1. Hypoxemia with PaO
2
< 60 mmHg, FIO
2
≤0.4 1. Pyrexia (temperature > 38.5°C)
2. Petechiae in a vest distribution 2. Tachycardia (heart rate > 110 beats per minute)
3. Pulmonary edema
4. Central nervous system depression
disproportionate to hypoxemia
3. Emboli visible in retina
4. Fat in sputum
5. Fat urine
6. Unexplained drop in hematocrit or platelet
count
7. Increasing erytrocite sedimentation rate (>71
mm/h)
There are no specific laboratory and imaging
methods for fat embolism syndrome (6). Hypoxia
and hypocapnia observed with the measurement
of arterial blood gases. Laboratory findings
include decrease in platelet and hematocrit levels,
increased sedimentation rate, increase in the level
of lipase, presence of fat globules in urine,
sputum, and bronchoalveolar lavage (3-8). Some
authors suggest bronchoalveolar lavage for rapid
and specific diagnosis of FES, but being an
invasive method restricts the availability of it (9).
Radiological findings are nonspecific in FES.
Radiographic examinations of the patients may be
normal. Although most patients had normal
radiographs initially after the trauma, symptoms
may occur within approximately 72 hours.
Resolution is expected usually in the second
week of the hospitalization (10). Frequently
observed CT findings include focal or diffuse
areas of consolidation, and/or ground-glass
opacities, nodules smaller than 10 mm and rarely
filling defects of fat density which were
determined with a Hounsfield unit in pulmonary
arteries. Filling defects consistent with
subsegmental pulmonary embolism were
observed in the chest CT of the first patient.
There were bilateral ground-glass density and
bilateral minimal pleural effusion in the second
patient.
In the third patient there were patchy infiltrates
bilaterally in the lower zones on chest radiograph
and areas with fat density in the atalectasis areas
in the lower lobes were observed on the thorax
CT. Although, often one of the first signs of FES
is respiratory failure, cerebral symptoms may be
prominent. In the acute phase cranial diffusion
MR has high sensitivity to detect cerebral fat
embolisms and may be preferred for diagnosis of
suspected patients (9).
Clinical approach to patients with FES
includes, general patient assessment involving the
traumatic situation, coordination of patient care,
active nutritional support, symptomatic treatment,
and adequate physical intervention (11).
Accepted treatment dosage and duration of
treatment with steroids is not known for FES
(12). However, heroically known beneficial
effects of steroids includes stabilizing pulmonary
capillary membrane, suppression of inflammatory
response, reduction of interstitial edema,
preventing activation of the complement system
and has such as preventing platelet activation
(12). The use of albumin in patients with fat
embolism syndrome, causes a decrease in free
fatty acid concentrations (11,12). Different
treatment methods with medications such as
heparin, ethanol, dextran, nonsteroidal anti-
inflammatory and heparin-glucose infusion had
been tried, but no contribution to a decrease in
morbidity and mortality had been reported.
Therefore, none of these are considered in routine
practice (11,12). On the other hand, Wang et al.
E. Tanrıverdio et al / Fat embolism
31
(13) reported that they achieved 92.3% success
with administering hydrocortisone, dextran 40
glucose and Dan Shen Root injection as a
treatment along supporting impaired respiratory
functions and restoring hypoxemia in patients
with fat embolism syndrome at 12 May 2008
Wenchuan earthquake.
A meta-analysis of studies showing the effects
of prophylactic steroid therapy in patients with
lower extremity fractures, revealed a reduction in
the incidence of hypoxemia and FES. Suggested
prophylactic treatment is to administer a total of
5 doses of 1 mg/kg intravenous
methylprednisolone in every 8 hours after
admission to hospital (14). Our first and second
cases were supported by oxygen inhalation
therapy; in the third case ventilator support was
needed. All three of our cases were not given
prophylactic steroid treatment. We preferred to
start steroid and low molecular weight heparin
therapy with strict monitoring of bleeding, after
the differential diagnosis.
As a result, in patients with confusion and
axillary petechial rash after bone fractures, fat
embolism should be considered in the diagnosis
and signs should be sought. Early diagnosis and
prompt supporting treatment is important in terms
of clinical course.
References
1. Taviloglu K, Yanar K. Fat embolism syndrome.
Surg Today 2007; 37: 5-8.
2. Oymak FS, Güven M, Bilgin M. et al. Fat embolism
syndrome: analysis of five cases. Solunum
Hastalıkları 2000; 11: 308-313.
3. Evarts CM. The fat embolism syndrome: A review. Surg
Clin North Am 1970; 50:493-507.
4. Costa AN, Mendes DM, Toufen C, et al. Adult
respiratory distress syndrome due to fat embolism in
the postoperative period following liposuction and
fat grafting. J Bras Pneumol 2008; 34: 622-625.
5. Yılmaz R, Ünüvar Atılmış Ü, Dokgöz H, Gürpınar
K. Fat embolism syndrome: An autopsy case.
Türkiye Klinikleri J Foren Med 2005; 2: 74-77.
6. Özyurt Y, Erkal H, Özay K, Arıkan Z. Traumatic fat
embolism syndrome: a case report. Turkish Journal
of Trauma & Emergency Surgery 2006; 12: 254-
257.
7. Yung GL, Fedullo PF. Pulmonary Thromboembolic
Disease. In: Fishman AP, eds. Fishman’s Pulmonary
Diseases and Disorders. 4th ed. Philadelphia:
McGraw-Hill Companies; 2008. p.1443-1444.
8. Müller C, Rahn BA, Pfister U, Meinig RP. The
incidence, pathogenesis, diagnosis and treatment of
fat embolism. Orthop Rev 1994; 23: 107-117.
9. Shaikh N, Parchani A, Bhat V, Kattren MA. Fat
embolism syndrome: Clinical and imaging
considerations. Case report and review of literature.
Indian J Crit Care Med 2008; 12: 32-36.
10. Muangman N, Stern EJ, Bulger EM, et al. Chest
radiographic evolution in fat embolism syndrome. J
Med Assoc Thai 2005; 88: 1854-1860.
11. Habashi NM, Andrews PL, Scalea TM. Therapeutic
aspects of fat embolism syndrome. Injury 2006; 37:
68-73.
12. Hekimoğlu Şahin S, Memiş D, Çolak A. Fat
embolism Associated with Anesthesia induction
with propofol-lidocaine combination: A case report.
Trakya Univ Tıp Fak Derg 2008; 25: 52-55.
13. Wang J, Yang H, Xiang J, et al. The early diagnosis
and treatment of fat embolism syndrome caused by
the injuries in Wenchuan earthquake. Zhonghua Wai
Ke Za Zhi 2008; 46: 1856-1858.
14. Cavallazzi R, Cavallazzi AC. The effect of
corticosteroids on the prevention of fat embolism
syndrome after long bone fracture of the lower
limbs: a systematic review and meta-analysis. J Bras
Pneumol 2008; 34: 34-41.
