
Summary
of
Safety
and
Effectiveness
Data
I.
General
Information
Product
Generic
Name:
Drug-Eluting
Coronary
Stent
System
(NIQ)
Endeavor
Zotarolimus-Eluting
Coronary
Stent
on
Product
Trade
Name:
the
Over-the-Wire
(OTW),
Rapid
Exchange
(RX),
or
Multi-Exchange
II (MX
2
)
Stent
Delivery
Systems
Applicant's
Name
and
Medtronic
Vascular
Address:
3576
Unocal
Place
Santa
Rosa,
CA
95403
Premarket
Approval
Application
(PMA)
Number:
Date
of
Panel
Recommendation:
October
10,
2007
Date
of
Notice
of
Approval
February
1,
2008
to Applicant:
II.
Indications
For
Use
The
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
(hereafter
referred
to
as
the
Endeavor
stent
system)
is
indicated
for
improving
coronary
luminal
diameter
in
patients
with ischemic
heart
disease
due
to
de
novo
lesions
of
length
<
27
mm
in
native coronary
arteries
with
reference
vessel
diameters
of>
2.5
mm
to
<
3.5
mm.
III.
Contraindications
The
Endeavor
Zotarolimus-Eluting
Coronary
Stent System
is
contraindicated
for
use
in
patients
with:
•
Known
hypersensitivity
to
zotarolimus
or
structurally-related
compounds.
*
Known
hypersensitivity
to
the
cobalt-based
alloy
(cobalt,
nickel,
chromium,
and
molybdenum).
*
Known
hypersensitivity
to
the
Phosphorylcholine
polymer
or
its
individual
components
(see
Section
V.
B
-
2.
Inactive
Ingredients
for
details).
Coronary
artery
stenting
is
contraindicated
for
use
in:
*
Patients
who
cannot
receive
recommended
anti-platelet
and/or
anticoagulant
therapy.
*
Patients
judged
to
have
a
lesion
that
prevents
complete
inflation
of
an
angioplasty
balloon
or
proper placement
of
the
stent
or
delivery
device.
IV.
Warnings
and
Precautions
The
warnings
and
precautions
can
be
found
in
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
labeling.
SSED
P060033
-
Page
1
of
59
c)
V.
Device
Description
The
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
is
a
device/drug
combination
product
comprised
of
device
components
(DriverTM
Coronary
Stent
and
Micro-DriverTM
Coronary
Stent
and
the
Endeavor
delivery
systems)
and
a
drug component
(a
formulation
of
zotarolimus
contained
in
a
polymer
coating).
The
characteristics
of
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
are
described
in
Table
1.
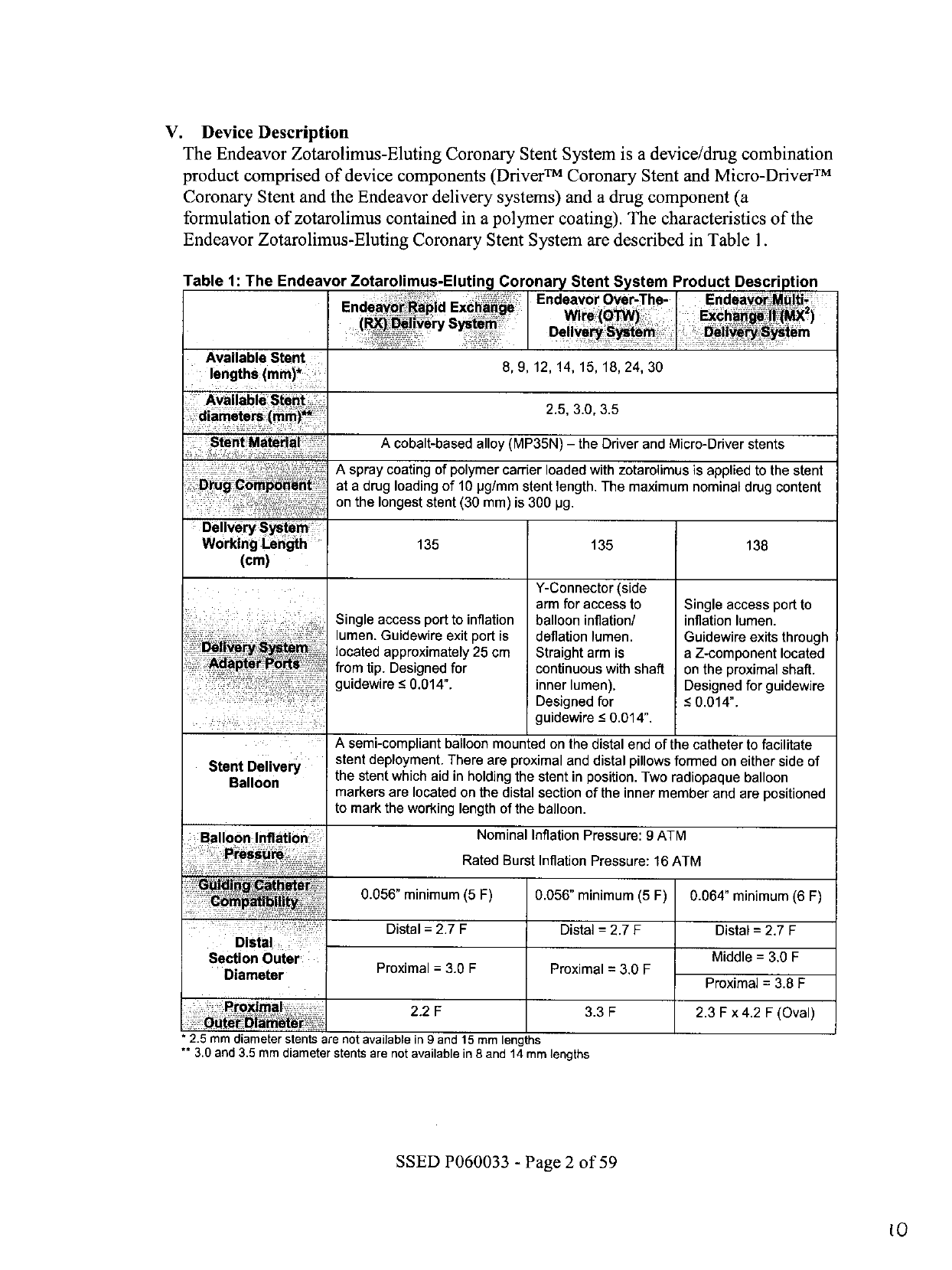
Table
1:
The
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
Product
Description
Endeavor
Over-The-
Endeavor
i-
2
Endeavor38p
id
Exchan
(RJQ~f~e!ry
System:l
Available
Stent
lengths
(mm)*
lengths (mm)'
~~~~~~8,9,
12,
14, 15,
18,
24,
30
Available
Stent
25
.,.
2.5,
3.0,
3.5
A
cobalt-based
alloy
(MP35N)
-
the
Driver
and
Micro-Driver
stents
A
spray
coating
of
polymer
carrier
loaded
with
zotarolimus
is
applied
to
the
stent
Ougt
omplb~onbnght-
at
a
drug
loading
of
10
pg/mm
stent
length.
The
maximum
nominal
drug
content
on
the
longest
stent
(30
mm)
is
300
pg.
Delivery
Stem
Working
Lengh
135
135
138
(cm)
Y-Connector
(side
arm
for
access
to
Single
access
port
to
Single
access
port
to
inflation
balloon
inflation/
inflation
lumen.
lumen.
Guidewire
exit
port
is
deflation
lumen.
Guidewire
exits
through
located
approximately
25
cm
Straight
arm
is
a
Z-component
located
from
tip.
Designed
for
continuous
with
shaft
on
the
proximal
shaft.
guidewire
<
0.014".
inner
lumen).
Designed
for
guidewire
Designed
for
<
0.014".
guidewire
•0.014".
A
semi-compliant
balloon
mounted
on
the
distal
end
of
the
catheter
to
facilitate
stent
deployment.
There
are
proximal
and
distal
pillows
formed
on
either
side
of
Stelont
the
stent
which
aid
in
holding
the
stent
in
position.
Two
radiopaque
balloon
Deliver
markers
are
located
on
the
distal
section
of
the
inner
member
and
are
positioned
to
mark
the
working
length
of
the
balloon.
Balloon
Initation
Nominal
Inflation
Pressure:
9
ATM
Pressur&
Rated
Burst
Inflation
Pressure:
16
ATM
0.056"
minimum
(5 F)
0.056"
minimum
(5 F)
0.064"
minimum
(6 F)
Distal
=
2.7
F
Distal
2.7
F
Distal
=
2.7
F
Distal
Section
Outer
Middle
=
3.0
F
S metion
Outer
Proximal
=
3.0
F
Proximal
=
3.0
F
Diameter
~~~~~~~~~~~~~~~Proximal
3.8
F
=
2.2
F
3.3
F
2.3
F
x
4.2
F
(Oval)
2.5
mm
diameter
stents
are
not available
in
9
and
15
mm
lengths
3.0
and
3.5 mm
diameter
stents
are
not
available
in S
and
14
mm
lengths
SSED
P060033
-
Page
2
of
59
to

A.
Device
Component
Description
The
device
component
consists
of
the
DriverTM
Coronary
Stent
or
Micro-DriverTM
Coronary
Stent
pre-mounted
onto
one
of
three
delivery
systems;
Over-The-Wire
(OTW),
Rapid
Exchange
(RX)
or
Multi-Exchange
II
(MX
2
)
Delivery
Systems.
Each
delivery
system
provides
a
means
for
delivering
the
stent
through
the
coronary
vasculature
and,
once
in
the
desired
location,
expands
the
stent
through
balloon
inflation.
The
delivery
systems
utilized
for
the
Endeavor
product
are
similar
in
materials,
design
and
construction
to
the
approved
Driver
(P030009,
approved
October
1,
2003;
Driver
MX
2
P030009/S001,
approved
on
August
4,
2004;
Driver
RX,
P030009/S003,
approved
on
December
22,
2005)
and
Micro-Driver
(P030009/S002,
approved
on
April
21,
2006)
delivery
systems.
The
Endeavor
stent
is
made
of
the
cobalt
alloy
MP35N
conforming
to
ASTM
F562.
The
modular
stent
segments
are
created
from
a
single
ring
formed
into
a
repeating
pattern
of
crowns
and
struts.
The
appropriate
ring
is
formed
into
alternating
upper
and
lower
crowns
with
seven
(2.5
mm
diameters)
or
ten
(3.0
-
3.5
mm
diameters)
crowns
per
end,
connected
by
axial
struts
in
a
sinusoidal
pattern.
The
stent
is
crimped
onto
various
size
delivery
catheter
balloons,
with
diameters
of
2.5
mm,
3.0
mm
or
3.5
mm.
The
Endeavor
stent
contains
10
jtg
zotarolimus
per
millimeter
of
stent
length
for
all
diameters.
Because
an
identical
dose
(l0~tg/mm
zotarolimus
per
mm
stent
length)
is
used
for
the
entire
Endeavor
2.5
mm
-
3.5
mm
diameter
range,
the
total
drug
per
stent
is
a
function
of
stent
length,
irrespective
of
stent
diameter.
B.
Drug
Component
Description
The
drug
component
of
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
consists
of
zotarolimus
(the
active
ingredient)
and
Phosphorylcholine
(PC)
polymer
(the
inactive
ingredient).
A.
1.
Zotarolimus
The
active
pharmaceutical
ingredient
utilized
in
the
Endeavor
stent
is
zotarolimus.
It
is
a
tetrazole-containing
macrocyclic
immunosuppressant.
The
chemical
name
of
zotarolimus
is:
[3S-[3R*[S*(1R*,3S*,4R*)],6S*,7E,9S*,I
OS*,
12S*,14R*,15E,17E,
19E,21R*,23R*,
26S*,27S*,34aR*]]-9,10,12,13,14,21,
22
,
23
,
24
,
25
,
26
,27,32,33,34,34a-
hexadecahydro-9,27-dihydroxy-3-[2-[3-methoxy-4-(
H-tetrazoyl-1
-yl)cyclohexyl]-
1-
methylethyl]-
10,21
-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-
pyrido[2,1-c]
[1,4]oxaazacyclohentriacontine-
1,5,11,28,29(4H,6H,31H)-pentone.
SSED
P060033
-
Page
3
of
59
tI

The
chemical
structure
of
zotarolimus
is
shown
below
(Figure
1).
N=N
/
\
3
CH·CH
O9
CH3
I
4
-
2H
26
0(,
0
'29k
2/T-%
25
24
Figure
1:
Chemical
Structure
of
Zotarolimus
Zotarolimus
has
extremely
low
water
solubility
and
is
a
lipophilic
compound
that
is
freely
soluble
in
propylene
glycol,
acetone,
toluene,
acetonitrile,
ethanol,
benzyl
alcohol
and
dimethyl
sulfoxide
(DMSO).
The
molecular
formula
of
zotarolimus
is
C52H79N5012
and
its
molecular
weight
is
966.2.
Zotarolimus
does
not
have
any
ionizable
groups
in
the
physiological
pH
range;
therefore,
its
solubility
is
expected
to
be
unaltered
in
this
range.
B.
2.
Inactive
Ingredients
The
only
inactive
ingredient
in
the
Endeavor
stent
is
the
Phosphorylcholine
(PC)
polymer,
which
acts
as
a
carter
for
zotarolimus.
The
PC
polymer
consists
of
2-
methacryloyloxyethyl
phosphorylcholine
that
is
synthesized
and
then
used
in
the
preparation
of
cross-linked
polymer
membranes
with
lauryl
methacrylate,
hydroxypropyl
methacrylate
and
trimethoxysilylpropyl
methacrylate
(crosslinker)
co-
monomers.
The
molecular
weight
of
PC
polymer
was
estimated
using
viscometry
and
resulted
in
values
of
Mw
ranging
from
160,000
to
270,000.
These
figures
were
supported
by
light
scattering
values
of
Mw
(g/mol)
ranging
from
100,000
to
200,000.
SSED
P060033
-
Page
4
of
59

PC
polymer
in
a
solvent
carrier
(ethanol)
is
applied
to
the
Driver
stent
to
form
the
base
layer
coat
of
the
Endeavor
stent.
The
polymer
is
also
mixed
with
the drug
zotarolimus
and
then
applied
to
the
base layer-coated
stents.
Finally,
a
drug-free
overspray
of
PC
polymer
is
applied
after
the
stent
has
been
coated
with
the
drug/polymer
formulation
and
it
has
been
crimped
onto
the
balloon.
The
drug/polymer
coating
is
applied
to the
entire
surface
(i.e.,
luminal
and
abluminal)
of
the
stent.
The
structural
formula
of
the
polymer
is
shown
in
Figure
2.
-
-" /vv
,/vvwJ~
("--.
~)
2
3
~("
) I
I
-"
-)
2 5
I-~
0
0
0
0
o
0
0
H
ISi''CH
O
H
3
C0
~_IOCH
3
i
OCH
3
N
+
Figure
2:
Chemical
Structure
of
PC
Polymer*
PC
Technology
T
is
licensed
under
patents
or
patent
applications
owned
by
Biocompatibles.
SSED
P060033
-
Page
S
of
59
t3

Table
2:
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
Product
Matrix
and
Nominal
Zotarolimus
Doses
Product
P
uProdut.;pl
Number
E X
-a
nNumE
d
W
NM
:;,
.
Stent
ID
l
tConent
(mm)~
(mm)
(pg:
EN25008W
EN25008UX
EN25008MX
2.50
8*
84
The
8
mm
and
14 mm
stent
lengths
have
a
total
nominal
drug
content
of
84
pg
and
144
pg.
respectively,
since
the
actual
stent
length
for
the
8
mm
stent
is
8.4
mm,
and
the
actual
stent
length
for
the
14
mm
stent
is
14.4
mm.
OTW
MVR
EN30009W
EN30009UX
EN30009MX
3.00
9
90
EN35009W
EN35009UX
EN35009MX
3.50
9
90
EN25012W
EN25012UX
EN25012MX
2.50
12
120
EN30012W
EN30012UX
EN30012MX
3.00
12
120
EN35012W
EN35012UX
EN35012MX
3.50
12
120
EN25014W
EN25014UX
EN25014MX
2.50
14*
144
EN30015W
EN30015UX
EN30015MX
3.00
15
150
EN35015W
EN35015UX
EN35015MX
3.50
15
150
EN25018W
EN25018UX
EN25018MX
2.50
18
180
EN30018W
EN30018UX
EN30018MX
3.00
18
180
EN35018W
EN35018UX
EN35018MX
3.50
18
180
EN25024W
EN25024UX
EN25024MX
2.50
24
240
EN30024W
EN30024UX
EN30024MX
3.00
24
240
EN35024W
EN35024UX
EN35024MX
3.50
24
240
EN25030W
EN25030UX
EN25030MX
2.50
30
300
EN3003OW
EN3003OUX
EN30030MX
3.00
30
300
EN35030W
EN3503OUX
EN35030MX
3.50
30
300
:
Note:
C.
Mechanism
of
Action
The
mechanism
(or
mechanisms)
by which
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
affects
neointimal
production
as
seen
in
clinical
studies
has not
been
established
conclusively.
In
vitro,
zotarolimus
inhibited
growth
factor-induced
proliferation
of
human
coronary
artery
smooth
muscle
cells
and
also
demonstrated
binding
affinity
with
FKBP
12
(binding
protein).
The
suggested
mechanism
of
action
of
zotarolimus
is
to
bind
to
FKBP
12,
leading
to
the
formation
of
a
trimeric
complex
with
the
protein
kinase
mTOR
(mammalian
target
of
rapamycin),
inhibiting
its
activity.
Inhibition
of
mTOR
activity
leads
to
inhibition
of
cell
cycle
progression
from
the
GI
to
the
S
phase.
SSED
P060033
-
Page
6
of
59
VI.
Alternative
Practices
and
Procedures
Treatment
of
patients
with
coronary
artery
disease
may
include
exercise,
diet,
drug
therapy,
percutaneous
coronary
interventions
(such
as
angioplasty,
bare
metal
stents.
coated
stents,
and
other
drug-eluting
stents),
and
coronary
artery
bypass
surgery
(CABG).
VII.
Marketing
History
The
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
is
commercially
available
in
the
following
countries:
*
Albania
*
Brazil
*
Germany
*
Lebanon
·
Pakistan
·
Sudan
*
Algeria
*
Bulgaria
*
Ghana
*
Libya
*
Panama
*
Sweden
*
Antigua
and
Barbuda
*
Cayman
Islands
*
Greece
·
Liechtenstein
*
Paraguay
*
Switzerland
*
Argentina
*
Chile
*
Guatemala
*
Lithuania
*
Peru
·
Syria
*
Armenia
*
China
*
Honduras
*
Luxembourg
*
Poland
*
Thailand
H
Trinidad
*
Aruba
*
Colombia
Hong
*
Malaysia
*
Portugal
and
*
Australia
*
Costa
Rica
·
Hungary
*
Malta
*
Qatar
Tobago
*
Tunisia
*
Austria
·
Cyprus
*
Iceland
*
Mauritius
·
Romania
*
Turkey
*
Bahamas
B
Czech
Republic
*
India
*
Mexico
*
Russia
·
Uganda
*
Bahrain
*
Denmark
·
Iran
*
Morocco
*
Saudi
Arabia
*United
Arab
Emirates
*Bangladesh
·
Dominican
Ban
h Republic
*Ireland
*
Mozambique
*
Senegal
*
United
Kingdom
*
Barbados
*
Ecuador
*
Israel
Nepal
·
*Serbia
&
Montenegro
*Uruguay
*
Belgium
*
Egypt
*
Italy
*
Netherlands
·
Singapore
*
Venezuela
*
Belize
*
-El
Belize
Salvador
·
Jamaica
*
New
Zealand
*
Slovakia
*
Virginia
Islands
,
Bermuda
a
Estonia
*
Jordan
*
Nicaragua
*
Slovenia
(British)
*
Yemen
,
Bolivia
·
Finland
*
Kenya
*
Nigeria
*
South
Africa
·
Zimbabwe
,
Bosnia-
Herzegovina
*
France
*
Kuwait
·
Norway
*
South
Korea
*
Botswana
*
Georgia
·
Latvia
*
Oman
*
Spain
As
of
December
31,
2007,
approximately
392,000
Endeavor
Zotarolimus-Eluting
Coronary
Stent
Systems
have
been
distributed
outside
of
the
United
States
(OUS).
No
products
have
been
withdrawn
from
the
market
in
any
country
for
any
reason.
SSED
P060033
-
Page
7
of
59

VIII.
Summary
of
Non-Clinical
Studies
A
series
of
non-clinical
laboratory
studies
were
performed,
pertaining
to
the
stent
and
the
stent
delivery
system
(i.e.,
the
stent
mounted
on
either
the
Endeavor
OTW,
RX
and
MX
2
stent
delivery
system),
the
polymer
substance
(i.e.,
Phosphorylcholine),
the
drug
substance
(i.e.,
zotarolimus),
and
the
finished
combination
product
(i.e.,
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System).
A.
Studies
of
the
Drug
Substance
Medtronic
Vascular
provided
a
letter
from
the
drug
substance
manufacturer,
Abbott
Laboratories
Inc.,
authorizing
FDA
access
to
a
Drug
Master
File
(DMF)
in
support
of
this
application.
In
vivo
and
in
vitro
pharmacology
and
toxicology
studies
as
well
as
animal
and
human
pharmacokinetic
studies
were
conducted
on
zotarolimus
to
provide
information
about
systemic
and
regional
toxicity,
distribution
profiles,
end-organ
disposition,
drug
metabolism
and
potential
drug-drug
interactions.
1.
Safety
Pharmacology
Safety
pharmacology
studies
have
been
conducted
to
determine
the
effects
of
zotarolimus
on
the
central
nervous
system
and
pulmonary
function
in
rats,
the
cardiovascular
system
in
conscious
primates,
hemodynamic
and
electrophysiologic
function
in
conscious
and
anesthetized
dogs,
canine
cardiac
Purkinje
fibers
repolarization
(in
vitro
assay),
hERG
current
(in
vitro),
antigenicity
in
guinea
pigs,
and
lymph
nodes
in
mice.
IV
administered
zotarolimus
has
no
effect
on
the
CNS
and
respiratory
system
parameters
in
the
rat
at
blood
concentrations
of-
30-times
the
estimated
Cma.
(maximum
blood
concentration)
from
one
stent.
In
the
anesthetized
dog
model,
IV
doses
of
zotarolimus
showed
no
significant
effect
on
heart
rate,
QTc
and
PR
interval.
The
blood
concentration
of
zotarolimus
is
estimated
to
be
about
5-fold
higher
than
the
Cmax
obtained
in
the
highest
IV
dose
tested
in
human
multiple
dose
study
for
safety
(800
jig
for
14
days).
Zotarolimus
did
not
reduce
hERG
current
at
concentration
up
to
181
ng/ml
(72
times
the
estimated
clinical
Cmax).
Zotarolimus
showed
minimal
prolongation
of
the
Purkinje
fiber
action
potential
duration
(by
6%)
at
concentration
up
to
21
ng/ml
(9
times
estimated
clinical
Cmax).
Zotarolimus
caused
in
vitro
inhibition
of
cell
proliferation
of
human
T
cell,
coronary
artery
smooth
muscle
and
endothelial
cells
at
IC
50
of
1.2,
2.9
and
2.6
nM,
respectively.
Zotarolimus
did
not
exhibit
receptor
interaction
when
tested
in
vitro
at
a
concentration
of
10
pM
for
binding
to
73
individual
assays.
At
levels
of
200
ng/ml
(100-fold
greater
than
a
typical
projected
Cmax
of
1.8
ng/ml),
zotarolimus
caused
no
direct
aggregation
of
human
platelets
in
whole
blood
or
did
not
enhance
aggregation
to
stimulation
by
platelet
agonists.
In
guinea
pigs
sensitized
once
per
week
for
four
weeks
by
subcutaneous
administration
of
zotarolimus
(30
tg/kg),
showed
no
induced
systemic
anaphylactic
or
passive
cutaneous
anaphylactic
reactions
indicating
that
zotarolimus
was
non-antigenic.
Topically
applied
zotarolimus
(50%
w/v)
on
the
dorsal
surface
of
both
ears
of
mice
showed
no
increase
in
SSED
P060033
-
Page
8
of
59

3
H-thymidine
incorporation
in
the
lymph
nodes
indicating
lack
of
skin
sensitizing
activity.
2.
Toxicology
Toxicology
studies
have
been
conducted
to
determine
the
general
toxicological
effects
in
various
species
and
the
potential
for
genetic,
reproductive
and
developmental
toxicology.
These
include
single
and
28
day
repeat
dose
IV
infusion
toxicokinetic
studies
in
the
rat
and
cynomolgus
monkey,
fertility
and
teratology
reproductive
toxicity
studies
in
the
rat
and
rabbit,
and
genotoxicity
studies
in
vitro
and
in
the
mouse.
Zotarolimus
was
embryo/feto-toxic
in
rats
at
IV
dosages
of
25
jig/kg/day
and
above
(approximately
3
times
the
cumulative
blood
exposure
provided
by
Endeavor
stents
coated
with
300
jig
zotarolimus).
Embryotoxicity
was
manifested
as
reduced
fetal
body
weights
and
fetal
ossification
delays,
but
no
major
fetal
malformations,
deaths,
or
minor
fetal
abnormalities
were
observed.
No
embryo-fetal
effects
were
observed
in
pregnant
rabbits
at
the
maternally
toxic
dosage
of
30
pg/kg/day
(approximately
13
times
the
cumulative
blood
exposure
provided
by
Endeavor
stents
coated
with
300
gg
zotarolimus).
The
Endeavor
stent
should
be
used
during
pregnancy
only
if
the
potential
benefit
outweighs
the
potential
risk
to
the
embryo/fetus.
Zotarolimus
was
not
genotoxic
in
the
in
vitro
bacterial
reverse
mutation
assay,
the
human
peripheral
lymphocyte
chromosomal
aberration
assay,
or
the
in
vivo
mouse
micronucleus
assay.
3.
Absorption,
Distribution,
Metabolism,
and
Excretion
(ADME)
Studies
Studies
were
conducted
to
characterize
the
absorption,
distribution,
metabolism,
and
excretion
profiles
in
various
species.
Absorption
studies
included
pharmacokinetic
evaluations
in
the rat,
rabbit,
mouse,
monkey,
and
pig
following
single
IV
or
oral
drug
dosing.
Distribution
studies
included
in
vitro
distribution
in
human
whole
blood,
in
vitro
binding
to
plasma
proteins
in
the
mouse,
rat,
rabbit,
dog,
pig,
monkey,
and
human
plasma,
and
tracing
of
radioactivity-labeled
drug
in
the
rat,
monkey,
and
pig
following
IV
dosing.
The
results
of
the
plasma
protein
binding
study
suggest
that
all
clinically
relevant
concentrations,
zotarolimus
would
be
extensively
protein
bound
in
plasma
and
would
undergo
significant
degradation.
Results
from
the
analysis
of
radioactive-labeled
drug
in
the
blood
and
plasma
are
consistent
with
zotarolimus
undergoing
rapid
degradation
within
plasma
while
drug
that
is
sequestered
within
blood
cells
is
relatively
more
stable
and
is
released
slowly.
All
concentrations
largely
decreased
in
parallel
with
blood
radioactivity
with
little
evidence
for
accumulation.
Metabolism
studies
included
analysis
of
metabolites
after
metabolism
by
human
liver
microsomes
in
vitro,
analysis
of
metabolites
in
blood,
plasma,
feces,
and
urine
in
the
rat,
pig,
monkey,
and
human
following
IV
dosing,
metabolism
and
excretion
in
rats,
SSED
P060033
-
Page
9
of
59
(-7

metabolism,
excretion,
and
tissue
distribution
in
rabbits,
metabolism
by
hepatocytes
from
the
rat,
dog,
monkey,
and
human,
and
the
effect
of
ketoconazole
on
PK
profile
in
the dog
following
IV
dosing.
Review
of
studies
of
the
metabolism
in
the
rat,
dog,
monkey,
and
human
indicated
that
in
all
cases
the
presence
of
hepatocytes
accelerated
the
loss
of
parent
compound
from
the
incubation
mixture.
Although
no
metabolites
or
breakdown
products
were
formally
identified
in
the
present
study,
it
is
likely
that
many
routes
of
metabolism
will be
shared.
In
the studies
of
metabolites
by
human
liver
microsomes,
the
ICs0
for
zotarolimus
determined
as
a
competitive
inhibitor
was
not
significantly
different
from the
potency
determined
after
pre-incubation,
suggesting
that
the
compound
is
not
a
mechanism-based
inhibitor
of
human
CYP3A
enzymes.
The
anticipated
clinical
plasma
levels of
zotarolimus
are
unlikely
to
exceed
the
IC
50
values
determined
in
this
study,
which
would
suggest
that
the
compound
is
unlikely
to
be
a
source
of
clinical
drug-drug
interactions
through
inhibition
of
CYP3A.
In
studies
of
the
effects
of
zotarolimus
on
seven
cytochrome
P450-dependent
monooxygenase
activities
in
human
liver
microsomes,
in
all
cases
the
extent
of
inhibition
of
each
enzyme
were
largely
the
same,
irrespective
of
the
absence
or
presence
of
a
pre-
incubation
step,
suggesting
that
the
compound
is
not
a
mechanism
based
inhibitor
of
any
of
the
human
P450
enzymes
tested.
Given
the
anticipated
clinical
plasma
levels
of
zotarolimus,
the
compound
is
unlikely
to
be
a
source
of
clinical
drug-drug
interactions
through
inhibition
of
CYP3A
or
the
other P450
enzymes
tested.
In
common
with
other
immunosuppressive
macrolides,
such
as
sirolimus,
the
metabolism
of
zotarolimus
appears
to
be
catalyzed
by
cytochromes
P450
of
the
CYP3A
subfamily,
CYP3A4
in
particular.
In
a
study
of
ketoconazole
interaction
with
zotarolimus
in
the
dog,
the
results
indicated
that
oxidative
metabolism
of
zotarolimus
can
be
blocked
by
the
CYP3A
selective
inhibitor
ketoconazole.
Co-dosing
with
ketoconazole
produced
a
statistically
significant
(p
<
0.05)
increase
in
the
zotarolimus
area
under
the
curve,
with
a
significant
decrease
in
the
clearance
values.
Excretion
studies
in
male
rats
included
assessment
of
physical
and
metabolic
stability
in
excreta.
The
results
indicated
that
elimination
occurs
primarily
via
the
feces.
4.
Intravenous
Administration
of
Zotarolimus
·
Pharmacokinetics
Zotarolimus
pharmacokinetic
activity
has
been
determined
following
intravenous
(IV)
administration
in
healthy
patients.
Table
3
provides
a
summary
of
the
pharmacokinetic
analysis.
SSED
P060033
-
Page
10
of
59

Table
3:
Pharmacokinetic
Parameters
(Mean
±
standard
deviation)
in
Patients
Following
Intravenous
Administration
of
Zotarolimus
meesUnlts
~
~;
6
Day
1
Day
14
Day
1
Day
14
Day
1
Day
14
Cmax
(ng/mL)
11.41
±
1.38*
11.93
±
1.25
21.99
±
3.79
23.31
±
3.15
37.72
±
7.00
41.79
±
6.68
Tmma
(h)
1.05
0.040
1.03
±
0.04
1.00
±
0.14
1.05
±
0.04
1.03
±
0.04
1.03
±
0.05
AUC
0
-
24
(ngoh/mL)
t
Wm$
(h)
34.19
±
4.39
*
47.70
±
6.68
32.9
±
6.8
68.43
±
15.41
100.47
±
18.02
37.6
±
4.5
123.48
±
13.34
174.43
±
19.88
36.0
±
4.7
CIL
(L/h)
4.2
±
0.6
4.2
±
0.6
4.0
±
0.9
4.0
±
0.9
4.6
±
0.4
4.6
±
0.4
N=
16
$
Harmonic
mean
±
pseudo-standard
deviation
£
Clearance
data
is
calculated
using
compartmental
methods.
All
other
data
presented
in
Table
3
is
calculated
using
non-
compartmental
methods,
When
administered
intravenously
for
14
consecutive
days,
zotarolimus
showed
dose
proportionality.
Renal
excretion
is
not
a
major
route
of
elimination
for
zotarolimus,
as
approximately
0.1%
of
the
dose
was
excreted
as
unchanged
drug
in
the
urine
per
day.
In
multiple
doses
of
200,
400,
and
800
pg,
zotarolimus
was
generally
well
tolerated
by
the
patients.
No
clinically
significant
changes
in
physical
examination,
vital
signs,
or
laboratory
measurements
were
observed
during
the
course
of
the
study.
For
a
total
stent
length
of
48
mm
(480
pg
drug
dose),
a
Cm.
of
4.0
ng/mL
and
AUCO-inf
(area
under
the
blood
concentration-time
curve
(AUC)
from
time
0
to
infinity)
of
162
ng.h/mL
were
estimated
as
seen
in
Table
4
below.
These
calculations
are
based
on
the
mean
Cmax
and
AUCO-inf
values
calculated
from
the
IV
dosing
studies
conducted
on
zotarolimus.
Table
4:
Zotarolimus
Dose
Exposure
F
~
~
~~~1
~~480
~
Stent
>EbuEMultiples
I~E
Cmax
(n
Iml
4.0
27.69
¥
AUJCo~f
n
*h/mL162
15.06
#
¥
Calculated
based
on
the
mean
C
value
(110.78)
from
the
highest
dose
group
(900
pg)
from
human
single
escalation
IV
dose
study
conducted
on
zotarolimus
#
Based
on
the
mean
all
day
AUC,,,
(Day
1
to 14):
2440
ng-h/mL)
value
from
the
highest
dose
regimen
(800
pg
QD
x
14
days)
from
human
multiple
escalation
IV
dose
study
conducted
on
zotarolimus
*
Adverse
Event
Profile
The
incidence
of
adverse
events
attributed
to
the
drug
zotarolimus
was
determined
in
IV
escalating
and
multiple-dose
studies.
In
the
single-escalating
dose
study,
the
proportion
of
patients
reporting
treatment-emergent
adverse
events
was
slightly
lower
among
patients
who
received
doses
of
zotarolimus
than
those
who
received
placebo
for
zotarolimus.
The
most
common
treatment-emergent
adverse
events
associated
with
zotarolimus
were
application
site
reaction,
injection
site
reaction,
pain,
and
hematuria.
There
were
no
deaths
or
other
serious
adverse
events
reported
in
this
study.
No
clinically
significant
changes
in
physical
examination,
vital
signs,
or
laboratory
measurements
were
observed
during
the
course
of
the
study.
Table
5
provides
a
summary
of
the
analysis.
SSED
P060033
-
Page
11
of
59

Table
5:
Summary
of
Treatment-Emergent
Adverse
Events
Reported
by
Two
or
More
Patients
in
Any
One
Treatment
by
Body
Systems
and
Coding
Symbols
for
a
Thesaurus
of
Adverse
Reaction
Terms
Term
in
the
Sti
nDose
Study
N8
(%/)~
.,N
=8(%/)
=
%
Headache
3
(15%)
0
(0%)
0
(0%)
0
(0%)
1
(13%)
0
(0%)
Body
as
a
whole
Injection
site
1(5%)
0
(0%)
0
(0%)
3
(38%)
0
(0%)
0
(0%)
Reaction
Pain
7(35%)
1
(13%)
0
(0%)
5
(63%)
5
(63%)
2
(25%)
Digestive
Diarrhea
2
(10%)
0
(0%)
0
(0%)
0
(0%)
0
(0%)
0
(0%)
System
Application
Skin
and
st
site
8
(40%)
1
(13%)
5
(63%)
2
(25%)
1
(13%)
5
(63%)
Appendage
Rato
Urogenmtal
Hematuria
1
(5%)
0
(0%)
System
I
1
(13%)
0
(0%)
1
(13%)
1
(13%)
I
__
__
_ __
I
_ __
_
__
_
I
I__
In
the
multiple-dose
study,
the
proportion
of
patients
reporting
treatment-emergent
adverse
events
was
similar
among
patients
who
received
doses
of
zotarolimus,
and
the
most
common
treatment-emergent
adverse
events
associated
with
zotarolimus
were
headache,
pain,
injection
site
reaction,
dry
skin,
abdominal
pain,
diarrhea,
and
rash.
There
were
no
deaths
or
other
serious
adverse
events.
Results
of
other
safety
analyses
including
individual
patient
changes,
changes
over
time
and
individual
clinically
significant
values
for
vital
signs,
laboratory
safety
assessments
and
physical
examinations
were
unremarkable
for
each
treatment
group.
No
clinically
significant
changes
in
physical
examination,
vital
signs,
or
laboratory
measurements
were
observed
during
the
course
of
the
study.
No
differences
were
seen
among
the
doses
with
respect
to
adverse
event
profiles
or
overall
drug
safety.
Table
6
provides
a
summary
of
the
analysis.
Table
6:
Summary
of
Treatment-Emergent
Adverse
Events
Reported
by
Two
or
More
Patients
in
Any
One
Tratment
b
Bod
S
stems
and
COSTART
Term
in
the
Multiple-dose
Study
~~td
OfA
TtCIAlTlaM21oo-
*S_
±4
pgdD
Term
.
(N)
_
_ _
_ _
_ _
_
_N_
(
)_
_
_
_
Headache
..
L
2
(13)
(4)
2
(13)
2
(13)
Pain
1
(4)
(13)
2
1
(6)
O
(0)
Injection
Site
Body
as
a
Ineto
ie2
(8)
O
(0)
0 (0)
2
(13)
Reaction
Injection
Site
Pain
2(8)
0(0)
0(0)
0(0)
Abdominal
Pain
1
(4)
1
0
(0)
Digestive
System
Diarrhea
1 (4)
0 (0)
1 (6)
0
(0)
Skin
and
Dry
Skin
0
(0)
(0)
2(13)
0
(0)
Appendage
Rash
0
01
61
0
(0)
SSED
P060033
-
Page
12
of
59
5.
In
Vivo
Pharmacokinetics
The
Endeavor
US
Pharmacokinetic
(PK)
study
was
conducted
to
evaluate
the
pharmacokinetic
profile
of
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
in
eligible
patients.
The
Endeavor
US
PK
Registry
was
a
prospective,
multi-center,
single-
arm,
open-label
study
designed
to
assess
the
acute
pharmacokinetics
and
safety
of
zotarolimus
administered
using
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
for
the
treatment
of
single
de
novo
lesions
in
native
coronary
arteries.
Pharmacokinetic
evaluation
was
conducted
on
all
patients
participating
in
the
Endeavor
US
PK
trial.
Forty-three
(43)
patients
received
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System;
six
patients
received
overlapping
stents.
Blood
samples
(5
mL)
were
collected
at
30
days
post-procedure.
Selected
pharmacokinetic
parameters
for
the
Endeavor
US
PK
study
analysis
are
provided
in
Table
7.
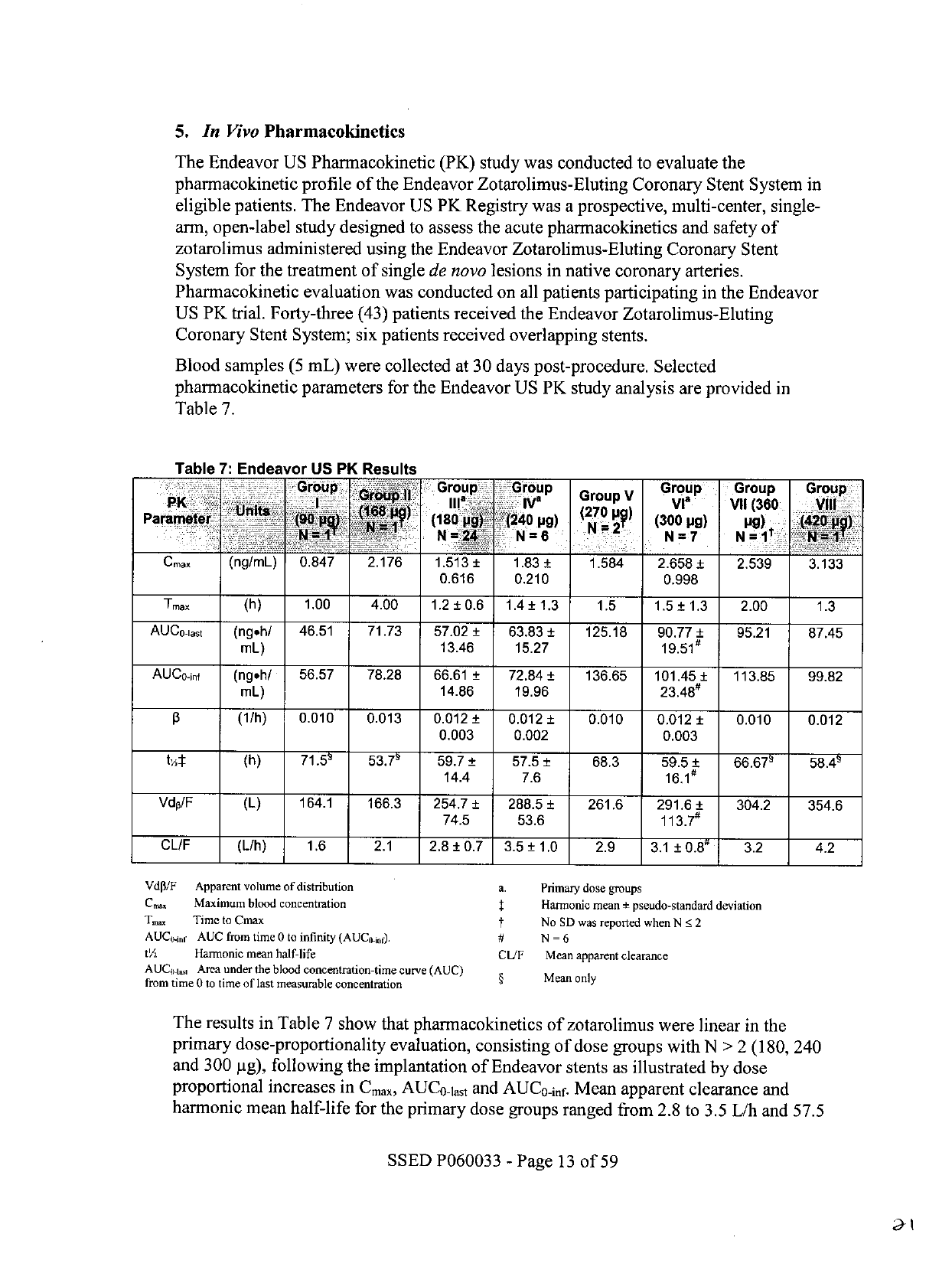
Table
7:
Endeavor
US
PK
Results
Group
Group
G
Grop
V
Group
Group
Group
Group
Pareter180
P
Un
: :'i
i
l
VI
2V
2240pg)(300
jig)
Vii
(360
g)
VIii
4
,
N=N
[]
6
N=7
N=
[
1
Cmax
(ng/mL)
0.847
2.176
1.513±
1.83
1.584
2.658±
2.539
3.133
0.616
0.210
0.998
Tmax
(h)
1.00
4.00
1.2
±
0.6
1.4
±
1.3
1.5
1.5
±
1.3
2.00
1.3
AUCo-iast
(ngeh/
46.51
71.73
57.02
±
63.83
±
125.18
90.77
±
95.21
87.45
mL)
13.46
15.27
19.51
#
AUCo-inf
(ngh/
56.57
78.28
66.61
±
72.84±
136.65
101.45±
113.85
99.82
mL)
14.86
19.96
23.48#
13
(1/h)
0.010
0.013
0.012
±
0.012
±
0.010
0.012
±
0.010
0.012
0.003
0.002
0.003
t4:l:
(h)
71.59
53.79
59.7
±
14.4
57.5
±
7.6
68.3
59.5
±
16.1
#
66.679
58.49
VdpIF
(L)
164.1
166.3
254,7
±
288.5
±
261.6
291.6
±
304.2
354.6
74.5
53.6
113.7#
CL/F
(L/h)
1.6
2.1
2.8
±
0.7
3.5
±
1.0
2.9
3.1
±
0.8'
3.2
4.2
Vdp/F
Apparent
volume
of
distribution
a.
Primary
dose
groups
Cm
Maximum
blood
concentration
t
Harmonic
mean
+
pseudo-standard
deviation
Tm.
Time
to
Cmax
t
No
SD
was
reported
when
N•
2
AUC-i.r
AUC
from
time
0
to
infinity
(AUConi
0
).
#
N
=
6
t'A
Harmonic
mean
half-life
CUF
Mean
apparent
clearance
AUCii.
Area
under
the blood
concentration-time
curve
(AUC)
from
time
0
to
time
of
last
measurable
concentration
§
Mean
only
The
results
in
Table
7
show
that
pharmacokinetics
of
zotarolimus
were
linear
in
the
primary
dose-proportionality
evaluation,
consisting
of
dose
groups
with
N
>
2
(180,
240
and
300
pg),
following
the
implantation
of
Endeavor
stents
as
illustrated
by
dose
proportional
increases
in
Cmax,
AUCO-lat
and
AUCO-inf.
Mean
apparent
clearance
and
harmonic
mean
half-life
for
the
primary
dose
groups
ranged
from
2.8
to
3.5
L/h
and
57.5
SSED
P060033
-
Page
13
of
59

to
59.7
hours,
respectively.
The
mean
time
to
reach
peak
systemic
concentration
(Tm.)
ranged
from
1.2
to
1.5
hours
after
stent
implantation.
Additionally,
this
study
showed
that
zotarolimus
is
released
gradually
into
the
systemic
circulation
without
any
evidence
of
dose
dumping.
6.
Drug
Interactions
The
effect
of
potential
drug
interactions
on
the
safety
or
efficacy
of
the
Endeavor
stent
has
not
been
investigated.
While
no
specific
clinical
data
are
available,
drugs,
like
sirolimus,
that
act
through
the
same
binding
protein
(FKBP
12)
may
interfere
with
the
efficacy
of
zotarolimus.
Zotarolimus
is
metabolized
by
CYP3A4,
a
human
cytochrome
P450
enzyme.
When
administered
concomitantly
with
200
mg
ketoconazole
bid,
a
strong
inhibitor
of
CYP3A4,
zotarolimus
produces
less
than
a
2-fold
increase
in
AUCO-inf
with
no
effect
on
Cmax.
Therefore,
consideration
should
be
given
to
the
potential
for
drug
interactions
when
deciding
to
place
an
Endeavor
stent
in
a
patient
who
is
taking
drugs
that
are
known
substrates
or
inhibitors
of
the
cytochrome
P450
isoenzyme
CYP3A4.
Systemic
exposure
of
zotarolimus
should
also
be
taken
into
consideration
if
the
patient
is
treated
concomitantly
with
systemic
immunosuppressive
therapy.
Formal
drug
interaction
studies
have
not
been
conducted
with
the
Endeavor
stent.
B.
Biocompatibility
Studies
A
series
of
GLP
biocompatibility
tests
and
USP
Physicochemical
tests
were
conducted
to
demonstrate
that
the
components
of
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
(OTW,
RX
and
MX
2
)
are
non-toxic.
Tests
were
conducted
on
ethylene
oxide-
sterilized
Endeavor
coated
stents,
stent
delivery
systems
(finished
product)
and
polymer-
only
coated
stainless
steel
(SS)
coupons.
These
test
articles
were
processed
in
the
same
manner
as
the
finished
Endeavor
product.
The
polymer-only
coated
coupons
did
not
include
drug
substance
but
were
manufactured
to
simulate
the
processing
of
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
with
equivalent
surface
treatment,
cross-
linking
and
sterilization
processes
utilized.
In
all
of
these
test
systems,
the
materials
were
non-reactive
and
met
all
acceptance
criteria.
The
results
of
the
biocompatibility
studies
indicated
that
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
was
biologically
safe
and
acceptable
for
clinical
use.
All
biocompatibility
testing
was
conducted
in
accordance
with
Good
Laboratory
Practices
Regulations
(21
CFR
§
58)
and
with
consideration
of
the
following
guidances:
*
Guidance
for
Industry
and
FDA
Staff,
Non-Clinical
Tests
and
Recommended
Labeling
for
Intravascular
Stents
and
Associated
Delivery
Systems.
Document
issued
on
January
13,
2005.
*
International
Standard
ISO
10993-1,
Biological
Evaluation
of
Medical
Devices:
Evaluation
and
Testing.
Table
8
provides
a
summary
of
the
biocompatibility
testing
conducted
in
support
of
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System.
SSED
P060033
-
Page
14
of
59

Table
8:
Summary
of
Biocompatibility
Testing
Test
Name
Test
Descpti--
i--
Re8
ISO
10993-5:
In
Vitro
·
EndeaVor
stent
Cytotoxicity
.
Cytotoxicity
and
delivery
Pass
(non-cytotoxic)
([929
MEM
Elution)
systems
*
Endeavor
stent
ISO-10993-11:
Systemic
*
Endeavor
stent
Pyrogenicity
Toxicity
(Material
Mediated
and
delivery
Pass
(non-pyrogenic)
Rabbit,
Injection)
systems
ISO-1
0993-10:
Sensitization
*
Endeavor
stent
Sensitization
and
delivery
Pass
(non-sensitizing)
(Guinea
pig
Maximization)
systems
*
Endeavor
stent
Acute
Intracutaneous
ISO-10993-10:
Irritation
*
Endeavor
stent
and
delivery
Pass
(non-irritant)
Reactivity
(Injection)
systems
*
Endeavor
stent
*
Endeavor
stent
Acute
Systemic
Toxicity
ISO-i
0993-11:
Systemic
and
delivery
Pass
(non-toxic)
Toxicity
(Acute)
systems
*
Endeavor
stent
ISO-10993-4:
In
Vivo
·
Endeavor
stent
Pass
(non-
Thromboresistance
and
delivery
thrombogenic)
systems
ISQ0-10993-4:
*
Endeavor
stent
Pass
(non-
C3a
Complement
Activation
and
delivery
complement
(In
Vitro)
systems
activating)
ISO-10993-4:
*
Endeavor
stent
Pass
(non-
SC5b9
Complement
and
delivery
complement
Activation
(In
Vitro)
systems
activating)
Hemocompatability
ISO-10993-4:
·
Endeavor
stent
Pass
(no
significant
Plasma
Recalcification
and
delivery
change
in
coagulation
systems
time)
*
Endeavor
stent
ISO-10993-4:
and
delivery
In
Vitro
Hemolysis
Study
systems
Pass
(non-hemolytic)
*
Endeavor
stent
ISO-10993-4:
*
Endeavor
stent
Pass
(no
change
in
White
Blood
Cell
Morphology
and
delivery
WBC
morphologically)
systems
SSED
P060033
-
Page
15
of
59
J3

Table
8:
Summary
of
Biocompatibility
Testing
Test
Name
T
cription
Test
Article
Result
ISO-10993-3:
Bacterial
Reverse
Mutation
*
Endeavor
stent
Pass
(non-mutagenic)
(AMES)
ISO-10993-3:
Genotoxicity
In
Vitro
Chromosomal
Pass
(non-
Aberration
in
Mammalian
*
Endeavor
stent
clastogenic)
Cells
ISO-10993-3:
In
Vivo
Mouse
Bone
Marrow
*
Endeavor
stent
Pass
(non-mutagenic)
Micronucleus
Test
Material
Characterization
USP
Physicochemical
*
Balloon
Material
CUSP
Physicochemical
Extracts
<66>
*
Polyethylene
Sheath
Pass
Testing)
(Aqueous)
·
PC
Polymer-only
Coated
Coupons
In
vivo
animal
testing
conducted
on
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
evaluated
the
effects
of
multiple
doses
and
device
exposure
in
a
porcine
coronary
or
rabbit
iliac
artery
model
for
up
to
180
days,
in
lieu
of
ISO
10993
sub-chronic
and
muscle
implantation
testing.
The
in
vivo
results
indicated
that
the
Endeavor
Zotarolimus-
Eluting
Coronary
Stent
System
was
biologically
safe
and
acceptable
for
clinical
use.
The
significant
animal
studies
are
summarized
separately
in
Section
VIII.
F
-
Animal
Studies.
Formal
carcinogenicity
and
reproductive
toxicity
testing
was
not
conducted
on
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System.
An
appropriate
rationale
has
been
provided
to
demonstrate
why
the
carcinogenic
potential
of
the
Endeavor
stent
is
minimal
based
on
the
types
and
quantities
of
materials
present
and
the
limited
period
of
zotarolimus
release.
The
genotoxicity
and
reproductive
toxicity
of
zotarolimus
have
been
investigated
in
bacterial
and
mammalian
cells
in
vitro
and
in
laboratory
animals
in
vivo.
There
is
no
evidence
to
suggest
that
any
chemical
interactions
occur
between
the
PC
polymer
and
zotarolimus
drug
under
established
processing
and
storage
conditions
that
would
lead
to
the
formation
of
covalent
bonds
or
that
would
alter
the
structure
of
the
drug
in
any
way
to
form
a
new
intermediate
or
molecular
entity.
Long
term
biocompatibility
of
the
drug/polymer
coating
on
the
stent
in
humans
is
unknown.
C.
In
Vitro
Engineering
Testing
In
vitro
engineering
testing,
in
accordance
with
FDA's
"Guidance
for
Industry
and
FDA
Staff:
Non-Clinical
Tests
and
Recommended
Labeling
for
Intravascular
Stents
and
Associated
Delivery
Systems"
(January
13,
2005),
was
conducted
on
the
Endeavor
stent
mounted
on
the
OTW,
RX,
and/or
MX
2
delivery
systems.
Some
in
vitro
engineering
tests
were
performed
on
the
uncoated,
bare
metal
version
of
the
Endeavor
stent
since
there
was
no
change
to
the
stent
substrate.
An
appropriate
rationale
was
provided
where
the
testing
of
the
bare
metal
stent
provided
a
worst
case
or
SSED
P060033
-
Page
16
of
59

representative
condition
for
the
attributes
evaluated.
The
effect
of
the
coating
is
assumed
to
be
negligible
when
evaluated
against
measurement
and
manufacturing
tolerances.
Additional
tests
were
conducted
to
support
the
integrity
of
the
coating
on
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
and
are
summarized
separately
in
Section
VIII.
D
-
Coating
Characterization
Testing.
The
in
vitro
engineering
studies
conducted
are
summarized
in
Table
9.
"Pass"
denotes
that
the
test
results
met
product
specifications
and/or
the
recommendations
in
the
referenced
guidance
documents.
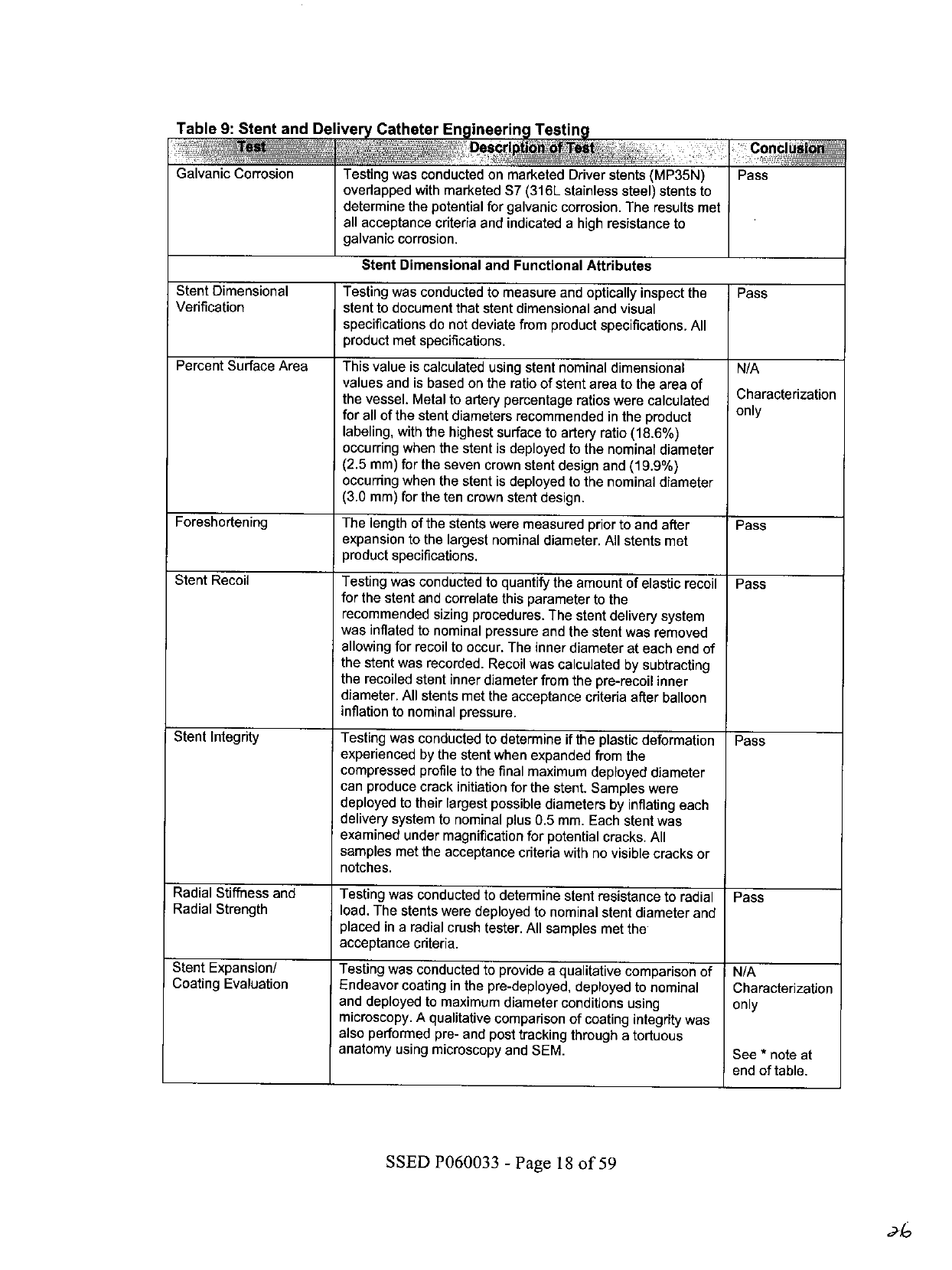
Table
9:
Stent
and
Delivery
Catheter
Engineering
Testing
on
of
Test
Conclus!o
Stent
Material
Specification
Conformance
Testing
Material
Characterization
Chemical
analysis
was
conducted
on
the
Co-Ni-Cr-Mo
alloy
provided
by
the
material
supplier
to
confirm
chemical
analysis
and
inclusion/impurity
content
as
provided
by
ASTM
F562
"Standard
Specification
for
Wrought
Cobalt-35
Nickel-20
Chromium-10
Molybdenum
Alloy
for
Surgical
Implant
Applications."
Pass
Surface
Contamination
SEM
analysis
was
conducted
to
detect
evidence
of
surface
contaminants
or
impurities.
Results
of
SEM
evaluation
showed
no
evidence
of
contamination
above
the
specified
limits.
Pass
Mechanical
Properties
Testing
was
conducted
to
characterize
the
following
properties
of
the
annealed
Co-Ni-Cr-Mo
alloy
bars:
0.2%
offset
yield
strength,
ultimate
tensile
strength,
percent
elongation,
and
reduction
of
area.
Bars
were
machined
from
barstock
conforming
to
ASTM
F562.
The
bars
were
tensile
tested
to
failure
while
engineering
stress
and
strain
were
continuously
recorded.
The
results
were
in
conformance
with
Pass
ASTM
F562.
Stent
Corrosion
Resistance
Endeavor
stents
were
tested
according
to
ASTM
F2129
"Standard
Test
Method
for
Conducting
Cyclic
Potentiodynamic
Measurements
to
Determine
the
Corrosion
Susceptibility
of
Small
Implant
Devices"
to
demonstrate
that
the
finished
stents
exhibit
corrosion
and
repassivation
characteristics
comparable
to
marketed
Driver
stents.
Results
were
comparable
to
the
control
stents
and
met product
specification
requirements.
Testing
was
also
conducted
to
evaluate
the
relative
susceptibility
to
pitting/crevice
corrosion
of
the
Endeavor
stent
utilizing
the
corrosion
techniques
outlined
in
ASTM
F746
and
using
sample
preparation
techniques
in
accordance
with
ASTM
F2129.
Results
were
comparable
to
marketed
Driver
stents
and
met
product
specification
requirements.
Pass
Fretting
Corrosion
Overlapped
bare
metal
Driver
or
Micro-Driver
stents
were
evaluated
post
fatigue
testing
to
determine
the
potential
for
fretting
corrosion.
The
results
met
all
acceptance
criteria
and
indicated
that
the
stents
possess
a
high
resistance
to
fretting
corrosion.
Pass
SSED
P060033
-
Page
17
of
59
Table
9:
Stent
and
De
ive
Catheter
Engineerin
Testing
Galvanic
Corrosion
Stent
Dimensional
Verification
Percent
Surface
Area
Foreshortening
Stent
Recoil
Stent
Integrity
Radial
Stiffness
and
Radial
Strength
Stent
Expansion/
Coating
Evaluation
Testing
was
conducted
on
marketed
Driver
stents
(MP35N)
overlapped
with
marketed
S7
(316L
stainless
steel)
stents
to
determine
the
potential
for
galvanic
corrosion.
The
results
met
all
acceptance
criteria
and
indicated
a
high
resistance
to
galvanic
corrosion.
Stent
Dimensional
and
Functional
Attributes
Testing
was
conducted
to
measure
and
optically
inspect
the
stent
to
document
that
stent
dimensional
and
visual
specifications
do
not
deviate
from
product
specifications.
All
product
met
specifications.
This
value
is
calculated
using
stent
nominal
dimensional
values
and
is
based
on
the
ratio
of
stent
area
to
the
area
of
the
vessel.
Metal
to
artery
percentage
ratios
were
calculated
for
all
of
the
stent
diameters
recommended
in
the
product
labeling,
with
the
highest
surface
to
artery
ratio
(18.6%)
occurring
when
the
stent
is
deployed
to
the
nominal
diameter
(2.5
mm)
for
the
seven
crown
stent
design
and
(19.9%)
occurring
when
the
stent
is
deployed
to
the
nominal
diameter
(3.0
mm)
for
the
ten
crown
stent
design.
The
length
of
the
stents
were
measured
prior
to
and
after
expansion
to
the
largest
nominal
diameter.
All
stents
met
product
specifications.
Testing
was
conducted
to
quantify
the
amount
of
elastic
recoil
for
the
stent
and
correlate
this
parameter
to
the
recommended
sizing
procedures.
The
stent
delivery
system
was
inflated
to
nominal
pressure
and
the
stent
was
removed
allowing
for
recoil
to
occur.
The
inner
diameter
at
each
end
of
the
stent
was
recorded.
Recoil
was
calculated
by
subtracting
the
recoiled
stent
inner
diameter
from
the
pre-recoil
inner
diameter.
All
stents
met
the
acceptance
criteria
after
balloon
inflation
to
nominal
pressure.
Testing
was
conducted
to
determine
if
the
plastic
deformation
experienced
by
the
stent
when
expanded
from
the
compressed
profile
to
the
final
maximum
deployed
diameter
can
produce
crack
initiation
for
the
stent.
Samples
were
deployed
to
their
largest
possible
diameters
by
inflating
each
delivery
system
to
nominal
plus
0.5
mm.
Each
stent
was
examined
under
magnification
for
potential
cracks.
All
samples
met
the
acceptance
criteria
with
no
visible
cracks
or
notches.
Testing
was
conducted
to
determine
stent
resistance
to
radial
load.
The
stents
were
deployed
to
nominal
stent
diameter
and
placed
in
a
radial
crush
tester.
All
samples
met
the
acceptance
criteria.
Testing
was
conducted
to
provide
a
qualitative
comparison
of
Endeavor
coating
in
the
pre-deployed,
deployed
to
nominal
and
deployed
to
maximum
diameter
conditions
using
microscopy.
A
qualitative
comparison
of
coating
integrity
was
also
performed
pre-
and
post
tracking
through
a
tortuous
anatomy
using
microscopy
and
SEM.
Pass
Pass
N/A
Characterization
only
Pass
Pass
Pass
Pass
N/A
Characterization
only
See
*
note
at
end
of
table.
SSED
P060033
-
Page
18
of
59

Table
9:
Stent
and
Del
ve
Catheter
Engineerin
Testing
__l
:tlonof
Test
Culuslon
Coating
Stress
Analysis
A
stress
analysis,
using
a
finite
element
analysis
(FEA),
demonstrated
that
the
addition
of
a
coating
to
a
Driver
or
Micro-Driver
stent
will
not
change
the
critical
location
of
stresses
during
expansion
and
during
in
vivo
loading.
Additionally,
the
coating
stress
analysis
showed
that
the
critical
stress
location
within
the
coating
is
in
the
same
location
found
on
a
bare
stent.
Fatigue
Analysis
(FEA)
An
in-depth
analysis
of
the
stent
was
conducted
to
ensure
that
the
implant
conditions
to
which
the
stent
will
be
subjected
would
not
result
in
failure
due
to
fatigue.
The
FEA
evaluated
the
structural
integrity
of
the
stent
when
subjected
to
the
expected
loading
conditions
generated
in
coronary
arteries.
The
analysis
took
into
account
manufacturing,
delivery,
implantation
and
clinical
loading
over
the
implant
life,
and
predicted
that
fatigue
failures
will
not
likely
occur.
Accelerated
Durability
This
testing
was
conducted
to
determine
the
fatigue
integrity
Testing
of
a
stent
under
accelerated
cycling
designed
to
model
in
vivo
conditions.
Stents
were
deployed
up
to
the
largest
intended
diameter
and
tested
through
a
simulated
10
year
life.
After
the
completion
of
the
ten
year
simulated
durability
testing,
the
stents
were
visually
inspected
using
microscopy
for
cracking
or
breaks.
All
units
tested
were
free
of
any
cracks
or
breaks.
There
were
no
stent
failures
noted
after
420
million
cycles
(equivalent
to
10
years
in
vivo).
Additionally,
Endeavor
stents
were
analyzed
using
SEM
post
accelerated
durability
testing.
Magnetic
Resonance
Non-clinical
testing
on
single
and
overlapped
stents
has
Imaging
(MRI)
Safety
demonstrated
the
Endeavor
stent
is
MR
Conditional.
It
can
be
and
Compatibility
scanned
safely
under
the
following
conditions:
Single
Stenting
Overlapped
(Stent
Length
30
mm)
Stenting
(Total
Length
55
mm)
Static
magnetic
field
of
3-
Static
magnetic
field
of
3-
Tesla
Tesla
Spatial
gradient
field
of
525
Spatial
gradient
field
of
720
Gauss/cm
Gauss/cm
Maximum
whole-body-
Maximum
whole-body-
averaged
specific
absorption
averaged
specific
absorption
rate
(SAR)
of
2
W/kg
for
20
rate
(SAR)
of
3
W/kg
for
15
minutes
of
scanning
minutes
of
scanning
In
non-clinical
testing,
the
In
non-clinical
testing,
the
Endeavor
stent
produced
a
Endeavor
stent
produced
a
temperature
rise
of
less
than
temperature
rise
of
less
than
0.50C
at
a
maximum
whole
0.50C
at
a
maximum
whole
body
averaged
specific
body
averaged
specific
absorption
rate
(SAR)
of
2
absorption
rate
(SAR)
of
3
W/kg
for
20
minutes
of
MR
W/kg
for
15
minutes
of
MR
scanning
in
a
3-Tesla,
Signa,
scanning
in
a
3-Tesla,
Excite,
General
Electric
Medical
General
Electric
Healthcare
Systems
(software
version
(software
version
G3.0-052B)
9.0)
MR
scanner.
The
MR
scanner.
The
maximum
maximum
whole
body
whole
body
averaged
SAR
averaged
SAR
was
displayed
was
displayed
on
MR
on
MR
scanner
console.
scanner
console.
Pass
Pass
Pass
See
*
note
at
end
of
table.
N/A
SSED
P060033
-
Page
19
of
59

Table
9:
Stent
and
Delivery
Catheter
Engineering
Testing
Test
ption
Testo~~nclusI
t~~
C>
The
Endeavor
stent
should
not
move
or
migrate
when
exposed
to
MR
scanning
immediately
post-implantation.
The
image
artifact
extends
The
image
artifact
extends
approximately
9
mm
from
the
approximately
10
mm
from
device/lumen
centerline
the
device/lumen
centerline
when
scanned
in
nonclinical
when
scanned
in
nonclinical
testing
using
a
3-Tesla,
testing
using
a
3-Tesla,
Signa,
General
Electric
Excite,
General
Electric
Medical
Systems
(software
Healthcare
(software
version
version
9.0)
MR
system
with
G3.0-052B)
MR
system
with
a
send-receive
RF
body
coil.
a
send-receive
RF
body
coil.
Radiopacity
The
radiopacity
of
the
Endeavor
stent
system
was
evaluated
at
delivery,
deployment
and
after
implantation
in
an
in
vivo
animal
study
and
radiopacity
characteristics
were
sufficiently
comparable
to
marketed
cobalt
alloy
stents.
Stent
ID
Uniformity
This
test
was
conducted
to
demonstrate
that
the
deployed
internal
diameter
of
the
Endeavor
stent
system
is
consistent
with
labeling.
Each
stent
was
deployed
to
nominal
pressure,
and
the
stent
inner
diameter
was
measured
at
each
end.
The
results
indicated
that
the
Endeavor
stent
system
expands
uniformly
at
all
diameters
and
maintains
this
uniformity
upon
withdrawal
of
the
balloon.
Delivery
System
Dimensional
and
Functional
Attributes
Delivery,
Deployment,
In
vitro
testing
was
conducted
to
evaluate
delivery,
and
Retraction
deployment,
and
retraction
of
the
Endeavor
stent
delivery
system
through
a
simulated
tortuous
path
according
to
the
instructions
for
use.
All
results
indicated
that
the
system
performed
as
intended
and
no
damage
to
the
stent
was
noted.
Balloon
Maximum
This
test
was
conducted
to
demonstrate
that
the
Endeavor
Pressure
stent
system
(with
mounted
stent)
will
not
experience
balloon,
shaft,
proximal
adaptation
or
proximal/distal
seal
loss
of
integrity
at
or
below
the
pressure
required
to
expand
the
stent
to
its
labeled
diameter.
Stent
delivery
systems
were
initially
pressurized
to
90
psi.
The
cycle
was
then
repeated,
increasing
inflation
pressure
by
15
psi
each
cycle
until
failure.
The
results
demonstrate
with
95%
confidence
that
at
least
99.9%
of
the
delivery
systems
will
not
experience
loss
of
integrity
at
or
below
the
rated
burst
pressure.
Balloon
Fatigue
Endeavor
stent
systems
were
required
to
complete
10
unconstrained
pressurization
cycles
to
rated
burst
pressure
(RSP).
The
stent/balloon
burst
results
show
statistically
that,
with
95%
confidence,
90%
of
the
delivery
systems
will
not
experience
balloon,
shaft,
or
proximal/
distal
seal
loss
of
integrity
at
or
below
the
maximum
recommended
RBP.
Stent
Diameter
vs.
Testing
was
performed
to
determine
how
the
stent
inner
Balloon
Pressure
diameter
varies
with
applied
inflation
pressures.
The
stent
(Compliance)
sizing
results
verify
that
the
stent
systems
meet
the labeled
compliance
curves.
Pass
Pass
N/A
Characterization
only
Pass
Pass
NA
Characterization
for
product
labeling
SSED
P060033
-
Page
20
of
59

Table
9:
Stent
and
Delivery
Catheter
Engineering
Testing
lon
frTest
ion
Catheter
Bond
Strength
Testing
was
performed
to
confirm
the
bond
strength
Pass
specifications
for
the
Endeavor
stent
delivery
system.
All
bonds
were
loaded
into
the
tensile
tester
and
pulled
to
failure.
All
samples
met
the
acceptance
criteria.
Stent/Catheter
Crossing
Endeavor
stents
and
delivery
systems
were
tested
to
verify
Pass
Profile
the
crossing
profile
per
label
claims.
All
samples
met
product
specifications.
Balloon
Inflation
and
Endeavor
delivery
systems
were
tested
for
inflation/deflation
Pass
Deflation
Time
times.
All
samples
met
the
product
specification.
Stent
Securement
*
Testing
was
conducted
to
assess
the
force
required
to
Pass
displace
a
crimped
Endeavor
stent
from
the
Endeavor
delivery
system
by
both
forward
and
reverse
motion.
All
stent
systems
met
the
stent
securement
specification.
*
Testing
was
performed
to
characterize
the
performance
of
the
Endeavor
stent
system
(undeployed)
to
cross
a
simulated
lesion
post
tracking
through
a
tortuous
artery
model.
The
Endeavor
product
performed
as
intended.
*
To
further
characterize
the
performance
of
the
Endeavor
stent
system
undeployed
stents
were
tracked
through
a
tortuous
artery
model
and
withdrawn
through
the
recommended
guide
catheter.
The
Endeavor
product
performed
as
intended.
Balloon
Deflatability
This
test
was
performed
to
provide
assurance
that
the
Pass
deflated
delivery
system
balloons
will
release
from
the
expanded
stent
without
interference.
All
samples
met
specification.
Catheter
Body
Testing
was
conducted
on
the
Endeavor
stent
delivery
Pass
Maximum
Pressure
systems
to
determine
the
ability
of
the
catheter
shaft
to
withstand
inflation
to
balloon
rated
burst
pressure.
All
samples
met
the
product
specification.
·
Note:
Microcracks
were
observed
in
the
drug/polymer
coating
layer
of
all
samples
both
pre-
and
post
simulated
use
and
pre-
and
post
durability
testing.
The
location
of
microcracks
did
not
appear
to
change
from
the
pre-
to
the
post
deployed
state.
Microcracks
were
not
observed
in
the
cross-linked
polymer
base
layer
(i.e.
the
coating
layer
that
remains
implanted
post
drug
exhaustion)
after
durability
testing.
Areas
of
apparent
coating
loss
were
observed
on
the
inner
and
outer
stent
diameters;
however
areas
of
coating
loss
were
predominantly
on
the
ID
surface
of
the
stent.
D.
Coating
Characterization
Testing
The
following
methods
were
developed
to
characterize
and
set
initial
specifications
for
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System.
The
coating
characterization
testing
conducted
on
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
includes
those
tests
summarized
in
Table
10.
SSED
P060033
-
Page
21
of
59

Table
10:
Coatin
Characterization
Testing
.~-
~
escrt~~~~r
Pton
of
Test
Materials
Analysis
-
Polymer
components
were
tested
to
ensure
conformity
to
raw
materials
Polymer
specifications
and
incoming
inspection
procedures.
Chemical
Analysis
-
Assays
were
conducted
to
determine
residual
monomer
content,
residual
Polymer
solvent
levels
and
water
content.
Chemical
Analysis
-
Drug
Drug
substance
was
tested
to
ensure
conformity
to
incoming
Certificate
of
Analysis.
Total
Drug
Content
Assay
was
conducted
to
quantitatively
determine
the
total
amount
of
drug
substance,
zotarolimus,
on
the
Endeavor
stent
system.
Dose
Density
Dose
per
mm
was
calculated.
Drug
Content
along
Stent
Testing
was
conducted
to
characterize
the
uniformity
of
distribution
of
Length
drug
along
the
length
of
the
Endeavor
stent
system.
Total
Drug
Related
Assays
were
conducted
to
quantitatively
determine
the
type
and
amount
Substances
of
impurities
and
degradation
products
on
the
Endeavor
stent
system.
Coating
Thickness
Testing
was
conducted
to
describe
the
coating
thickness
along
the
length
of
the
stent.
In
Vitro
Elution
Assay
was
developed
to
measure
the
in
vitro
release
kinetics
of
zotarolimus
from
the
Endeavor
stent
system.
Particulates
Particulate
levels
were
determined
for
the
Endeavor
stent
system
post
tracking
and
deployment.
All
samples
met
product
specifications.
Medtronic
Vascular
provided
a
letter
from
the
polymer
manufacturer,
Biocompatibles
Ltd.,
authorizing
access
to
a
Master
File
(MAF)
in
support
of
this
submission.
SSED
P060033
-
Page
22
of
59
30

E.
Chemistry
Manufacturing
and
Controls
(CMC)
Testing
Where
applicable,
International
Conference
on
Harmonization
(ICH)
Guidelines
were
followed
for
the
testing
routinely
performed
on
Endeavor
stents
as
part
of
CMC.
This
testing
is
summarized
in
Table
11.
Information
to
support
the
stability
of
the
Endeavor
stent
is
summarized
separately
in
Section
VIII.
G
-
Stability
below.
Table
11:
CMC
Release
Testing
Test
:_
_
_ _
_
_
_
_
_ _
_
_
_
Material
Analysis
-
Polymer
The
polymer
was
tested
to
ensure
conformity
to
specifications.
The
polymer
met
specifications
prior
to
utilization
in
finished
goods.
Drug
Identity
Assay
was
conducted
to
verify
the
identity
of
the
drug
substance,
zotarolimus,
on
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System.
The
product
met
specifications
established
for
finished
goods
release.
Drug
Content/Impurities
Assays
were
conducted
to
quantitatively
verity
the
amount
of
drug
and
the
type
and
amount
of
impurities
on
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System.
The
product
met
specifications
established
for
finished
goods
release.
Drug
Content
Uniformity
Multiple
stents
were
assayed
to
verify
the
uniformity
of
the
drug
content
between
the
individual
stents
was
within
specifications
established
for
finished
good
release.
Residual
Solvents
Assay
was
conducted
on
the
Endeavor
stent
system
to verify
that
residual
levels
of
solvents
used
in
the
manufacturing
process
were
below
acceptable
limits
established
for
finished
goods
release.
In
Vitro
Elution
The
in
vitro
release
profile for
zotarolimus
was
measured
on
the Endeavor
stent
system.
Specifications
were
based
on
the
elution
characteristics
of
stents
evaluated
in
the
clinical
investigation.
The
product
met
specifications
established
for
finished
goods
release.
Particulates
Particulate
levels were
monitored
to
verify
that
they
remain
below
acceptable
levels
as
established
in
the
product
specifications.
F.
Animal
Studies
Detailed
arterial
histopathology
and
histomorphometry
are
not
obtainable
through
human
clinical
trials.
Consequently,
a
series
of
animal
studies
were
conducted
to
evaluate
safety,
proof
of
concept
and
overall
product
performance.
Medtronic
Vascular
conducted
a
series
of
animal
studies
evaluating
various
zotarolimus-
eluting
stent
formulations
(e.g.,
drig
dosages),
polymer-coated
control
stents
and/or
bare
metal
control
stents.
These
studies
were
conducted
in
coronary
arteries
of
pigs,
or
iliac
arteries
of
rabbits.
Data
from
these
studies
served
as
the
basis
for
the
dose
selection
for
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
used
in
the
Endeavor
clinical
studies
and
provided
an
assessment
of
the
safety
of
the
product
over
a
range
of
timepoints.
The
intravascular
safety
and
biocompatibility
of
zotarolimus-eluting
stents
were
evaluated
in
a
series
of
animal
studies
in
a
porcine
model
of
stent-mediated
vascular
injury.
Non-GLP
studies
were
conducted
in
order
to
provide
additional
background
data
for
non-safety
related
areas.
All
study
phases
(feasibility,
safety,
pharmacokinetic
and
SSED
P060033
-
Page
23
of
59

________
________
acute)
are
represented
by
studies
that
were
conducted
in
accordance
with
§
21CFR
58
(Good
Laboratory
Practices),
except
where
noted.
The
results
of
these
studies
support
the
safety
and
biocompatibility
of
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System.
Summaries
of
the
major
animal
studies
performed
to
support
product
safety
are
included
in
Table
12.
Table
12:
Summary
of
Major
Supportive
Animal
Studies
Stent
Design
Stento
)o!in
FS102
Test
Article:
Diameter:
Domestic
Swine
Test:
32
7
days
*Angiographic
patency
zotarolimus-eluting
*
3.0,
3.5,
4.0
Test
and
Control:
Control:
20
*Histologic
and
Endeavor
stent
mm
1
histomorphometric
system,
multiple
doses
Lengths:
evaluation
of
single
Control:
BMIS
*12,
18mm
(LAD,
LCX,
RCA)
and
overlapping
GLP:
Yes
One
stent/vessel
stents
GLP:
Yes
Cell
proliferation
!.~~~~~~~~~~~~~~~~~~~~
Animals
received
·
Acute
delivery
both
test
and
control
FS1
35
Test
Article:
Diameter:
Juvenile
Test:
24
28
days
*
Anglographic
patency
zotarolimus-eluting
·
2.25,
2.5
mm
Yorkshire
Swine
*
Histologic
and
Control:
20
hsoohmti
Endeavor
stent
Lengths:
Test
and
Control:ic
system,
multiple
doses
·
8
mm
21
Control
evaluation
of
Control:
BIMIS
and
(Polymer
exaggerated
dose
Polymer
only coated
(LAD,
LCX,
RCA)
Coated
and
Endeavor
stent
stents
One
stent
per
Stents):
12
systems
GLP:
Yes
vessel.
Animals
*Acute
Delivery
received
both
test
*Chronic
vascular
and
control.
response
at
28
days
FS99
Test
Article:
Diameter:
Domestic
Swine
Test:
99
28
days
*
Angiographic
patency
zotarolimus-eluting
·
2.5,
3.0,
3.5,
Test
and
Control:
Control:
64
*Histologic
and
Endeavor
stent
4.0
mm
histomorphometric
system,
multiple
doses
Lengths:
Control
evaluation
of
Control:
BMVIS
and
12,
18mm
(LAD,
LCX,
RCA)
(Polymer
exaggerated
dose,
Polymer
only
coated
One
stent
per
Coated
single
and
Stents):
13
overlapping
stents
stents
vessel.
Animals
13
sents
*
Acute
Delivery
GLP:
Yes
received
both
test
and
control.
*
Chronic
vascular
response
at
28
days
FS100
Test
Article:
Diameter:
Yucatan
Test:
58
90
days
·
Angiographic
patency
zotarolimus-eluting
·
2.5,
3.0
3.5,
Miniswine
*
Histologic
and
Endeavor
stent
Endeavor
stent
~~~~~~~~Control:
4.0
mm
Test
&
Control:
31
43
histomorphometric
system,
multiple
doses
Lengths:
Control
evaluation
of
Control:
BMIS
and
*12
mm
(LAD,
LCX,
RCA)
(Polymer
exaggerated
dose,
Polymer
only
coated
One
stent
per
Coated
single
and
Stents):
overlapping
stents
stents
vessel.
Animals
GLP:
Yes
received
both
test
9
Acute
Delivery
and
control.
*
Chronic
vascular
_________
_
______
________
_
______
________response
at
90
days
SSED
P060033
-
Page
24
of
59
3a

________
Table
12:
Summary
of
Major
Supportive
Animal
Studies
FS101
Test
Article:
Diameter:
Yucatan
Test:
55
180
days
*
Angiographic
patency
zotarolimus-eluting
*2.5,
3.0,
3.5,
Miniswine
Coto:4
Histologic
and
Endeavor
stent
4.0
mm
Test
&
Control:
30
Cnrl40histomorphometric
system,
multiple
doses
Legh:Cnrlevaluation
of
Control:
BMS
and
*12
mm
(LAD,
LCX,
RCA)
(Polymer
exaggerated
dose,
Polymer
only
coated
One
stent
per
~~Coated
single
and
stlyents
l
oae
vnessel.
Anial
Stents):
1 1
overlapping
stents
stents
vessel.
Animals
~~~~~~~~~~~*
Acute
delivery
GLP:
Yes
received
both
test
and
control.
*
Chronic
vascular
__________________
at
180
days
~~~~~~~~~~~response
FS114
Test
Article:
Diameter:
Domestic
Swine
48
1,
2,
3,
5,7,
Evaluation
of
drug
zotarolimus-eluting
*
3.0
mm
(ALXRC)10,
14
and
release
rate,
arterial
Endeavor
stent
system
Lengths:
(ALXRC)28
days
drug
levels
&
systemic
GLP:
Yes
*12
mm
One
stent
per
drug
levels
over
time.
vessel.
FS161
Test
Article:
Diameter:
Juvenile
Test:
ACT
level:
0,
*
Microscopic
zotarolimus-eluting
*
3.0
mm
Yorkshire
Swine
3,
6,9
and
evaluation
of
the
Endeavor
stent
system
Legh:Endeavor
II
days
overall
subacute
Lengths:
~Test
and
Control:
RX:
6
Control:
BMS
*15
mm
22
divided
into
3
Angiography:
tissue
reaction
of
the
tes
1an
ontolEndeavor
1 1
days
vessel
and
the
GLP:
Yes
temst
an
otol
TW:
5
surrounding
(Arms
LXRC
Enevrmyocardium;
Endeavor
One
tentper
MX2:
6
thrombogenicity
of
Onesstent
pr
Control:
the
stent
and
delivery
vessel
~~~~~~~~~~device
(LAD,
LCX,
RCA)
examination
of
the
at
1
1-days
0riv:
post-stenting
*
Acute
delivery
*
Angiographic
patency
*
Histologic
and
histomorphometric
evaluation
of
test
and
controls
*
ACT
levels
at
time
of
implant,
3, 6,
and
9
days
post-implant,
at
I
~~~~~~~~~~~~~~~~~~~~~~explant
BMS
=
bare
metal
stent
-
Drnver
for
these
studies.
The
systemic
toxicity
of
the
intact
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
has
been
investigated
in
a
number
of
studies
that
were
intended
principally
to
define
the
local
tolerance
and
healing
response
to
the
implanted
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
(Studies
FS97,
FS99,
FSlOO0,
FSlI
l,
FSlO
2,
FSllOI
,
FS124
and
FS
135).
In
these
studies,
body
weight
data,
clinical
laboratory
measurements,
necropsies,
and
histopathological
examination
of
selected
tissues
from
the
animals
implanted
with
stents
demonstrated
only
infrequent
adverse
systemic
effects
and
no
systemic
effects
that
could
be
attributed
to
stent
implantation.
SSED
P060033
-
Page
25
of
59

The
efficacy
of
the
zotarolimus
incorporated
into
the
coating
of
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
in
inhibiting
smooth
muscle
proliferation
at
the
site
of
implantation
has
been
examined
in
studies
FS102
and
FS107.
In
both
studies,
there
were
significantly
fewer
proliferating
cells
found
in
sections
of
stented
tissue
at
7
days
post-implantation
when
zotarolimus
was
incorporated
into
the
stent
coating
at
5
pg/mm
or
10
jtg/mm
than
when
bare
Driver
stents
were
implanted.
The
rate
of
elution
of
zotarolimus
from
the
implanted
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
and
the
distribution
of
zotarolimus
from
the
stent
to
surrounding
arterial
tissues,
plasma,
and
distant
organs
has
been
measured
using
the
rabbit
iliac
artery
model
(FS97)
and
the
swine
coronary
artery
model
(FS1
14).
These
studies
demonstrate
that
the
drug
rapidly
elutes
from
the
stent,
with
less
than
50%
of
the
drug
remaining
on
the
stent
at
24
hours
after
implantation
and
less
than
6%
remaining
at
7
days.
Blood
levels
rapidly
decline
to
undetectable
levels
by
7
days
after
implantation
and
peripheral
tissues
have
highly
limited
exposures.
Thus,
while
systemic
exposure
is
limited,
drug
remains
at
the
implantation
site
for
at
least
28
days.
The
local
tolerance
and
healing
of
coronary
vessels
after
implantation
of
the
Endeavor
stent
have
been
evaluated
at
various
times
(i.e.,
7,
28,
90,
and
180
days)
after
stent
implantation
into
swine
coronary
arteries
(Studies
FS99,
FS
I00,
FS
01,
FS
102,
FS
135)
and
rabbit
iliac
arteries
(Study
FS
107).
These
studies
demonstrate
that
initially
the
stent
produced
a
slightly
greater
level
of
inflammation
surrounding
stents
than
the
bare
control
Driver
stents.
This
effect
was
observed
early
after
implantation
but
did
not
persist.
Also,
a
slight
delay
in
endothelialization
and
fibrin
removal
between
overlapped
bare
and
overlapped
drug-coated
stents
was
observed.
However,
no
differences
in
long-term
healing
attributable
to
the
presence
of
the
drug
coating
on
the
stents
were
observed.
The
slightly
greater
inflammation
observed
with
the
Endeavor
stent
did
not
result
in
greater
levels
of
restenosis.
The
effect
of
the
stents
on
vascular
healing
after
stent
placement
was
also
evaluated
in
both
swine
and
rabbits.
All
of
the
stents
exhibited
complete
endothelialization
at
both
the
90
and
180
day
timepoints.
G.
Stability
Manufacturing
site-specific
stability
studies
were
conducted
to
establish
a
shelf-
life/expiration
date
for
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System.
Testing
to
establish
package
integrity
and
functional
testing
of
the
stent
system
were
conducted
on
aged
product.
Testing
evaluation
included
drug
identity,
assay,
degradants,
in
vitro
elution,
particulates,
sterility,
drug
content
uniformity,
residual
solvents,
and
endotoxins.
Appropriate
engineering
tests
were
also
performed
on
aged
product
and
compared
to
baseline
to
ensure
that
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
performed
acceptably.
The
data
generated
support
a
shelf
life
of
one
year.
H.
Sterilization
The
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
(OTW,
RX,
and
MX
2
delivery
systems)
is
sterilized
using
ethylene
oxide
sterilization,
and
has
been
validated
per
SSED
P060033
-
Page
26
of
59

AAMIIISO
11135:1994
"Medical
Devices
-
Validation
and
Routine
Control
of
Ethylene
Oxide
Sterilization."
Results
obtained
from
the
sterilization
studies
show
that
the
product
satisfies
a
minimum
Sterility
Assurance
Level
(SAL)
of
10
-6
.
The
amount
of
bacterial
endotoxins
was
verified
to
be
within
the
specification
limit
for
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System.
IX.
Overview
of
Clinical
Studies
The
principal
safety
and
efficacy
information
for
the
Endeavor
stent
is
presented
from
the
following
clinical
studies
-
the
ENDEAVOR
I
trial,
the
ENDEAVOR
II
trial,
the
ENDEAVOR
III
trial
and
the
ENDEAVOR
IV
trial.
These
studies
have
evaluated
the
performance
of
the
Endeavor
Stent
in
patients
with
symptomatic
ischemic
heart
disease
in
single
de
novo
lesions
of
native
coronary
arteries.
The
ENDEAVOR
I
trial
was
the
first-in-man
study
for
the
Endeavor
stent.
ENDEAVOR
I
was
a
non-randomized,
prospective,
multi-center,
single-arm
trial.
The
purpose
of
the
trial
was
to
assess
the
initial
safety
of
the
Endeavor
stent.
The
primary
endpoints
in
this
trial
were
the
rate
of
major
adverse
cardiac
events
(MACE)
defined
as
composite
of
death,
myocardial
infarction
(MI),
emergent
bypass
surgery,
or
target
lesion
revascularization
(TLR)
at
30
days
and
in-segment
late
loss
at
4
months
as
measured
by
quantitative
coronary
angiography
(QCA).
Post-procedure,
patients
received
aspirin
indefinitely
and
clopidogrel
or
ticlopidine
for
a
minimum
of
3
months.
The
ENDEAVOR
II
trial
was
a
prospective,
multi-center,
double-blind,
two-arm
randomized
and
controlled,
superiority
trial
that
compared
the
Endeavor
stent
to
a
control
bare
metal
stent
(the
Driver
stent).
Eligibility
was
based
on
assessments
of
lesion
reference
vessel
diameter
and
lesion
length.
The
primary
endpoint
in
this
trial
was
the
target
vessel
failure
(TVF)
rate,
defined
as
the
composite
of
cardiac
death,
MI,
or
clinically-driven
target
vessel
revascularization
(TVR)
of
the
treated
vessel
at
9
months
post-procedure.
The
powered
secondary
endpoint
was
in-segment
late
loss
at
8
months
measured
by
QCA.
Post-procedure,
patients
received
aspirin
indefinitely
and
clopidogrel
or
ticlopidine
for
a
minimum
of
3
months.
The
ENDEAVOR
III
trial
was
a
prospective,
multi-center,
single-blind,
two-arm
randomized
and
controlled,
non-inferiority
trial
that
compared
the
Endeavor
stent
to
a
control
DES
(the
Cypher
stent).
Eligibility
was
based
on
the
assessments
of
a
lesion
reference
vessel
diameter
and
lesion
length.
The
primary
endpoint
of
this
study
was
in-
segment
late
loss
at
8
months
as
measured
by
QCA
and
defined
as
the
difference
between
post-procedure
minimum
lumen
diameter
(MLD)
and
the
MLD
at
time
of
follow-up
within
the
stented
region
and
5
mm
proximal
and
distal
to
the
edges
of
the
stent.
Post-
procedure,
patients
received
aspirin
indefinitely
and
clopidogrel
or
ticlopidine
for
a
minimum
of
3
months.
The
ENDEAVOR
IV
trial
was
a
prospective,
multi-center,
single-blind,
two-arm
randomized
and
controlled,
non-inferiority
trial
that
compared
the
Endeavor
stent
to
a
SSED
P060033
-
Page
27
of
59
¢:5

_________
________
control
DES
(the
Taxus
stent).
Eligibility
was
based
on
the
assessments
of
a
lesion
reference
vessel
diameter
and
lesion
length.
The
primary
clinical
endpoint
in
this
non-
inferiority
study
was
the
TVF
rate,
defined
as
the
composite
of
cardiac
death,
MI,
or
clinically-driven
TVR
of
the
treated
vessel
at
9
months
post-procedure.
The
powered
secondary
endpoint
was
in-segmnent
late
loss
at
8
months,
measured
by
QCA.
Post-
procedure,
patients
received
aspirin
indefinitely
and
clopidogrel
for
a
minimum
of
6
months.
Table
13:
Clinicai
Trial
Comparisons
ENDEAVOR
I
EDA61EN
AVO
ENDEAVOR1V1
Il
.
Multi-center
(n=8)
Mult-center
(n=7
Multi-center
(n=29)
Multi-center
(n=80)
Study
Type
Prospective
Prospective
Prospective
Prospective
Non-randomized
Randomized
Randomized
Randomized
Number
of
10
~
'
Tota:
117(ndeavor:
Total:
436
(Endeavor:
Total:
1548
(Endeavor:
Patients
Total:
1
(ndeavor)
598,
Driver:
599)
323,
Cypher:1
13)
773,
Taxus:
775)
Single
de
novo
lesion
Single
de
novo
lesion
Single
de
novo
lesion
Single
de
novo
lesion
Lesion
in
native
coronary
in
native
coronary
in
native
coronary
in
native
coronary
Critria
artery
<515
mm
in
artery
>
14
mm
and
<
Criteria
length
and
>
3.0
mm
to
27
mm
in
length
and
>
artery>14
mm
and
<
artey<2mmi
27
mm
in
length
and>
an
mm
2
.
2.Smm
to
<
3a5mm
i
leryt
27
5
in
35
~~~~
2
5
mm
to
<3.Smm
in
lngt
n~
m
t
35mm
in
diameter
diameter
diameter
3.5
mm
in
diameter
Product
Endeavor
Stent
on
the
Endeavor
Stent
on
the
Endeavor
Stent
on
the
Endeavor
Stent
on
the
Used
Rapid
Exchange
Stent
Rapid
Exchange
Stent
Over-The
-Wire
Stent
Over-The
-Wire
Stent
Delivery
System
Delivery
System
Delivery
System
Delivery
System
Aspirin
indTefi~nitely
and
~Aspirin
indefinitely
and-
Aspirin
indefinitely
and
Aspirin
indefinitely
and
Antiplatelet
clopidogrel
or
clopidogrel
or
clopidogrel
or
clopidogrel
or
Therapy
ticlopidine
for
~:
3
ticlopidine
for
Ž:
3
ticlopidine
for
>
3
ticlopidine
for
~:
6
months,
months,
months,
months
30
days:
clinical
30
days:
clinical
30
days:
clinical
30
days:
clinical
4
&
12
months:
clinical
8
months:
clinical
and
8
months:
clinical
and
8
months:
clinical
and
Follow
up
and
angiographic/IVUS
angiographic/IVUS
angiographic/IVUS
angiographic/IVUS
9
month:
clinical
9
month:
clinical
9
month:
clinical
9
month:
clinical
1-5
years:
telephone
6
month,
1-5
years:
6
month,
I-5
years:
6
month,
1-5
years:
telepon
telehn
telepon
48
month
follow-up
36
month
follow-up
is
24
month
follow-up
is
9
month
follow-up
is
Status
complete.
Yearly
follow
complete.
Yearly
follow
complete.
Yearly
follow
complete.
Yearly
follow
up
to
5
years
is
up
to
5
years
is
up
to
5
years
is
up
to
5
years
is
__________ongoing.
ongoing
.
oina
on
I
onaninn
Two
additional
single-arm
non-randomized
trials
were
reviewed
by
FDA:
the
ENDEAVOR
II
Continued
Access
study
and
the
ENDEAVOR
PK
study.
The
objective
of
the
ENDEAVOR
II
Continued
Access
registry
was
to
collect
additional
acute
safety
information
and
performance
data
of
the
Endeavor
stent.
The
primary
endpoint
was
MACE
at
30
days.
The
objective
of
the
ENDEAVOR
PK
study
was
to
assess
the
pharmnacokinetic
profile
of
the
Endeavor
stent
(see
Section
VIII.
A
-
S
In
Vivo
Pharmacokinetics).
These
trials
provide
additional
data
on
Endeavor
stent
use.
Results
of
these
studies
have
been
pooled
with
the
patients
treated
with
Endeavor
stents
in
Endeavor
I,
II,
III,
and
IV
studies
described
above
in
a
post-hoc
patient-level
analysis
to
provide
an
enhanced
estimate
of
the
incidence
of
low-frequency
events
and
outcomes
in
specific
patient
subgroups
(see
Section
XI.
F
Overall
Results
of
the
ENDEAVOR
Clinical
Program
(ENDEAVOR
I,
II,
II-CA,
III,
IV
and
USPK)).
SSED
P060033
-
Page
28
of
59

The
Endeavor
MX
2
delivery
system
was
not
used
in
the
Endeavor
clinical
investigations
(ENDEAVOR
I
to
IV).
The
Endeavor
MX
2
utilizes
a
modified
shaft
to
facilitate
the
multi-exchange
feature
of
the
delivery
system.
The
distal
section
of
the
Endeavor
MX
2
Zotarolimus-Eluting
Coronary
Stent
System,
including
the
stent,
balloon,
drug
coating
and
stent
interfaces
are
identical
to
that
of
the
Endeavor
RX and
OTW
delivery
systems
which
were
studied
clinically.
Appropriate
pre-
clinical
testing
was
provided
to
support
the
Endeavor
MX
2
delivery
system.
Acute
and
long
term
safety
and
performance
data
is
provided
in
the
PMA
application
(ENDEAVOR
I
to
IV
clinical
investigations,
over
2000
patients)
to
support
the
clinical
safety
and
efficacy
of
the
Endeavor
stent
mounted
on
the
Endeavor
delivery
systems.
X.
Adverse
Events
A.
Observed
Adverse
Events
Observed
adverse
event
experience
with
the
Endeavor
stent
comes
from
four
clinical
studies:
the
ENDEAVOR
IV,
the
ENDEAVOR
III,
the
ENDEAVOR
II,
and
the
ENDEAVOR
I
trials.
The
ENDEAVOR
IV,
III,
II,
and
I
trials
have
evaluated
the
performance
of
the
Endeavor
stent
in
patients
with
symptomatic
ischemic
heart
disease
in
single
de
novo
lesions
of
native
coronary
arteries.
Principal
adverse
events
are
shown
in
Table
14.
SSED
P060033
-
Page
29
of
59
3-)

Table
14:
ENDEAVOR
IV,
III,
Ii
and
I
-
Principal
Adverse
Events
from
Post-procedure
to
Latest
Foi~ow-
p
~JbrAVORIV
yr
Taxcus
Ed>CpDriver,
.J4V
N=775
N
In-Hospital
MACE
0.9%
(7/773)
2.6%
(20/775)
0.6%
(2/323)
3.5%
(4/113)
2.5%
(15/597)
2.9%/
(1
7/596)
0%
(0/100)
Total
Death
0%
(0/773)
0%
(0/775)
0.0%
(0/323)
0.0%
(0/113)
0.2%
(1/597)
0.0%
(0/596)
0.0%
(0/100)
Cardiac
Death
0.0%
(0/773)
0.0%
(0/775)
0.0%
(0/323)
0.0%
(0/113)
0.2%
(1/597)
0.0%
(0/596)
0.0%
(0/100)
Non-Cardiac
0.0%
(0/773)
0.0%
(0/775)
0.0%
(0/323)
0.0%
(0/113)
0.0%
(0/597)
0.0%
(0/596)
0.0%
(0/100)
DeathI
ml
0.8%
(6/773)
2.1%
(16/775)
0.6%
(21323)
3.5%
(4/113)
2.5%
(15/597)
2.7%
(16/596)
0.0%
(0/100)
0
wave
Ml
0.3%
(2/773)
0.1%
(1/775)
0.0%
(01323)
0.0%
(0/113)
0.2%
(1/597)
0.3%
(2/596)
0.0%
(0/100)
Non-Q
wave
Ml
0.5%
(4/773)
(15/775)
~(2/323)
3.5%
(4/113)
2.3%
(14/597)
2.3%
(14/596)
0.0%
(0/100)
1.9%9/
0T.6%
TVR
--
_0.4%
-(3/773)
0.6%
(51775)
0.0%
(0/323)
0.0%1
~(0/113)
0.5%
(3/597)
0.3%
(2/596)
0.0%
(0/100)
TLR
0.4%
(3/773)
0.5%
(4/775)
0.0%1
-(0/323)
0.0%
(0/113)
0.5%
(3/597)
0.3%
(2/596)
0.0%
(0/100)
Non-TLR
0.0%
(0/773)
0.3%/
(2/775)
0.0%
(0/323)
0.0%
(0/113)
0.0%
(0/597)
0.0%
(0/596)
0.0%
(0/100)
Cardiac
death
or
0.8%
(6/773)
2.1
%
(16/775)
0.6%
(2/323)
3.5%
(4/113)
2.5%
(15/597)
2.7%
(16/596)
0.0%
(0/100)
ml
TVF
0.9%
(7/773)
2.6%(2-0/775)
0.6%
.(2/323)
3.5%
(4/113)
2.9%o_
2.5%
(15/597)
(1-7/5
96)
0.0%
(0/100)
Stent
thrombosis
0.3%
(2/773)
0.0%
(0/775)
0.0%
(0/323)
0.0%
(0/113)
0.3%
(2/597)
0.3%
(2/596)
0.0%
(0/100)
(protocol)
Data
at
9
Months
MACE
5.%(274)
57(4/3)
75%0(24/321)
7.1%
(8/113)
7.3%
(43/692)
14.4%
(85/591)
2.0%
(2/100)
Total
Death
(70.7%(5/740)
680/.8%(6/734)
0.6%/
~(2/321)
0.0%/
~(0/113)
-1.2%9
(7/5-92)
0.~5%/
~(3/591)
0.0%
(0/100)
Cardiac
Death
0.4%/(3/740)
0.3%2/734
0.%0/21
0.0%/
~(0/1113)
0.-8%/
(5/592)
50.5%(3/591)
0.0%
(0/100)
Non-Cardiac
0.3%
.(2/740)
05%
(4/734)
06%/
(2/321)
00(0/1
13)
03%~/(2/592)
.%(/9)
0.0%
(0/100)
Death
ml
1.5%
(11/740)
2.5%
(18/734)
_0.6%/6(2/321)
3.5%
(41113)
2.7%(16/592)
3.9%
(23/591)
1.0%
(1/100)
QOwaveMl
30.3%(2/740)
0.1%
(1/734)
0.0%(0/321)
0_O-.0(0/113)
06._3-%(2/592)
0_.~8%/(5/591)
0.0%
(0/100)
Non-0
wave
Ml
1.2%/
(9/740)
_2.3%/
(17/734)
0.6_6%
3.5%~(4/1
13)
/
(14/592)
3.0%
(18/591)
1.0%
(1/100)
(2/3-21)
24%
TVR
5.5%
(41/740)
5.0%
(37/734)
11.2%
(36/321)
8.0%
(9/113)
5.6%
(33/592)
12.5%
(74/591)
2.0%
(2/100)
TLR
4.2%
(31/740)
2.7%
(20/734)
6.2%1
(20/32
1)
3.T5%/
(4/1-13)
4._~/6%(27/592)
171.8%
~(70/691-)
.0%(2/100)
2--
Norn-TLR
2.0%
(15/740)
2.9%
(21/734)
5.90%
(19/321)
5.3%
(6/1
13)
1.59%(9/592)
2.2%
(13/591)
0.0%
(0/100)
Cardiac
death
or
1.9%
(14/740)
2.7%
(20/734)
0.6%
(2/321)
3.5%
(4/113)
3.4%
(20/592)
4.4%
(26/591)
1.0%
(1/100)
ml
TVF
6.8%
(0/740)
7.4
(54/734)
11.8%
TVE
6.8%(50/740)
7.4%(54/734)
~~~~~(38/321)
7.9%
(47/592)
15.1%
(89/591)
2.0%
(2/100)
11.5%
(13/113)
Stent
thrombosis
0.8%
(6/740)
0.1%
(1/734)
0.0%
(0/321)
0.0%
(0/113)
0.5%
(3/592)
1.2%
(7/591)
1.0%
(1/100)
(protocol)
1-year
MACE
NA
NA
7.8%
(25/320)
8.0%
(9/112)
88
(52/590)
15.6%
(92/589)
2.0%
(2/99)
2-erMACE
NAN
.%(933
1.%(312
.%(58/587)
18.1%
(10-6/586)
3.0%
(3/99)
_Y'
M
NA
~~~~~~~~~~~~~~~~12.0%
~3-year
M
A
rCE
N
AN
A(
9 5
7
0 7
1
0 5
9
. %
(
/ 8
SSED
P060033
-
Page
30
of
59

Table
14:
ENDEAVOR
IV,
Ill,
II
and
I
-
Princi
al
Adverse
Events
from
Post-procedure
to
Latest
Follow-up
VoRIR
m
...
.
ENDEAVOR.
4-year
MACE
NANA
NA
NA
NA
7.2%
(7/97)
Latest
Data
Available
9
Months
24
Months
36
Months
48
Months
MACE
5.7%
(42/740)
5.7% (42/734)
9.3%
(29/313)
11.6%
(13/112)
12.0%
(69/%577)
20.7%
(120/579)
7.2%
(7197)
Total
Death
0.7%
(5/740)
0.8%
(6/734)
1.6%
(5/313)
4.5%
(5/112)
3.3%
(19/577)
4.5%
(26/579)
4.1%
(4/97)
Cardiac
Death
0.4%
(3/740)
0.3%
(2/734)
0.0%
(0/313)
0.9%
(1/112)
1.6%
(9/577)
2.4%
(14/579)
0.0%
(0/97)
Non-Cardiac
Death
0.3%
(2/740)
0.5%
(4/734)
1.6%
(5/313)
3.6%
(4/112)
1.7%
(10/577)
2.1%
(12/579)
4.1%
(4/97)
MI
1.5%
(11/740)
2.5%
(18/734)
0.6%
(2/313)
3.6%
(4/112)
3.3%
(19/577)
4.3%
(25/579)
1.0%
(1/97)
Q
wave
MI
0.3%
(2/740)
0.1%
(1/734)
0.0%
(0/313)
0.0%
(0/112)
0.3%
(2/577)
1.0%
(6/579)
0.0%
(0/97)
Non-Q
wave
MI
1.2%
(9/740)
2.3%
(17/734)
0.6%
(2/313)
3.6%
(4/112)
2.9%
(17/577)
3.3%
(19/579)
1.0%
(1/97)
TVR
TV R
5.5%
(41/740)
5.5%
(41/740)
5.0%
(37/734)
~~~~13.7%
13)
9.8%
(11/112)
(43/313)
9.5%
(55/577)
17.6%
(102/579)
5.2%
(5/97)
TLR
4.2%
(31/740)
2.7%
(20/734)
7.0%
(22/313)
4.5%
(5/112)
7.3%
(42/577)
14.7%
(85/579)
3.1%
(3/97)
Non-TLR
2.0%
(15/740)
2.9%
(21/734)
8.3%
(26/313)
6.3%
(7/112)
2.9%
(17/577)
4.8%
(28/579)
2.1%
(2/97)
Cardiac
death
or
ml
1.9%
(14/740)
2.7%
(20/734)
0.6%
(2/313)
3.6%
(4/112)
4.5%
(26/577)
6.7%
(39/579)
0.0%
(0/0)
TVF
6.8%
(50/740)
7.4%
(54/734)
14.4%
(45/313)
13.4% (15/112)
(45/313)
12.8%
(7/7)
~~~~~(74/577)
21.4% (124/579)
5.2%
(6/97)
Stent
thrombosis
0.8%
(6/740)
0.1%
(1/734)
0.0%
(0/313)
0.0%
(0/112)
0.5%
(3/577)
1.2%
(7/579)
1.0%
(1/97)
NA=
Not
Applicable;
variable
and/or
time
point
not
calculated
N
=
The
maximum
number
of eligible
patients.
The
numbers
are
%
(Count/Sample
Size).
Major
adverse
cardiac
events
(MACE)
is
defined
as
composite
of
death,
MI
(Q
wave
and
non-Q
wave),
emergent
bypass
surgery,
or
target
lesion
revascularization
(repeat
PTCA
or
CABG).
Q
wave
MI
(QMI)
defined
when
any
occurrence
of
chest
pain
or
other
acute
symptoms
consistent
with
myocardial
ischemia
and
new
pathological
Q
waves
in
two
or
more
contiguous
ECG
leads
as
determined
by
an
ECG
core
laboratory
or
independent
review
of
the
CEC,
in
the
absence
of
timely
cardiac
enzyme
data,
or
new
pathologic
Q
waves
in
two
or
more
contiguous
ECG
leads
as
determined
by
an
ECG
core
laboratory
or
independent
review
of
the
CEC
and
elevation
of
cardiac
enzymes.
In
the
absence
of
ECG
data
the
CEC
may
adjudicate
Q
wave
MI
based
on
the
clinical
scenario
and
appropriate
cardiac
enzyme
data.
Non-Q
Wave
MI
(NOMI)
is
defined
as
elevated
CK
Ž
2X
the
upper
laboratory
normal
with
the
presence
of
elevated
CK-
MB
(any
amount
above
the
institution's
upper
limit
of
normal)
in
the
absence
of
new
pathological
Q
waves.
Stent
Thrombosis:
See
section
XI.F1
for
the
per
protocol
stent
thrombosis
definition.
Target
vessel
failure
(TVF)
is
defined
as
a
composite
of
cardiac
death,
myocardial
infarction,
or
clinically-driven
target
vessel
revascularization.
Target
lesion
revascularization
(TLR)
is
defined
as
any
clinically-driven
repeat
intervention
of
the
target
lesion
by
PCI
or
CABG
of
the
target
vessel.
Target
vessel
revascularization
(TVR)
is
defined
as
any
clinically
driven
repeat
intervention
of
the
target
vessel
by
PCI
or
CABG.
SSED
P060033
-
Page
31
of59

B.
Potential
Adverse
Events
Adverse
events
associated
with
using
this
product
are
those
associated
with
percutaneous
coronary
diagnostic
(including
angiography
and
IVUS)
and
treatment
procedures.
These
risks
may
include,
but
are
not
limited
to,
the
following:
*
Abrupt
vessel
closure
*
Access
site
pain,
hematoma
or
hemorrhage
*
Allergic
reaction
(to
contrast,
antiplatelet
therapy,
stent
material,
or
drug
and
polymer
coating)
*
Aneurysm,
pseudoaneurysm,
or
arteriovenous
fistula
(AVF)
*
Arrhythmias
•
Balloon
rupture
*
Cardiac
tamponade
*
Coronary
artery
occlusion,
perforation,
rupture,
or
dissection
*
Coronary
artery
spasm
*
Death
*
Embolism
(air,
tissue,
device,
or
thrombus)
*
Emergency
surgery:
peripheral
vascular
or
coronary
bypass
*
Failure
to
deliver
the
stent
•
Hemorrhage
requiring
transfusion
·
Hypotension/hypertension
*
Incomplete
stent
apposition
*
Infection
or
fever
*
Late
or
very
late
thrombosis
*
Myocardial
infarction
(MI)
*
Myocardial
ischemia
*
Peripheral
ischemia/peripheral
nerve
injury
*
Renal
failure
*
Restenosis
of
the
stented
artery
*
Rupture
of
native
or
bypass
graft
*
Shock/pulmonary
edema
*
Stent
deformation,
collapse,
or
fracture
*
Stent
migration
*
Stent
misplacement
*
Stroke/transient
ischemic
attack
*
Thrombosis
(acute
and
subacute)
*
Unstable
angina
*
Ventricular
fibrillation
Patients'
exposure
to
zotarolimus
is
directly
related
to
the
total
amount
of
stent
length
implanted.
The
adverse
events
that
have
been
associated
with
the
intravenous
injection
of
zotarolimus
in
humans
include:
SSED
P060033
-
Page
34
of
59
'tO

*Anemia
*Application
site
reaction
*Diarrhea
*Dry
skin
*Headache
*Hematuria
*Infection
*Injection
site
reaction
*Pain
(abdominal,
arthralgia,
injection
site)
*Rash
In
addition,
thirty-day
adverse
event
information
was
provided
on
over
2000
patients
treated
with
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
to
support
the
safety
profile
of
zotarolimus.
There
may
be
other
potential
adverse
events
that
are
unforeseen
at
this
time.
XI.
Summary
of
Clinical
Studies
A.
Results
of
the
ENDEAVOR
IV
Trial
Primary
Objective:
To
demonstrate
the
non-inferiority
in
safety
and
efficacy
of
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
when
compared
to
the
Taxus
Express
2
Paclitaxel-Eluting
Coronary
Stent
System
for
the
treatment
of
single
de
novo
lesions
in
native
coronary
arteries
with
a
reference
vessel
diameter
of
2.5
mm
to
3.5
mm
and
lesion
length
of<~
27
mm.
Desi
n:
This
was
a
prospective,
multi-center,
single-blind,
two-arm,
randomized
and
controlled
non-inferiority
trial
that compared
the
Endeavor
stent
to
a
control
DES
(the
Taxus
stent).
A
total
of
1548
patients
were
enrolled
at 80
study
sites
in
the
United
States
who
presented
with
symptomatic
ischemic
heart
disease
attributable
to
stenotic
lesions
of
the
native
coronary
arteries
that
were
amenable
to
treatment
by
stenting.
Patients
were
stratified
by
diabetic
status
and
subsequently
randomized
to
receive
either
the
Endeavor
or
Taxus
stent
in
a
1:1I
ratio.
Multiple
stents
were
allowed
for
bailout
only.
Follow-up
was
performed
at
30
days,
6,
8,
and
9
months,
and
will
be
performed
at
12
months,
and
annually
thereafter
out
to
5
years.
The
first
328
consecutively
enrolled
patients
(across
all
sites)
were
scheduled
to
have
angiographic
and
IVUS
evaluations
at
8
months.
Following
the
index
procedure,
patients
were
treated
with
aspirin
indefinitely
and
clopidogrel
or
ticlopidine
for
a
minimum
of
6
months.
Demographics:
The
mean
age
was
63.5
years
for
patients
in
the
Endeavor
arm
and
63.6
years
for
patients
in
the
Taxus
arm.
The
Endeavor
arm
had
66.9%
(5
17/773)
males,
and
the
Taxus
arm
had
68.5%
(53
1/775)
males.
In
the
Endeavor
arm,
28.2%
(2
18/773)
of
patients
had
prior
percutaneous
coronary
revascularization,
compared
to
29.5%
(229/775)
of
patients
in
the
Taxus
arm.
In
the
Endeavor
arm,
3
1.2%
(241/773)
of
patients
had
a
history
of
diabetes
mellitus,
compared
to
30.5%
(236/775)
of
patients
in
the
Taxus
arm.
Patients
were
well-matched
for
baseline
demographics
with
no
statistically
significant
differences
between
treatment
arms.
SSED
P060033
-
Page
33
of
59

Results:
The
primary
and
secondary
endpoints,
protocol-defined
stent
thrombosis,
and
the
latest
available
follow-up
results
are
presented
below
(Table
15,
Table
16,
Table
17
and
Figure
3).
The
primary
endpoint
of
TVF
at
9
months
was
met
with
6.8%
(50/740)
for
the
Endeavor
arm
and
7.4%
(54/734)
for
the
Taxus
arm
(p <
0.001
for
non-inferiority).
The
pre-specified
secondary
endpoint
of
in-segment
late
loss
at
8
months
was
not
met
with
measurements
of
0.36
+
0.47
mm
(143)
for
the
Endeavor
arm
and
0.23
±
0.45
mm
(135)
for
the
Taxus
arm
(p
=
0.0890
for
non-inferiority)
Table
15:
ENDEAVOR
IV
Clinical
Results
PRIMARY
ENDPOINT
TVF
§
6.8%
(50/740)
7.4%
(54/734)
<
0.001*
§
9-month
primary
endpoint.
*
Test
for
non-inferiority.
EFFICACY
TVR
5.5%
(41/740)
5.0%
(37/734)
0.727**
TLR
4.2%
(31/740)
2.7%
(20/734)
0.154*t
TLR,
PCI
3.8%
(28/740)
1.9%
(14/734)
0.041**
TLR,
CABG
0.5%
(4/740)
0.8%
(6/734)
0.546**
Non-TLR
2.0%
(15/740)
2.9%
(21/734)
0.316**
Non-TLR,
PCI
1.8%
(13/740)
2.5%
(18/734)
0.370**
Non-TLR,
CABG
0.4%
(3/740)
0.4%
(3/734)
1.000**
SAFETY
Total
Death
0.7%
(5/740)
0.8%
(6/734)
0.773**
Cardiac
Death
0.4%
(3/740)
0.3%
(2/734)
1.000**
Non-Cardiac
Death
0.3%
(2/740)
0.5%
(4/734)
0.450**
Cardiac
Death
or
MI
1.9%
(14/740)
2.7%
(20/734)
0.303**
MI
1.5%
(11/740)
2.5%
(18/734)
0.194**
Q
wave
MI
0.3%
(2/740)
0.1%
(1/734)
1.000'*
Non-Q
wave
Mi
1.2%
(9/740)
2.3%
(17/734)
0.117**
Stent
Thrombosis
(protocol)
0.8%
(6/740)
0.1%
(1/734)
0.124**
**
P-values
for
outcome
differences
are
not
adjusted
for
multiple
comparisons.
Notes:
Fisher's
Exact
test
was
used
for
P-values.
This
trial
was
not
adequately
powered
to
compare
the
rate
of
low
frequency
events,
nor
was
it
sized
to
determine
the
rate
of
low
frequency
events
with
a
pre-specified
precision.
Numbers
are
%
(Count/Sample
Size).
To
be
included
in
the
event
rate
calculation
for
a
given
interval,
a
patient
either
had
to
have
an
event
before
the
time
of
interest
or
they
had
to
be
event-free
before
the
lower
window
of
the
follow-up.
SSED
P060033
-
Page
34
of
59

0
1
00%'
E
'~
95%'
--
ENDEAVOR
--
TAXUS
90%
I
I
I
,
I
0
30
60
90
120
150
180
210
240
270
Time
after
Initial
Procedure
(days)
: 'TV
i
tree:
IENDEAVOR
93.3%96.7%6%
ITAXUS
1
92.8%
1
7.2%I
_
0.626
ILog-rank
P-value.
P-value
is
not
adjusted
for multiple
comparisons.
Figure
3:
Survival
Free
from
Target
Vessel
Failure
(at
270
days)
SSED
P060033
-
Page
35
of
59
'43

Table
16:
Endeavor
IV
8-Month
An
iographic
and
IVUS
Results
SECONDARY
ENDPOINT
Late
Loss,
In-segment
(mm)
¥
0.36
±
0.47
(143)
0.23
±
0.45
(135)
0.089*
¥
Powered
secondary
endpoint.
*
Test
for
non-inferiority.
OTHER
ANGIOGRAPHIC
RESULTS
MLD
(mm),
In-stent
Post-Procedure
2.62
±
0.43
(763)
2.61
±
0.44
(763)
0.703**
8-Month
1.95
±
0.61
(143)
2.25
±
0.61
(135)
<
0.001**
MLD
(mm),
In-segment
Post-Procedure
2.22
±
0.47
(770)
2.19
±
0.50
(772)
0.196*
8-Month
1.80
±
0.55
(144)
1.98
±
0.56 (135)
0.008**
%
DS,
In-stent
Post-Procedure
5.50
±
9.61
(763)
5.01
10.49(763)
0.348**
8-Month
26.41
±
19.74
(143)
16.09
±
17.99
(135)
<
0.001*
%
DS,
In-segment
Post-Procedure
20.47
9.54(770)
20.97
±11.12(772)
0.344**
8-Month
32.28
17.02
(144)
26.61
±
15.52
(135)
0.004**
Late
Loss, In-stent
(mm)
0.67
±
0.49
(142)
0.42
±
0.50
(135)
< 0.001*
Binary
Restenosis
In-stent
Restenosis
13.3% (19/143)
6.7%
(9/135)
0.075**
In-segment
Restenosis
15.3%
(22/144)
10.4%
(14/135)
0.284**
IVUS
RESULTS
Neointimal
Volume
(mm
3
)
24.14
±19.38
(74)
14.88
±16.62
(77)
0.002**
%
Volume
Obstruction
15.72
±10.40
(74)
9.88
±9.24
(77)
<
0.001'*
Incomplete
Apposition
Post-procedure
12.5%
(17/136)
11.8%
(15/127)
1.000'*
8-Month
10.0%
(12/120)
14.7%
(17/116)
0.324'
Resolved
3.8%
(4/106)
2.1%
(2/95)
0.686**
Persistent
8.5%
(9/106)
10.5%
(10/95)
0.638**
Late Acquired
0.9% (1/106)
3.2%
(3/95)
0.346**
**
P-values
for
outcome
differences
are
not
adjusted
for
multiple
comparisons.
Note:
Fisher's
Exact
test
or
Student's
t-test
was
used
for
P-values.
SSED P060033
-
Page
36
of
59

Table
17:
ENDEAVOR
IV
Protocol-Defined
Stent
Thrombosis*
Throu
h
9
Months
Cumulative
ST
through
9
Months
0.8%
(6/740)
0.1%
(1/734)
0.124**
Acute
ST
(<
24 hrs)
0.0%
(0/770)
0.0%
(0/771)
-
Subacute
ST
(>
24
hrs
and
•
30days)
0.4%
(3/770)
0.1%
(1/771)
0.374**
Late
ST
(>
30
days
and
•
9
months)
0.4%
(3/740)
0.0%
(0/734)
0.250**
*
See
section
XI.F1
for
the
per
protocol
stent
thrombosis
definition.
**
P-values
for
outcome
differences
are
not
adjusted
for
multiple
comparisons.
Notes:
Fisher's
Exact
test
was used
for
P-values.
This
trial
was
not
adequately
powered
to
compare
the
rate
of
low
frequency
events,
nor
was
it
sized
to
determine
the
rate
of
low
frequency
events
with
a
pre-specified
precision,
To
be
included
in
the
event
rate
calculation
for
a
given
interval,
a
patient
either
had
to
have
an
event
before
the
time
of
interest
or
they
had
to
be event-free
before
the
lower
window
of
the
follow-up.
Numbers
are
%
(Count/Sample
Size).
B.
Results
of
the
ENDEAVOR
III
Clinical
Trial
Primary
Objective:
To
demonstrate
non-inferiority
in
in-segment
late
loss
at
8
months
between
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
and
the
Cypher
Sirolimus-Eluting
Coronary
Stent
System
for
the
treatment
of
single
de
novo
lesions
in
native
coronary
arteries
with
a
reference
vessel
diameter
of
2.5
mm
to
3.5
mm
and
lesion
lengths
ofŽ>
14
mm
and
<
27
mm.
Design:
This
was
a
prospective,
multi-center,
single-blind,
two-arm,
randomized
and
controlled
non-inferiority
trial
that
compared
the
Endeavor
stent
to
a
control
DES
(the
Cypher
stent).
A
total
of
436
patients
were
enrolled
at
29
study
sites
in
the
United
States
who
presented
with
symptomatic
ischemic
heart
disease
attributable
to
stenotic
lesions
of
native
coronary
arteries
that
were
amenable
to
treatment
by
stenting.
Patients
were
randomized
to
receive
either
an
Endeavor
or
a
Cypher
stent
in
a
3:1
ratio.
Multiple
stents
were
allowed
for
bailout
only.
Follow-up
was
performed
at
30
days,
6, 8,
9,
12
months,
and
at
2
years,
and
will
be
performed
annually
thereafter
out
to
5
years.
All
patients
were
scheduled
to
have
angiographic
and
IVUS
evaluations
at
8
months.
Following
the
index
procedure,
patients
were
treated
with
aspirin
indefinitely
and
clopidogrel
or
ticlopidine
for
a
minimum
of
3
months.
Demographics:
The
mean
age
was
61.4
years
for
patients
in
the
Endeavor
arm
and
61.7
years
for
patients
in
the
Cypher
arm.
The
Endeavor
arm
bad
65.3%
(211/323)
males
and
the
Cypher
arm
had
81.4%
(92/113)
males.
In
the
Endeavor
arm,
22.6%
(73/323)
of
patients
had
prior
percutaneous
coronary
revascularization
compared
to
16.8%
(19/113)
of
patients
in
the
Cypher
arm.
In
the
Endeavor
arm,
29.7%
(96/323)
of
patients
had
a
history
of
diabetes
mellitus
compared
to
28.3%
(32/113)
of
patients
in
the
Cypher
arm.
Patients
were
well
matched
for
baseline
demographics,
with
gender
being
the
only
significant
difference
between
treatment
arms.
Results:
The
primary
and
secondary
endpoints,
protocol-defined
stent
thrombosis,
and
the
latest
available
follow-up
results
are
presented
below
(Table
18,
Table
19,
and
Table
20).
SSED
P060033
-
Page
37
of
59

The
primary
endpoint
of
in-segment
late
loss
at
8
months
was
not
met
with
measurements
of
0.36
±
0.46
mm
(277)
for
the
Endeavor
arm
and
0.13
±
0.33
mm
(94)
for
the
Cypher
arm
(p
<
0.791
for
non-inferiority).
Differences
noted
in
baseline
demographics
(gender)
did
not
result
in
a
significant
impact
on
study
outcomes.
Table
18:
Endeavor
Iii
8-Month
Angao
raphic
and
IVUS
Results
*
i~~~:,;:
:Ende
7
PRIMARY
ENDPOINT
Late
Loss,
In-segment
(mm)
§
0.36
±
0.46
(277)
0.13
±
0.33
(94)
0.791*
§
8-month
primary
endpoint.
*
Test
for
non-inferiority.
OTHER
ANGIOGRAPHIC
RESULTS
MLD
(mm),
In-stent
Post-Procedure
2.67
±
0.42
(323)
2.67
±
0.40
(112)
0.993**
8-Month
2.06
±
0.57
(277)
2.52
±
0.56
(94)
<
0.001**
MLD
(mm),
In-segment
Post-Procedure
2.27
±
0.45
(323)
2.28
±
0.47
(113)
0.836**
8-Month
1.91
±
0.53 (277)
2.16
±
0.50
(94)
<
0.001*
%
DS,
In-stent
Post-Procedure
4.33
±
9.77
(323)
5.92
±
9.07
(112)
0.132**
8-Month
24.90
17.45(277)
11.01
+15.91
(94)
<0.001*
%
DS,
In-segment
Post-Procedure
19.38
±
9.25
(323)
20.17
±
11.74
(113)
0.522**
8-Month
30.42
±
15.57
(277)
23.86_+
13.87
(94)
<
0.001'*
Late
Loss,
In-stent
(mm)
0.62
±
0.49
(277)
0.15
±
0.34
(94)
<
0.001*
Binary
Restenosis
In-stent
Restenosis
9.7%
(27/277)
2.1%
(2/94)
0.014**
In-segment
Restenosis
12.3%
(34/277)
4.3%
(4/94)
0.029**
IVUS
RESULTS
Neointimal
Volume
(mm
3
)
24.09
±
21.16
(209)
3.74
±
5.20
(67)
<
0.001**
%
Volume
Obstruction
15.94
±
10.94
(187)
2.66
±
3.11
(61)
<
0.001'
Incomplete
Apposition
Post-procedure
12.4%
(31/251)
17.7%
(17/96)
0.224**
8-Month
7.5%
(17/226)
17.1%
(13/76)
0.025**
Resolved
5.8%
(11/189)
7.4%
(5/68)
0.770**
Persistent
7.9%
(15/189)
11.8%
(8/68)
0.332**
Late
Acquired
0.5%
(1/189)
5.9%
(4/68)
0.018*
P-values
for
outcome
differences
are
not
adjusted
for
multiple
comparisons.
Note:
Fisher's
Exact
test
or
Student's
t-test
was
used
for
P-values.
SSED
P060033
-
Page
38
of
59

Table
19:
ENDEAVOR
III
Clinical
Results
EFFICACY
TVF
11.8%(38/321)
11.5%(13/113)
1.000*
14.4%(45/313)
13.4%(15/112)
0.875*
TVR
11.2%
(36/321)
8.0%
(9/113)
0.375**
13.7%
(43/313)
9.8%
(11/112)
0.325*
TLR
6.2%
(20/321)
3.5%
(4/113)
0.346**
7.0%
(22/313)
4.5%
(5/112)
0.498**
TLR,
PCI
5.3%
(17/321)
3.5%
(4/113)
0.612'*
5.8%
(18/313)
4.5%
(5/112)
0.808*t
TLR,
CABG
0.9%
(3/321)
0.0%
(0/113)
0.571**
1.3%
(4/313)
0.0%
(0/112)
0.577`*
Non-TLR
5.9%
(19/321)
5.3%
(6/113)
1.000'*
8.3%
(26/313)
6.3%
(7/112)
0.545**
Non-TLR,
PCI
5.6%
(18/321)
5.3%
(6/113)
1.000'*
7.7%
(24/313)
6.3%
(7/112)
0.832**
Non-TLR,
CABG
0.3%
(1/321)
0.0%
(0/113)
1.000'*
1.0%
(3/313)
0.0%
(0/112)
0.570**
SAFETY
Total
Death
0.6%
(2/321)
0.0%
(0/113)
1.000*
1.6%
(5/313)
4.5%
(5/112)
0.138**
Cardiac
Death
0.0%
(0/321)
0.0%
(0/113)
-
0.0%
(0/313)
0.9%
(1/112)
0.264**
Non-Cardiac
Death
0.6%
(2/321)
0.0%
(0/113)
1.000**
1.6%
(5/313)
3.6%
(4/112)
0.252*`
Cardiac
Death
or
MI
0.6%
(2/321)
3.5%
(4/113)
0.042**
0.6%
(2/313)
3.6%
(4/112)
0.044**
Ml
0.6%
(2/321)
3.5%
(4/113)
0.042**
0.6%
(2/313)
3.6%
(4/112)
0.044**
Q
wave
MI
0.0%
(0/321)
0.0%
(0/113)
--
0.0%
(0/313)
0.0%
(0/112)
--
Non-Q
wave
MI
0.6%
(2/321)
3.5%
(4/113)
0.042**
0.6%
(2/313)
3.6%
(4/112)
0.044**
Stent
Thrombosis
(protocol)
0.0%
(0/321)
0.0%
(0/113)
--
0.0%
(0/313)
0.0%
(0/112)
--
**
P-values
for
outcome
differences
are not
adjusted
for
multiple
comparisons.
Notes:
Fisher's
Exact
test
was
used
for
P-values.
This
trial
was
not
adequately
powered
to
compare
the
rate
of
low
frequency
events,
nor
was
it
sized
to
determine
the
rate
of
low
frequency
events
with
a
pre-specified
precision.
Numbers
are
%
(Count/Sample
Size).
To
be
included
in
the
event
rate
calculation
for
a
given
interval,
a
patient
either
had
to
have
an
event
before
the
time
of
interest
or
they
had
to
be
event-free
before
the
lower
window
of
the
follow-up.
SSED
P060033
-
Page
39
of
59
'-C7

Table
20:
ENDEAVOR
III
Protocol-Defined
Stent
Thrombosis*
Through
24
Months
Epdeavor
Cyhr
Cumulative
ST
through
24
Months
0.0%
(0/313)
0.0% (0/112)
-
Acute
ST
(•
24
hrs)
0.0%
(0/323)
0.0%
(0/113)
--
Subacute
ST
(>
24
hrs
and
•
30
days)
0.0%
(0/323)
0.0%
(0/113)
-
Late
ST
(>
30
days
and
<
12
months)
0.0%
(0/320)
0.0%
(0/112)
-
Very
late
ST
(>
12
months
and
_<
24
months)
0.0%
(0/313)
0.0%
(0/112)
-
See
section
XI.F1
for
the
per
protocol
stent
thrombosis
definition.
Notes:
This
trial
was
not
adequately
powered
to
compare
the
rate
of
low
frequency
events,
nor
was
it
sized
to
determine
the
rate
of
low
frequency
events
with
a
pre-specified
precision.
To
be
included
in
the
event
rate
calculation
for
a
given
interval,
a
patient
either
had
to
have
an
event
before
the
time
of
interest
or
they
had
to
be
event-free
before
the
lower
window
of
the
follow-up.
Numbers
are
%
(Count/Sample
Size).
C.
Results
of
the
ENDEAVOR
II
Clinical
Trial
Primary
Objective:
To
demonstrate
superiority
in
the
safety
and
efficacy
of
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
when
compared
to
the
Driver
Coronary
Stent
System
for
the
treatment
of
single
de
novo
lesions
in
native
coronary
arteries
with
a
reference
vessel
diameter
of
2.25
mm
to
3.5
mm
in
diameter
and
lesion
lengths
of
>
14
mm
and
<
27
mm.
Design:
This
was
a
prospective,
multi-center,
double-blind,
two-arm
randomized
and
controlled
superiority
trial
that
compared
the
Endeavor
stent
to
a
control
bare
metal
stent
(BMS),
the
Driver
stent.
A
total
of
1197
patients
were
enrolled
at
72
study
sites
in
Asia,
Australia,
Europe,
Israel
and
New
Zealand
who
presented
with
symptomatic
ischemic
heart
disease
attributable
to
stenotic
lesions
of
native
coronary
arteries
that
were
amenable
to
treatment
by
stenting.
Patients
were
randomized
to
receive
either
an
Endeavor
or
a
Driver
stent
in
a
1:1
ratio.
Multiple
stents
were
allowed
for
bailout
only.
Follow-up
was
performed
at
30
days,
6,
8,
9,
12
months,
at
2
and
3
years,
and
will
be
performed
annually
thereafter
out
to
5
years.
The
first
600
consecutively
enrolled
patients
(across
all
sites)
were
scheduled
to
receive
angiographic
evaluation
at
8
months,
and
300
patients
were
scheduled
to
receive
IVUS
evaluation
at
8
months
at
pre-specified
sites.
Following
the
index
procedure,
patients
were
treated
with
aspirin
indefinitely
and
clopidogrel
or
ticlopidine
for
a
minimum
of
3
months.
Demographics:
The
mean
age
was
61.6
years
for
patients
in
the
Endeavor
arm
and
61.9
years
for
patients
in
the
Driver
arm.
The
Endeavor
arm
had
77.2%
(461/597)
males,
and
the
Driver
arm
had
75.3%
(449/596)
males.
In
the
Endeavor
arm,
21.7%
(129/595)
of
patients
had
prior
percutaneous
coronary
revascularization,
compared
to
18.0%
(107/594)
of
patients
in
the
Driver
arm.
In
the
Endeavor
arm,
18.2%
(108/595)
of
patients
had
a
history
of
diabetes
mellitus,
compared
to
22.2%
(132/595)
of
patients
in
the
Driver
arm.
Patients
were
well
matched
for
baseline
demographics,
with
no
statistically
significant
differences
between
treatment
arms.
SSED
P060033
-
Page
40
of
59

Results:
The
primary
and
secondary
endpoints,
protocol-defined
stent
thrombosis,
and
the
latest
available
follow-up
results
are
presented
below
(Table
21,
Table
22,
Table
23
and
Figure
4).
The
primary
endpoint
of
TVF
at
9
months
was
met
with
7.9%
(47/592)
for
the
Endeavor
arm
and
15.1%
(89/591)
for
the
Driver
arm
(p
< 0.001
for
superiority).
The
pre-specified
secondary
endpoint
of
in-segment
late
loss
at
8
months
was
met,
with
measurements
of
0.36
mm
±
0.46
mm
(264)
for
the
Endeavor
arm
and
0.72
mm
±
0.61
mm
(263)
for
the
Driver
arm
(p
<
0.001
for
superiority).
Table
21:
ENDEAVOR
II
Clinical
Results
ths
Endeava
river
PRIMARY
ENDPOINT
TVF
§
7.9%
(47/592)
15.1%
(89/591)
<0.001*
12.8%
21.4%
(124/579)
<0
0'01
(74/577)
§
9-month
primary
endpoint.
Test
for
superiority.
EFFICACY
TVR
5.6%
(33/592)
12.5%(74/591)
<0.001'*
9.5%
(55/577)
17.6%
(102/579)
<
0.001
TLR
4.6%
(27/592)
11.8%(70/591)
<0.001'*
7.3%
(42/577)
14.7%
(85/579)
<
0.001'*
TLR,
PCI
4.2%
(25/592)
11.3%
(67/591)
<
0.001**
6.9%
(40/577)
13.8%
(80/579)
<
0.001*
TLR, CABG
0.3% (2/592)
0.5%
(3/591)
0,687**
0.5%
(3/577)
1.0%
(6/579)
0.506**
Non-TLR
1.5%
(9/592)
2.2%
(13/591)
0.400**
2.9%
(17/577)
4.8%
(28/579)
0.128**
Non-TLR,
PCI
1.4%
(8/592)
2.2%
(13/591)
0.282**
2.8%
(16/577)
4.7%
(27/579)
0.119**
Non-TLR,
CABG
0.2%
(1/592)
0.0%
(0/591)
1.000**
0.2%
(1/577)
0.3% (2/579)
1.000'*
SAFETY
Total
Death
1.2%
(7/592)
0.5%
(3/591)
0.342**
3.3%
(19/577)
4.5%
(26/579)
0.362**
Cardiac
Death
0.8%
(5/592)
0.5%
(3/591)
0.726**
1.6%
(9/577)
2.4%
(14/579)
0.400**
Non-Cardiac
Death
0.3%
(2/592)
0.0%
(0/591)
0.500**
1.7%
(10/577)
2.1%
(12/579)
0.830**
Cardiac
Death
or
MI
3.4%
(20/592)
4.4%
(26/591)
0.372**
4.5%
(26/577)
6.7%
(39/579)
0.125-*
Ml
2.7%
(16/592)
3.9%
(23/591)
0.260**
3.3%
(19/577)
4.3%
(25/579)
0.443**
Q
wave
Ml
0.3%
(2/592)
0.8%
(5/591)
0.287**
0.3%
(2/577)
1.0%
(6/579)
0.287**
Non-Q
wave
MI
2.4%
(14/592)
3.0%
(18/591)
0.481**
2.9%
(17/577)
3.3%
(19/579)
0.866**
Stent
Thrombosis
0.5% (3/592)
1.2%
(7/591)
0.224**
0.5%
(3/577)
1.2%
(7/579)
0.342**
(protocol)
*'
P-values
for
outcome
differences
are
not
adjusted
for
multiple
comparisons.
Notes:
Fisher's
Exact
test
was
used
for
P-values.
This
trial
was
not
adequately
powered
to
compare
the
rate
of
low
frequency
events,
nor
was
it
sized
to
determine
the
rate
of
low
frequency
events
with
a
pre-specified
precision.
Numbers
are
%
(Count/Sample
Size).
To
be
included
in
the
event
rate
calculation
for
a
given
interval,
a
patient
either
had
to
have
an
event
before
the
time
of
interest
or
they
had
to
be
event-free
before
the
lower
window
of
the
follow-up.
SSED
P060033
-
Page
41
of
59

1
00%-
I--
90%-
E
-'
85%-
80%-
75%.
,
0
90
180
II
270
-Endeavor
--
Driver
;
-
I I I
360
450
540
630
720
Time
after
Initial
Procedure
(days)
I
,_i
I
810
900
990
1080
Endeavo
87.4%
12.6%1
<0.001
Drv
78.9%
21.1%
*
Log-rank
P-value. P-value
is
not
adjusted
for
multiple
comparisons.
Figure
4:
Survival Free
from
Target
Vessel
Failure
(at
1080
days)
SSED
P060033
-
Page
42
of
59
5'0

Table
22:
Endeavor
II
8-Month
Angio
ra
hic
and
IVUS
Results
SECONDARY
ENDPOINT
Late
Loss, In-segment
(mm)
¥
0.36
±
0.46
(264)
0.72
±
0.61
(263)
<
0.001*
¥
Powered
secondary
endpoint.
*
test
for
superiority.
OTHER ANGIOGRAPHIC
RESULTS
MLD
(mm),
In-stent
Post-Procedure
2.59
±
0.43
(588)
2.61
±
0.44
(589)
0.436**
8-Month
1.99
±
0.56
(264)
1.62
±
0.70
(265)
<
0.001**
MLD
(mm),
In-segment
Post-Procedure
2.21
±
0.49 (589)
2.24
±
0.49
(590)
0.302**
8-Month 1.86
±
0.55
(264)
1.56
±
0.67
(265)
<
0.001'*
%
DS,
In-stent
Post-Procedure
6.04
±
10.43 (588)
6.23
±
10.03
(589)
0.757**
8-Month
27.91
±
17.30
(264) 42.24
±
21.73
(265)
< 0.001t*
%
DS,
In-segment
Post-Procedure
20.39
10.26
(589)
20.11
±9.38
(590)
0.622**
8-Month
32.67
±
16.27
(264)
44.33
±
20.45
(265)
<
0.001**
Late Loss, In-stent
(mm) 0.62
±
0.46 (264)
1.03
±
0.59 (263)
<
0.001'*
Binary
Restenosis
In-stent
Restenosis
9.5%
(25/264)
33.2%
(88/265)
<
0,001*
In-segment
Restenosis
13.3%
(35/264) 34.7%
(92/265)
<
0.001*
IVUS
RESULTS
Neointimal Volume (mm
3
)
30.15
±-21.66
(90)
53.51
±
39.80
(81)
<
0.001**
%
Volume
Obstruction 17.34
±
10.27 (90) 29.55
±
17.58
(81)
<
0.001**
Incomplete
Apposition
Post-procedure
24.8%
(36/145)
19.6%
(28/143)
0.322**
8-Month 16.8%
(21/125)
14.5%
(16/110)
0.721**
Resolved
7.0% (8/114)
6.7% (7/104)
1.000**
Persistent
17.5%
(20/114)
14.4%
(15/104)
0.583**
Late
Acquired 0.0%
(0/114)
0.0%
(0/104)
--
P-values
for
outcome
differences
are
not
adjusted
for
multiple
comparisons.
Note:
Fisher's
Exact
test
or
Student's
t-test
was
used
for
P-values.
SSED
P060033
-
Page
43
of
59

Table
23:
ENDEAVOR
II
Protocol-Defined
Stent
Thrombosis*
T
hrou
36
Months
j,,
aue
Cumulative
ST
through
36
Months
0.5%
(3/577)
1.2%
(7/579)
0.342**
Acute
ST
(•<
24
hrs)
0.2%
(1/596)
0.2%
(1/594)
1.000'*
Subacute
ST
(>
24
hrs
and
•
30
days)
0.3%
(2/596)
1.0%
(6/594)
0.178*t
Late
ST (>
30
days
and
<
12
months)
0.0%
(0/590)
0.0% (0/589)
-
Very
late
ST
(>
12
months
and
•
36
months)
0.0%
(0/577)
0.0%
(0/579)
--
*
See
section
XI.Flfor
the
per
protocol
stent
thrombosis
definition.
P-values
for
outcome
differences
are
not
adjusted
for
multiple
comparisons.
Notes:
Fisher's
Exact
test
was
used
for
P-values.
This
trial
was
not
adequately
powered
to
compare
the
rate
of
low
frequency
events,
nor
was
it
sized
to
determine
the rate
of
low
frequency
events
with
a
pre-specified
precision.
To
be
included
in
the
event
rate calculation
for
a
given
interval,
a
patient
either
had
to
have
an event
before
the
time
of
interest
or
they
had
to
be
event-free
before
the
lower
window
of
the
follow-up.
Numbers
are
%
(Count/Sample
Size).
D.
Results
of
the
ENDEAVOR
I
Clinical
Trial
Primary
Obiective:
To
demonstrate
the
safety
and
efficacy
of
the
Endeavor
Zotarolimus-
Eluting
Coronary
Stent
System
for
the
treatment
of
single
de
novo
lesions
in
native
coronary
arteries
with
a
reference
vessel
diameter
of
3.0
mm
to
3.5
mm
and
lesion
length
of•<
15
mm.
Design:
The
ENDEAVOR
I
trial
was
the
first-in-man
study
for
the
Endeavor
stent.
This
was
a
non-randomized,
prospective,
multi-center,
single-arm
trial.
A
total
of
100
patients
were
enrolled
at
8
study
sites
in
Australia
and
New Zealand
who
presented
with
symptomatic
ischemic
heart disease
attributable
to
stenotic
lesions
of
the
native
coronary
arteries
that
were
amenable
to
treatment
by
stenting.
Follow-up
was
performed
at
30
days,
4,
9,
12
months,
at
2,
3
and
4
years,
and
will
be
performed
at
5
years.
All
patients
were
scheduled
to
have
angiographic
follow-up
at
4
and
12
months.
Following
the
index
procedure,
patients
were
treated
with
aspirin
indefinitely
and
clopidogrel
for
a
minimum
of
3
months.
Demographics:
The
mean
age
was
59
years,
and
79%
were
male.
Diabetes
was
present
in
16%,
and
47%
had
a
prior
MI.
Results:
The
primary
and
secondary
endpoint,
protocol-defined
stent
thrombosis,
and
the
latest
available
follow-up
results
are
presented
below
(Table
24,
Table
25,
and
Table
26).
The
primary
endpoint
of
30-day
MACE
was
1.0%
(1/100),
and
the
co-primary
endpoint
of
in-segment
late
loss
at
4
months
was
0.22
±
0.43
mm
(98).
SSED
P060033
-
Page
44
of
59
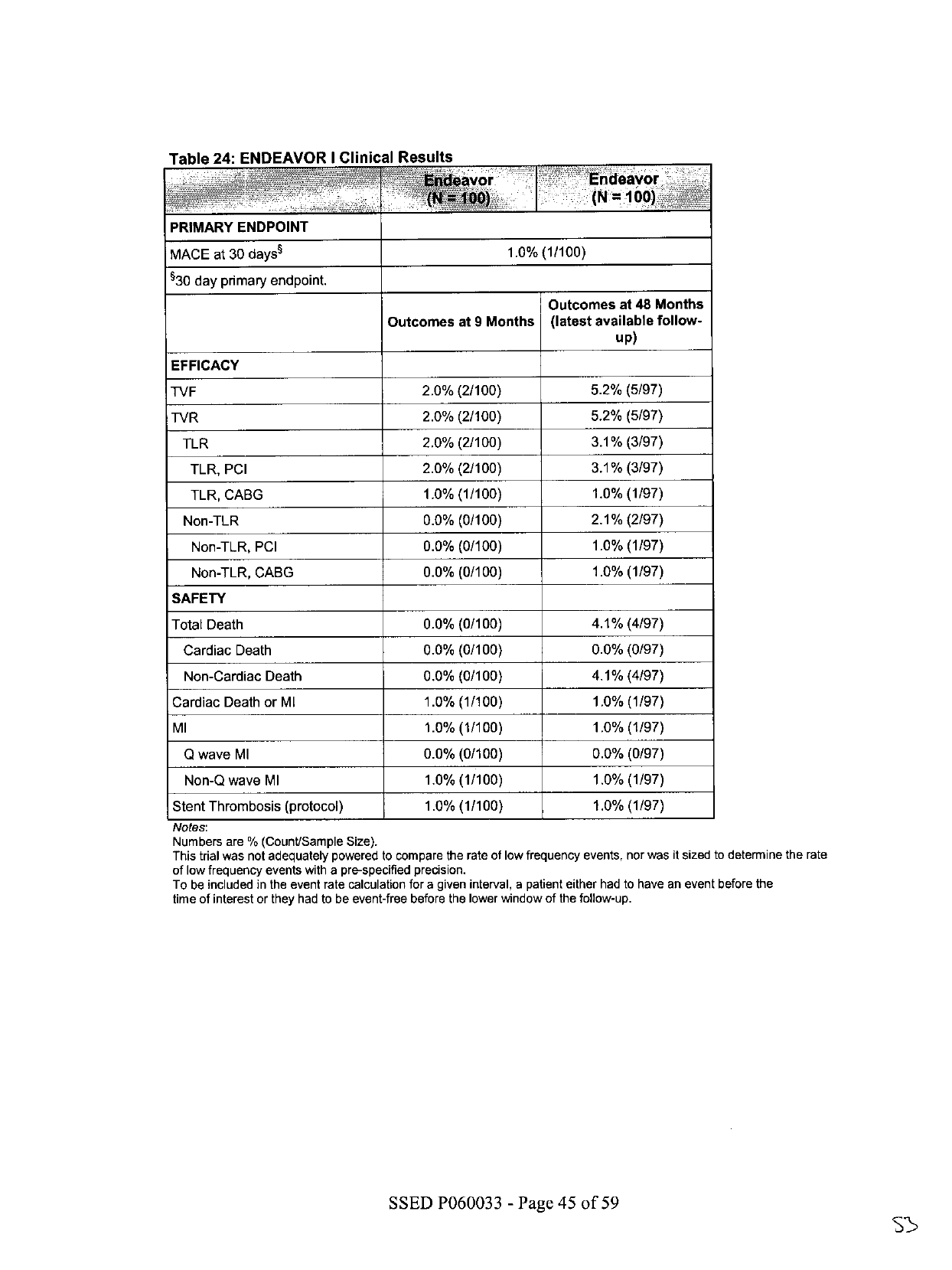
Table
24:
ENDEAVOR
I
Clinical
Results
-
~
~
vor
::i'Endeavor
(N1=
00)
PRIMARY
ENDPOINT
MACE
at
30
days
§
1.0%
(1/100)
530
day
primary
endpoint.
Outcomes
at
48
Months
Outcomes
at
9
Months
(latest
available
follow-
up)
EFFICACY
TVF
2.0%
(2/100)
5.2%
(5/97)
TVR
2.0%
(2/100)
5.2%
(5/97)
TLR
2.0%
(2/100)
3.1%
(3/97)
TLR,
PCI
2.0%
(2/100)
3.1%
(3/97)
TLR,
CABG
1.0%
(1/100)
1.0%
(1/97)
Non-TLR
0.0%
(0/100)
2.1%
(2/97)
Non-TLR,
PCI
0.0%
(0/100)
1.0%
(1/97)
Non-TLR,
CABG
0.0% (0/100)
1.0%
(1/97)
SAFETY
Total
Death
0.0%
(0/100)
4.1%
(4/97)
Cardiac
Death
0.0%
(0/100)
0.0%
(0/97)
Non-Cardiac
Death
0.0%
(0/100)
4.1%
(4/97)
Cardiac
Death
or
MI
1.0%
(1/100)
1.0%
(1/97)
Ml
1.0%
(1/100)
1.0%
(1/97)
Q
wave
MI
0.0% (0/100)
0.0%
(0/97)
Non-Q
wave
MI
1.0%
(1/100)
1.0%
(1/97)
Stent
Thrombosis
(protocol)
1.0%
(1/100)
1.0%
(1/97)
Notes:
Numbers
are
%
(Count/Sample
Size).
This
trial
was
not
adequately
powered
to
compare
the
rate
of
low
frequency
events,
nor
was
it
sized
to
determine
the
rate
of
low
frequency
events
with
a
pre-specified
precision.
To
be
included
in
the event
rate
calculation
for
a
given
interval,
a
patient
either
had
to
have
an
event
before
the
time
of
interest
or
they
had
to
be
event-free
before
the
lower
window
of
the
follow-up.
SSED
P060033
-
Page
45
of
59
ss

________
___
______
Table
25:
ENDEAVOR
1
12-
Month
Angiographic
and
IVUS
Results
ANGIOGRAPHIC
RESULTS
MLD (mm),
In-stent
Post-Procedure
12-Month
MED
(mm),
In-segment
Post-Procedure
12-Month
%
DS,
In-stent
Post-Procedure
12-Month
%
DS,
In-segment
Post-Procedure
12-Month
Late
Loss,
In-stent
(mm)
Late
Loss,
In-segment
(mm)
Binary
Restenosis
In-stent
Restenosis
In-segment
Restenosis
IVUS
RESULTS
Neojntjmnal
Volume
(mm')
%
Volume
Obstruction
Incomplete
Apposition
Post-procedure
12-Month
Resolved
Persistent
Late
Acquired
2.84
±0.35
(100)
2.26
±0.49
(92)
2.52
±0.42
(100)
2.08
±0.47
(92)
5.37
±7.51
(1
00)_
21.75
±15.35
(92)
16.54±8.40
(100)
28.00
±13.41
(92)
0.58
±0.44
(92)
0.43
±0.44
(92)
4.3%
(4/92)
5.4% (5/92)
14.15
±11.82
(86)
9.73
±8.50
(86)
12.6%
(12/95)
4.7%
(4/86)
8.1%
(7/86)
4.7%
(4/86)
0.0%
(0/86)
SSED P060033
-
Page
46
of
59

Table
26:
ENDEAVOR
I
Protocol-Defined
Stent
Thrombosis*
Through
48
Months
Cumulative
ST
through
48
Months
1.0%
(1/97)
Acute
ST
(•
24
hrs)
0.0%
(0/100)
Subacute
ST
(>
24
hrs
and
<
30
days)
1.0%
(1/100)
Late
ST
(>
30
days
and
<
12
months)
0.0%
(0/99)
Very
Late
ST
(>
12
months
and
•
48
months)
0.0%
(0/97)
*
See
section
XI.Flfor
the
per
protocol
stent
thrombosis
definition.
Notes:
To
be
included
in
the
event
rate
calculation
for
a
given
interval,
a
patient
either
had
to
have
an
event
before
the
time
of
interest
or
they
had
to
be
event-free
before
the
lower
window
of
the
follow-up.
Numbers
are
%
(Count/Sample
Size).
E.
Gender
Bias
The
gender
selection
in
this
series
of
clinical
trials
was
completely
random,
and
solely
based
upon
exclusion
and
inclusion
criteria.
In
the
ENDEAVOR
IV
study
(conducted
in
the
US),
women
represented
32.3%
of
the
population.
In
the
ENDEAVOR
III
study
(conducted
in
the
US),
women
represented
30.5%
of
the
population.
In
the
ENDEAVOR
II
study
(conducted
OUS),
women
represented
23.7%
of
the
population.
In
the
ENDEAVOR
I
feasibility
trial
(conducted
OUS),
women
represented
21.0%
of
the
population.
According
to
the
American
Heart
Association
Heart
Disease
and
Stroke
Statistics
(2008
Update),
women
represent
approximately
40%
of
patients
age
60-79
with
coronary
heart
disease.
Although
the
ratio
of
men
to
women
in
the
ENDEAVOR
trials
does
not
match
the
prevalence
of
coronary
artery
disease
in
the
general
U.S.
population,
it
is
reflective
of
the
underlying
distribution
of
the
disease
for
the
types
of
patients
that
would
meet
the
study
inclusion/exclusion
criteria
(i.e.,
younger,
less
complex
disease).
No
selection
bias
on
the
basis
of
gender
was
identified
during
the
review.
In
addition,
data
were
analyzed
to
determine
if
gender
was
an
independent
predictor
of
clinical
outcomes
of
death,
cardiac
death,
all
MI,
QMI,
Non-Q
MI,
cardiac
death
or
MI,
TVF,
TVR,
TLR
and
stent
thrombosis
(all
definitions).
No
differences
in
safety
or
effectiveness
were
found,
with
respect
to
gender.
F.
Overall
Results
of
the
Endeavor
Clinical
Program
(ENDEAVOR
I,
II,
II-CA,
III,
IV
and
USPK)
In
order
to
better
estimate
the
incidence
of
low-frequency
events
or
outcomes
in
various
specific
patient
subgroups,
a
patient-level
pooled
analysis
was
conducted.
This
analysis
compared
pooled
Endeavor
stent
patients
(across
all
trials)
to
Driver
stent
patients
from
ENDEAVOR
II.
Although
ENDEAVOR
I
(100),
ENDEAVOR
1I-CA
(296)
and
ENDEAVOR
USPK
(43)
are
not
randomized
trials,
for
the
purpose
of
this
analysis,
they
are
pooled
with
the
randomized
trials
--
ENDEAVOR
11
(596),
ENDEAVOR
III
(323)
and
ENDEAVOR
IV
(770)
--
to
allow
the
broadest
comparison
of
the Endeavor
stent
(1287
patients)
vs.
the
Driver
stent
patients
(599)
to
2
years
of
follow-up.
Across
the
ENDEAVOR
program,
2133
patients
received
the
Endeavor
stent.
The
patient-level
data
SSED
P060033
-
Page
47
of
59

was
included
until
the
latest
available
time
point
depending
on
the
follow-up
status
for
each
trial
--
ENDEAVOR
1
(97%
complete
at
4
years),
ENDEAVOR
11
(97.8%
completed
at
3
years),
ENDEAVOR
I1-CA
(97.3%
complete
at
2
years),
ENDEAVOR
III
(96.9%
complete
at
2
years),
ENDEAVOR
IV
(96.1%
complete
at
9
months),
and
ENDEAVOR
USPK
(97.7%
complete
at
9
months).
Table
27:
Patient
Follow-up
"77'-tA~"o<'Wi7
1
2
24
48
nths
ii~~~~~~~
i~nhs:
2&onth
:i
ENDEAVOR
I
100
100
100
99
99
98
97
-
ENDEAVOR
II
596
593
592
590
587
577
ENDEAVOR
II
CA
296
295
293
292
288
-
ENDEAVOR
III
323
321
321
320
313
-
-
ENDEAVOR
IV
770
766
740
ENDEAVOR
PK
43
43
42
Total
2128
2118
2088
1301
1287
675
97
It
is
acknowledged
that
the
results
of
such
retrospective
pooled
analyses
are
hypothesis-
generating
in
nature.
Definitive
proof
of
the
presence
or
absence
of
any
differences
between
sub-groups
requires
prospectively
powered
assessment
in
dedicated
clinical
trials.
The
results
of
the
pooled
analysis
show
the
Endeavor
stent
significantly
reduces
the
need
for
repeat
revascularization
vs.
the
Driver
stent
that
is
maintained
throughout
long-term
follow-up
as
shown
in
Figure
5.
SSED
P060033
-
Page
48
of
59

100•
95%
-T
E
0
E
90%_~~~~~~
8s.s%
(D)
'.
85%'
p
=
<
0.001
-
Endeavor
--
Driver
80%
I
o
90
180
270
360
450
540
630 720
810
900
990
1080
Time
after
Initial
Procedure
(days)
100%~
_
95%-''-
89.4%
(E)
80%
-
u5_
--
Endeavor
--
Driver
0
980
180
270
360
450
540
630
720
610
900
990
080
Time
after
Initial
Procedure
(days)
Figure
5:
Efficacy-Target
Lesion
and
Target Vessel
Revascularization
in
ENDEAVOR
Pooled
Analysis
Kaplan-Meier
rates
%.
P-values
are
from
the
Log-rank
test
and
are
not
adjusted
for multiple
comparisons.
The
Endeavor
stent
is
more
effective
than
the
Driver
stent
in
reducing
the need
for
revascularization,
as
shown
in
Figure
5.
The analyses
shown
in
Figure
6
suggest
a
lower
rate
of
cardiac
death
in
pooled Endeavor
patients
compared
to
Driver patients
from
ENDEAVOR
II.
The
pooled
analysis
addressed
total
death
as
well
as
cardiac
death
and
non-cardiac
death
as
its
components.
There
were
no
differences
noted
in
non-cardiac
or
total death
between
groups.
SSED
P060033
-
Page
49
of
59

100%_
--
96.9%
(E)
96%-
-n~~~~~~
94)
95.5%
(D)
92%-
P
=
0.
199
-
Endeavor
--
Driver
90%
I
o
go
180
270
360
450
540
630
720
810
900
990
1080
Time
after
Initial
Procedure
(days)
99.0%
(E)
~98%_
97.6%
(D)
12
96%-
a
E
0
94%-
E
0)
P
92%-
92%-=
0.025
-
Endeavor
--
Driver
90%
1
,
I
I
I
--
,- - -
I
I
I
0
90
180
270
360
450
540
630
720
810
900
990
1080
Time
after
Initial
Procedure
(days)
lO%--
....
97.9%
....
(E)
....
98%-
97.8%
(D)
q)
96%.
0
z
92%
P
0.799
-Endeavor
--
Driver
90%
1
1
I
I
I
I I
I
o
90
180
270
360
450
540
630
720
810
900
990
1080
Time
after
Initial
Procedure
(days)
Figure
6:
Safety-Mortality
in
ENDEAVOR
Pooled
Analysis
Kaplan-Meier
rates
%.
P-values
are
from
the
Log-rank
test
and
are
not
adjusted
for
multiple
comparisons.
SSED
P060033
-
Page
50
of
59
5'g

The
MI
rates
in
patients
receiving
the
Endeavor
stent
vs.
the
Driver
control
stent
were
also
examined.
At
three
years,
any
differences
noted
favored
the
Endeavor
stent
as
shown
in
Figure
7.
100
E~~~~~~~~~~~~~~~~~~~~~~~E
0
94%-95.8%
(D)
=0.047
92%-
P
-Endeavor
-Driver
o
90
16~0
270
36~0
450
540
630
72'0
61'0
9010
990
1080
Time
after
Initial
Procedure
(days)
99.8%
(E)
96%
~~~~~~~~~~~~~~~~~99.0%
(D)
E
98%-
ci
E
S
4%-
U-
92%-
P
0.016
-Endeavor
-
-Driver
0
90
180
270
360
4
50
540
630
72'0
810
900
990
10O80
Time
after
Initial
Procedure
(days)
o
~~~~~~~~~~~~~~~~~~~~96.5%(D)
0 9%
92%-
P
=0.256
-
Endeavor
-
-Driver
0
90
180
270
360
450
540
630 720
810
900
990
1080
Time
after
Initial
Procedure
(days)
Figure
7:
Safety-MI
in
ENDEAVOR
Pooled
Analysis
Kaplan-Meier
rates
%.
P-values
are
from
the
Log-rank
test
and are
not
adjusted for
multiple
comparisons.
SSED
P060033
-
Page
51
of
59

1.
Stent
Thrombosis
in
Endeavor
Pooled
Analysis
For
the
critical
safety
endpoint
of
stent
thrombosis,
Endeavor
rates
have
been
reported
using
two
different
reporting
mechanisms:
the
pre-specified
protocol
definition
and
the
retrospective
Academic
Research
Consortium
(ARC)'
definition.
Stent
thrombosis
was
defined
(per
protocol)
in
the
ENDEAVOR
clinical
trials
as
the
occurrence
of
any
of
the
following:
*
Angiographic
thrombus
or
subacute
closure
within
the
stented
vessel
at
the
time
of
the
clinically-driven
angiographic
restudy
for
documented
ischemia
(chest
pain
and
ECG
changes).
*
Any death
not
attributed
to
a
non-cardiac
cause
within
the
first
30
days.
·
Late stent
thrombosis
is
reported
according
to
the
following
criteria:
MI
>
30
days
after
index
and
attributable
to
the
target
vessel,
angiographic
documentation
(site-reported
or
by
QCA)
of
thrombus
or
total
occlusion
at
the
target
site
and
freedom
from
interim
revascularization
of
the
target
vessel.
All
events
were
re-adjudicated
based
on
FDA
recommendation
using
the
ST
definitions
proposed
by
ARC.
This
was
performed
by
an
independent
events
committee
blinded
to
the
treatment
groups
of
the
individual
patients.
According
to
ARC,
each
incident
of
ST
is
categorized
by
timing,
level
of
evidence,
and
relationship
to
TLR
as
follows:
Timing:
Acute
stent
thrombosis
2
:
0-24
hours
post
stent
implantation
Subacute
stent
thrombosis
2
:
>
24
hours-30
days
post
stent
implantation
Late
stent
thrombosis:
>
30
days-l
year
post
stent
implantation
Very
late
stent
thrombosis:
>
1
year
post
stent
implantation
Level
of
Evidence:
*
Definite
stent
thrombosis:
Definite
stent
thrombosis
is
considered
to
have
occurred
by
either
angiographic
or
pathologic
confirmation.
*
Probable
stent
thrombosis:
Clinical
definition
of
probable
stent
thrombosis
is
considered
to
have
occurred
after
intracoronary
stenting
in the
following
cases:
*
Any
unexplained
death
within
the
first
30
days
*
Irrespective
of
the
time
after
the
index
procedure,
any
MI
that
is
related
to
documented
acute
ischemia
in
the
territory
of
the
implanted
stent
without
angiographic
confirmation
of
stent
thrombosis
and
in
the
absence
of
any
other
obvious
cause
*
Possible
stent
thrombosis:
Clinical
definition
of
possible
stent
thrombosis
is
considered
to
have
occurred
with
any
unexplained
death
from
30
days
following
intracoronary
stenting
until
end
of
trial
follow-up.
SSED
P060033
-
Page
52
of
59
GOO

Stent
Thrombosis
After
TLR:
Censored
vs.
Non-Censored:
Censoring
stent
thrombosis
events
that
occur
post-TLR
performed
for
stent
restenosis
may
be
appropriate,
as
the
thrombosis
may
be
related
to
the
treatment
chosen
to
treat
restenosis
(e.g.,
brachytherapy)
rather
than
the
type
of
stent
used
in
the
index
procedure.
Alternatively,
censoring
stent
thrombosis
events
that
occur
after
TLR
may
bias
results
in
favor
of
devices
with
higher
restenosis
risks.
Therefore,
stent
thrombosis
data
presented
in
this
review
will
report
both
TLR-censored
and
TLR-
uncensored
rates
as
follows:
*
ARC
Definite
+
probable
(TLR-censored):
Adjudicated
stent
thrombosis
meeting
the
definite
or
probable
ARC
definition
with
censoring
of
any
definite
or
probable
stent
thrombosis
events
that
may
have
occurred
after
a
TLR.
*
ARC
Definite
+
probable
(TLR-uncensored):
Adjudicated
stent
thrombosis
meeting
the
definite
or
probable
ARC
definition
including
any
definite
or
probable
stent
thrombosis
events
that
may
have
occurred
after
a
TLR.
In
the
ENDEAVOR
clinical
program
comprised
of
six
multi-center
trials,
2133
patients
were
assigned
to
receive
the
Endeavor
Stent
(1287
patients
were
followed
out
to
two
years
and
675
patients
out
to
three
years).
When
all
patients
who
received
the
Endeavor
stent
across
trials
were
pooled
and
compared
to
the
patients
who
received
the
Driver
stent
in
ENDEAVOR
II,
the
Endeavor
stent
did
not
appear
to
pose
an
increased
stent
thrombosis
risk.
Regardless
of
the
method
for
reporting
the
pre-specified
protocol
definition
or
the
retrospective
ARC
definition,
in
the
randomized
ENDEAVOR
II
trial
and
the
FDA-requested
pooled
analysis,
the
Endeavor
stent
exhibited
low
event
rates
that
were
similar
to
or
lower
than
the
Driver
stent.
The
cumulative
rates
of
stent
thrombosis
(per
protocol
and
per
the
ARC
definite
+
probable
definitions)
in
patients
treated
with
Endeavor
stents
from
the
pooled
ENDEAVOR
trials
are
shown
in
Table
28
below.
(Stent
thrombosis
rates
observed
in
patients
treated
with
Driver
stents
in
ENDEAVOR
II
are
shown
for
reference.)
ARC
definite
+
probable
stent
thrombosis
is
reported
both
as
TLR-censored
and
uncensored.
SSED
P060033
-
Page
53
of
59

Table
28:
Stent
Thrombosis
Protocol
and
Definite
+
Probable
Stent
Thrombosis
(ARC
I
Thrombosis
(0-30
Days)
Stent
Thrombosis
(Protocol)
0.3%
(7/2128)
[0.1%,
0.7%]
1.2%
(7/594)
[0.5%,
2.4%]
ARC
Definite
+
Probable
(TLR-censored)
0.3%
(7/2128)
[0.1%,
0.7%]
1.2%
(7/594)
[0.5%,
2.4%]
[0.5%,
2.4%]
ARC
Definite
+
Probable
(TLR-uncensored)
0.3%
(7/2128)
[0.1%,
0.7%]
1.2%
(7/594)
Thrombosis
(0-6
Months)
Stent
Thrombosis
(Protocol)
0.5%
(10/2118)
[0.2%,
0.9%]
1.2%
(7/593)
[0.5%,
2.4%]
[0.5%,2.4%]
ARC
Definite
+
Probable
(TLR-censored)
0.5%(11/2118)
[0.3%,00.9%]
1.2%(7/593)
[0.3%,
0.9%]
1.2%
(7/593)
[0.5%,
2.4%]
ARC
definite
+
probable
(TLR-uncensored)
0.5%
(11/2118)
Thrombosis
(0-12
Months)
Stent
Thrombosis
(Protocol)
0.3%
(4/1301)
[0.1%,
0.8%]
1.2%
(7/589)
[0.5%,
2.4%]
ARC
Definite
+
Probable
(TLR-censored)
0.4%
(5/1301)
[0.1%,
0.9%]
1.4%
(8/589)
[0.6%,
2.7%]
ARC
Definite
+
Probable
(TLR-uncensored)
0.5%
(6/1301)
[0.2%,
1.0%]
1.4%
(8/589)
[0.6%,
2.7%]
Thrombosis
(0-24
Months)
Stent
Thrombosis
(Protocol)
0.3%
(4/1287)
[0.1%,
0.8%]
1.2%
(7/586)
[0.5%,
2.4%]
ARC
Definite
+
Probable
(TLR-censored)
0.5%
(6/1287)
[0.2%,
1.0%]
1.4%
(8/586)
[0.6%,
2.7%]
[0.2%,
1.1%]
1.4%
(8/586)
[0.6%,2.7%]
ARC
Definite
+
Probable
(TLR-uncensored)
0.5%
(7/1287)
Thrombosis
(0-36
Months)
Stent
Thrombosis
(Protocol)
0.6%
(4/675)
[0.2%,
1.5%]
1.2%
(7/579)
[0.5%,
2.5%]
ARC
Definite
+
Probable
(TLR-censored)
0.9%
(6/675)
[0.3%,
1.9%]
1.4%
(8/579)
[0.6%,
2.7%]
ARC
Definite
+
Probable
(TLR-uncensored)
0.9%
(6/675)
[0.3%,
1.9%]
1.6%
(9/579)
[0.7%,
2.9%]
Beyond
one
year,
the
Endeavor
stent
showed
zero
stent
thrombosis
by
the
pre-specified
protocol
definition
and
one
stent
thrombosis
event
by
the
post
hoc
ARC
definition
(definite
+
probable).
SSED
P060033
-
Page
54
of
59
IS

100.0%
(E)
10
-~98%-
g
96%-
E
94%
I-.
E
92%
P =
n/a
~~-Endeavor
-Driver
E
0
o,
90%]
2?
360
450
540
630
720
810
900
§90
1090
Time
after
Initial
Procedure
(days)
99.9%
(E)
100%-
----
r-
---
99.8%
(D)
~98%-
4
E
96%_
94%-
0E
92%-
P
0.737
-Endeavor
--
Driver
0
90%-
360
4605
0
630
720
81,0
g00
990
1080
Time
after
Initial
Procedure
(days)
Figure
8:
Freedom
from
Stent
Thrombosis
(Protocol)
and
Definite/Probable
Thrombosis
(ARC)
Kaplan-Meier
rates
%.
P-values
are
from
the
Log-rank
test
and
are
not
adjusted
for
multiple
comparisons.
2.
Diabetic
Patients
in
ENDEAVOR
Pooled
Analysis
Diabetic
patients
comprise
an
important
patient
subgroup
that
is
at
increased
risk
for
cardiovascular
morbidity
and
mortality.
Although
diabetic
patients
were
included
in
the
Endeavor
clinical
trials,
there
were
no
pre-specifled
hypotheses
or
trial
design
features
to
warrant
a
specific
labeled
indication
for
the
use
of
the
Endeavor
stent
in
diabetic
individuals.
Table
29
shows
clinical
outcomes
through
9
months
in
patients
pooled
from
the
ENDEAVOR
trials
and
stratified
by
non-diabetics,
all
diabetics,
insulin-dependent
diabetics,
and
non-insulin
dependent
diabetics.
As
expected,
TLR
and
TVR
rates
were
numerically
increased
in
diabetics
vs.
non-diabetics,
with
no
observed
safety
signals
with
respect
to
the
rates
of
death,
cardiac
death,
MI,
or
stent
thrombosis.
SSED
P060033
-
Page
55
of
59

Table
29:
Clinical
Events
Through
9
Months
Death
0.8%
0.8%
0.7%
0.8%
Cardiac
Death
0.5
0.6
0.0%
0.8%
~MI
2.4%
1.5%
2.0%
1.4%
Cardiac
Death
or
MI
2.0%
1.9%
Protocol
ST
0.5%
0.6%
0.7%
0.5%
Definite
and
Probable
ST
ARC
(TLR.
censored)
~
~~~0,5%
censored)
0,8%
1,3%
0,5%
Definite
and
Probable
ST
ARC
(TLR
0
uncensored)
~
~~0,5%
0.8%
1.3%
uncensored)
0.5%
TLR
4.1%
6.3%
6.0%
6.5%
TVR
5.8%
9,4%
8.0%
9.8%
From
the
pooled
ENDEAVOR
studies,
clinical
outcomes
through
9
months
are
shown
in
Table
30
stratified
by
all
diabetics,
insulin-dependent
diabetics,
and
non-insulin
dependent
diabetics.
Event
rates
for
the
Driver
patients
in
the
ENDEAVOR
II
study
are
shown
for
reference.
These
data
show
no
observed
safety
signals
with
respect
to
the
rates
of
death,
cardiac
death,
MI,
or
stent
thrombosis
with
the
Endeavor
stent
compared
to
the
Driver
stent.
Table
30:
Clinical
Events
in
Diabetics
Endeavor
Compared
to
Driver
BMS
uh
9
Months
Death~~~~~~~~~~~~~~~~~~~~~~
0
.
.
.
'.......
Death
~~~~~0.8%
Cardiac
Death
0.6%
MI
1.5%
Cardiac
Death
or
Ml
1.9%
Protocol
ST
0.6%
Definite
and
Probable
ST
ARC
(TLR-
0.8%
1.5%
1.5%
3.8%
5.3%
2.3%
2.3%
0.7%
0.0%
2.0%
2.0%
0.7%
1.3%
2.3%
2.3%
2.3%
4.5%
0.0%
0.0%
0.8%
0.8%
1.4%
1.9%
0.5%
0.5%
1.1
%
1.1%
4.5%
5.7%
3.4%
3.4%
censored)
Definite
and
Probable
ST
ARC
(TLR-
0.8%
2.3%
1.3%
0.0%
0.5%
3.4%
uncensored)
TLR
TVR
6.3%
9.4%
15.2%
15.9%
6.0%
8.0%
13.6%
13.6%
6.5%
9.8%
15.9%
17.0%
SSED
P060033
-
Page
56
of
59

XII.
Conclusions
Drawn
from
the
Studies
The
safety
and
effectiveness
of
the
Endeavor
Zotarolimus-Eluting
Coronary
Stent
System
are
based
on
the
results
obtained
from:
biocompatibility;
in
vivo
pharmacokinetics;
in
vitro
engineering
testing;
coating
characterization;
chemistry,
manufacturing
and
controls
information;
in
vivo
animal
testing;
sterilization
and
stability
testing;
and
clinical
studies.
These
test
results
revealed
the
following:
The
biocompatibility,
in
vivo
pharmacokinetics,
and
in
vivo
animal
testing
that
were
conducted
demonstrated
that
the
acute
and
chronic
in
vivo
performance
characteristics
of
the
product
provide
reasonable
assurance
of
safety
and
are
acceptable
for
clinical
use.
The
in
vitro
engineering
testing
conducted
on
the
stent
and
delivery
system(s)
demonstrated
that
the
performance
characteristics
met
the
product
specifications
and
the
coating
characterization
testing
adequately
described
the
important
attributes
of
the
zotarolimus/polymer
coating.
The
chemistry,
manufacturing,
and
controls
information
ensures
that
product
meeting
specifications
will
be
released.
The
test
results
obtained
from
the
sterilization
testing
demonstrated
that
the
product
can
be
adequately
sterilized
and
is
acceptable
for
clinical
use.
The
stability
testing
demonstrated
that
the
product
can
be
labeled
with
a
shelf
life
of
12
months.
The
clinical
testing
conducted
demonstrated
that
the
product
provides
a
reasonable
assurance
of
safety
and
effectiveness
when
used
as
indicated
in
accordance
with
the
instructions
for
use.
Specifically,
the
Endeavor
stent
was
shown
to
be
superior
to
an
approved
bare
metal
stent
with
respect
to
both
clinical
outcomes
and
angiographic
data.
In
addition,
clinical
outcomes
with
the
Endeavor
stent
were
non-inferior
to
those
obtained
using
an
approved
drug-eluting
stent.
Although
the
Endeavor
stent
did
not
demonstrate
non-inferiority
to
two
approved
drug-eluting
stents
based
on
angiographic
data,
the
aggregate
of
clinically
relevant
safety
and
effectiveness
data
outweighed
the
results
of
surrogate
angiographic
measurements.
XIII.
Panel
Recommendation
At
an
advisory
meeting
held
on
October
10,
2007,
the
Circulatory
Systems
Devices
Panel
unanimously
recommended
that
Medtronic's
PMA
for
the
Endeavor
Zotarolimus
Drug-
Eluting
Coronary
Stent
System
&
Over-the-Wire
(OTW),
Rapid
Exchange
(RX),
and
Multi-Exchange
II
(MX
2
)
Stent
Delivery
Systems
be
approved
subject
to
submission,
and
approval
by,
the
Center
for
Devices
and
Radiological
Health
(CDRH)
of
the
following:
1.
The
sponsor
should
conduct
a
>
5,000
patient
single-arm
post-approval
study
with
endpoints
of
very
late
stent
thrombosis
and
cardiac
death
+
myocardial
infarction,
rigorous
monitoring,
and
follow-up
through
at
least
5
years.
2.
The
labeling
regarding
antiplatelet
therapy
use
should
be
consistent
with
current
ACC/AHA
Guidelines
as
FDA
has
proposed
for
currently
approved
drug-eluting
stents
following
the
December
2006
panel
meeting.
Specifically,
the
labeling
should
describe
the
use
of
antiplatelet
therapy
in
the
clinical
trials
and
suggest
that
use
through
1
year
may
be
beneficial
per
the
published
consensus
guidelines.
SSED
P060033
-
Page
57
of
59

XIV.
CDRH
Decision
CDRH
concurred
with
the
Panel's
recommendations
of
October
10,
2007.
Medtronic's
post-approval
study
proposal
developed
with
FDA
and
presented
to
the
Panel
addresses
the
Panel's
first
recommendation.
Specifically,
data
from
5,300
Endeavor
patients
(2,000
from
the
US
registry
and
3,300
OUS
from
the
Endeavor
arm
of
the
PROTECT
study)
will
be
collected
and
pooled
for
an
analysis
of
rates
of
stent
thrombosis
and
cardiac
death
plus
myocardial
infarction.
Data
will
be
analyzed
separately
for
the
patients
enrolled
in
accordance
with
the
labeled
indication
and
collectively
for
all
patients
enrolled
in
the
studies.
Medtronic
expects
the
PROTECT
trial
and
the
U.S.
Post
Approval
Registry
will
include
a
sufficient
number
of
patients
who
have
received
the
Endeavor
stent
in
accordance
with
the
approved
indication
to
allow
evaluation
of
both
endpoints
noted
above.
Data
from
at
least
2000
patients
will
be
available
to
provide
80%
power
to
demonstrate
that
the
rate
of
stent
thrombosis
per
year
is
<
1.0%
in
Endeavor
patients
who
receive
the
Endeavor
stent
in
accordance
with
the
approved
indication.
This
sample
size
also
provides
>
80%
power
to
demonstrate
that
the
rate
of
cardiac
death
+
myocardial
infarction
at
annual
timepoints
through
5
years
in
Endeavor-stented
patients
is
less
than
the
incidence
in
patients
who
received
the
Driver
stent
in
the
ENDEAVOR
II
trial
plus
a
50%
non-inferiority
margin.
To
address
the
Panel's
second
recommendation,
Medtronic
provided
revised
labeling
in
a
PMA
amendment.
The
final
labeling
describes
the
use
of
dual
antiplatelet
therapy
in
the
ENDEAVOR
trials
and
further
states
that
"Current
guidelines
recommend
that
patients
receive
aspirin
indefinitely
and
that
clopidogrel
therapy
be
extended
to
12
months
in
patients
at
low
risk
of
bleeding
(ref:
ACC/AHA/SCAI
PCI
Practice
Guidelines3,
4
)."
Additionally,
Medtronic
has
agreed
to
conduct
or
participate
in
a
study
that
will
develop
clinical
data
to
identify
the
optimal
duration
of
dual
antiplatelet
therapy
following
percutaneous
intervention
with
the
Endeavor
drug-eluting
stent.
The
applicant's
manufacturing
and
sterilization
facilities
were
inspected
and
found
to
be
in
compliance
with
relevant
Quality
System
Regulations
(21
CFR
820)
and
pharmaceutical
current
Good
Manufacturing
Practice
(cGMP)
regulations.
FDA
issued
an
approval
order
on
February
1,
2008.
XV.
Approved
Specifications
Directions
for
Use:
See
product
labeling.
Hazard
to
Health
from
Use
of
the
Product:
See
Indications,
Contraindications,
Warnings,
Precautions,
and
Adverse
Events
in
the
labeling.
Post
Approval
Requirements
and
Restrictions:
See
Approval
Order.
SSED
P060033
-
Page
58
of
59
66

XVI.
References
ICutlip
DE,
Windecker
S,
Mehran
R,
et
al.
Clinical
end
points
in
coronary
stent
trials:
a
case
for
standardized
definitions.
Circ
2007;
115:2344-51.
2Acute
or
subacute
can
also
be
replaced
by
the
term
early
stent
thrombosis.
Early
stent
thrombosis
(0
-
30
days)
will
be
used
in
the
remainder
of
this
document.
3Smith
et
al.
ACC/AHA/SCAI
2005
Guideline
Update
for
Percutaneous
Coronary
Intervention.
JACC,
2006;
47:
el-121.
4King
III
et
al.
2007
Focused
Update
of
the
ACC/AHA/SCAI
2005
Guideline
Update
for
Percutaneous
Coronary
Intervention.
JACC,
2008;
51:172-209.
SSED
P060033
-
Page
59
of
59
