
Health and Care Act 2022
Impact assessments summary document and analysis
of additional measures
Published 4 November 2022
2
Contents
1. Introduction ................................................................................................................... 3
2. Policies .......................................................................................................................... 4
3. Interactions between policies ........................................................................................ 6
4. Specific Impact Tests .................................................................................................... 7
5. Post Implementation Review (PIR) ............................................................................... 9
6. Impact assessments of additional measures ............................................................... 10
7. Annex A: Licensing of non-surgical cosmetic procedures: Illustrative examples ......... 69
8. Annex B: Initial assessment of impact and costs for a cosmetic licensing scheme ..... 71
9. Glossary ...................................................................................................................... 76
3
1. Introduction
The Health and Care Act 2022 builds on the proposals brought forward by the NHS following the
publication of the Long-Term Plan. These proposals built on extensive engagement by the NHS
in 2019 and were further developed in the 2021 White Paper Integration and Innovation:
Working Together to Improve Health and Social Care for All. The Act advances on the
collaborative working seen throughout the pandemic, to shape a system which is best placed to
serve the needs of the population.
The core measures in the Act follow three core themes, all of which are integral for helping the
system to recover from the pandemic and transform patient care for decades to come.
Firstly, the Act removes barriers which stop the system from being truly integrated, with different
parts of the NHS working better together, alongside local government, to tackle the nation’s
health inequalities.
Secondly, the Act reduces bureaucracy across the system. DHSC wants to remove barriers
which make sensible decision-making harder and distracts staff from delivering what matters –
the best possible care.
Lastly, DHSC wants to ensure appropriate accountability arrangements are in place so that the
health and care system can be more responsive to both staff and the people who use it.
All of these measures are intended to complement, not distract from, the transformation that is
already taking place across the system. These policies should be seen in the context of those
broader reforms.
Alongside the core measures, there are additional policies to make targeted changes to allow
the government to support the social care system, to improve quality and safety in the NHS, to
grant the flexibility to take public health measures and to implement worldwide reciprocal
healthcare agreements.
These measures will provide a foundation to build upon and our aim is to use legislation to
provide a supportive framework for health and care organisations to continue to pursue
integrated care for service users and taxpayers in a pragmatic manner.
As the health and care system further emerges from the pandemic, these legislative measures
will assist with recovery by bringing organisations together, removing more of the bureaucratic
and legislative barriers between them and enabling the changes and innovations they need to
make.

4
2. Policies
The Health and Care Act legislates for multiple policy objectives and therefore brings forward a
number of different measures. All of the policies where costs and benefits have been identified
have an impact assessment (IA) which discusses the options, rationale, costs and benefits in
detail.
Several of the policies relate to enabling powers in the Act which do not have quantifiable
benefits or costs, as the impact of the policy will ultimately depend upon how the powers are
used. Nevertheless, a qualitative assessment of the potential costs, benefits, risks and
mitigations have been included as part of this package of IAs.
Furthermore, given that there are multiple policies, several of which do not have quantifiable
benefits, it was not deemed appropriate to calculate an overall Net Present Value for the relative
costs and benefits across the entirety of the Act. Rather, if costs and benefits have been
quantified, then an NPV will be included in that policies respective IA and will be considered in
isolation.
Table 1 presents a summary of the IAs published alongside the Act and the individual IA title in
which they have been incorporated. Policies on Health Services Safety Investigations Body
(HSSIB), and, introducing a 2100-0530 watershed on TV and online ban for paid advertising of
food and drink that are High in Fat, Salt and Sugar (HFSS) products, each have their own
standalone document dedicated to that respective policy.
Table 1: Summary of policies and where to find their associated IAs
Policy
Impact assessment
Commercial dealings in organs for transplantation: extra-territorial
offences
Additional measures IA
Eradicating slavery and human trafficking in supply chains
Additional measures IA
Food information for consumers: power to amend retained EU Law
Additional measures IA
Hospital Food Standards
Additional measures IA
Increasing gamete and embryo storage limits to a maximum of 55 years
for all
Additional measures IA
Information about payments to persons in the health care sector,
enforcement and consent
Additional measures IA
Licensing of cosmetic procedures
Additional measures IA
Medical examiners
Additional measures IA
Medicines information systems
Additional measures IA
Powers allowing further products to be centrally stocked and supplied
free of charge to community pharmacies without the need to reimburse
them under the standard NHS arrangements
Additional measures IA
Professional regulation
Additional measures IA
Rest of World reciprocal healthcare
Additional measures IA
Water fluoridation
Additional measures IA
Abolishing Local Education Training Boards
Core IA
Accountability and Transparency of Mental Health Spending
Core IA
NHS England mandate: cancer outcome targets
Core IA
Arm’s-Length Bodies transfer of functions power
Core IA
Care Quality Commission reviews of Integrated Care Systems
Core IA

5
Climate change duties
Core IA
Competition
Core IA
Data sharing
Core IA
Designating Integrated Care Boards as Operators of Essential Services
under NIS Regulation
Core IA
Duty to cooperate
Core IA
Establishing Integrated Care Boards and Integrated Care Partnerships in
law
Core IA
Foundation Trusts capital spend limit
Core IA
Further embedding research in the NHS
Core IA
General power to direct NHS England
Core IA
ICB and NHSE inequalities duty extension
Core IA
Information about inequalities
Core IA
Joint Committees, Collaborative Commissioning and Joint Appointments
Core IA
Merging NHS England and NHS Improvement
Core IA
National Tariff
Core IA
New trusts
Core IA
Provider selection and Choice
Core IA
Public Health power of direction
Core IA
Reconfiguration of services: intervention powers
Core IA
Special Health Authorities Time Limits
Core IA
NHS England Mandate: general (and Better Care Fund)
Core IA
Triple Aim
Core IA
Workforce accountability
Core IA
Adult social care – assurance
Social Care IA
Adult social care – discharge to assess
Social Care IA
Adult social care – provider payments
Social Care IA
Policies with standalone IAs
Virginity Testing Ban
Health Services Safety Investigations Body (HSSIB)
Introducing a 2100-0530 watershed on TV and online ban for paid advertising of food and drink that
are High in Fat, Salt and Sugar (HFSS) products
Hymenoplasty Ban
There are several policies in the Act which include a requirement to consult, to undertake a
review or to produce a report. These policies are: Child safeguarding etc in health and care:
policy about information sharing, and; Review into disputes relating to treatment of critically ill
children. For these policies an impact assessment has not been included as the impact of the
policy will depend upon the outcome of the consultation, its recommendations, and whether or
how they are acted upon, or any commitments to action in the report. An impact assessment will
be undertaken following consultation/publication as appropriate.
Impact assessments on the policies regarding early medical termination of pregnancy and
mandatory training on learning disability and autism are in development and will be published in
due course.
6
3. Interactions between policies
The policies covered in the Act should be seen as mutually reinforcing, rather than policies to be
viewed in isolation. Therefore, there are interdependencies between the Act provisions,
whereby the success of one policy may depend on the impact of another. This is particularly
true of provisions relating to the three principles underlying the Act, which are being put in place
to foster collaboration across the health and care system and are covered in the Core IA.
Potential interdependencies are outlined below, although this list is not exhaustive and further
details can be found in the specific analyses for each policy.
The Triple Aim and Duty to Cooperate provisions make it more likely that other policies covered
in the Act will have a system benefit (e.g. appropriate joint working with ICBs and their system
partners). The benefits derived from these policies will depend on the success of other
measures to deliver beneficial system change. Further detail can be found in the respective
sections in the Core IA.
The Professional Regulation provisions have potential interdependencies with the ALB transfer
function policy, and, with other existing policies related to health and social care. This is
explored in more detail in the Professional Regulation section of the Additional Measures IA.
For the public health measures related to obesity, namely those concerning the advertising of
HFSS foods and Food information for consumers: power to amend retained EU Law, the impact
of these policies on public health may be difficult to disaggregate as they are part of a wider
programme of supporting the public to make better informed choices about their diet.
7
4. Specific Impact Tests
In some cases, the policies included within the Health and Care Act introduce enabling powers,
and so the impacts will not materialise until secondary legislation is finalised and implemented.
Therefore, at secondary legislation stage, more detailed analysis of the finalised policy will be
undertaken, which will also include detailed analysis of specific impacts, such as those on the
justice system, trade and the environment where appropriate.
Equality
The policy measures in the accompanying IAs have undergone a equalities assessments as
appropriate.
Privacy
The powers that enable data to be required from adult social care providers may have an
impact on privacy depending on the form of data required. Any requests that relate to
identifiable information will be subject to existing data protection legislation and individual
privacy tests will be undertaken as appropriate. Similarly, the power to extend NHS Digital’s
(NHSD) powers to enable it to require data from private providers may also have an impact on
privacy and NHSD will ensure that appropriate safeguards are in place.
Justice system
Justice impacts are anticipated for the HSSIB, Medicines Information Systems, Organ
Transplantation, Virginity Testing, Hymenoplasty, Information about payments, Licensing of
Cosmetic Procedures Eradicating slavery and human trafficking in supply chains and
Commercial dealings in organs for transplantation: extra territorial-offenses.
Restrictions on HFSS advertising may result in some enforcement actions reaching the courts,
although this number is expected to be very small.
New burdens for local government
No new burdens on local authorities are anticipated at this stage from the primary legislation.
However, this will be under review as the Government continues to implement these policies
through guidance and secondary legislation. We expect to produce a new burdens assessment
for the Adult Social Care Assurance policies but will continue to keep other areas of the Act
under review.
Competition and innovation
The policy in the Core IA relating to competition intends to change the roles in respect of
competition of the Competition and Markets Authority (CMA) and NHS Improvement (Monitor
functions). The policy aims to create a more nuanced approach to certain NHS transactions that
gives greater weight to collaboration. The potential impacts of this on competition are outlined in
the Core IA.
8
The policies relating to provider selection and choice, licensing of cosmetic procedures and
eradicating slavery and human trafficking in supply chains may have impacts on competition at
secondary legislation stage. This will be explored as part of an impact assessment as
appropriate at that point.
Restrictions on HFSS advertising may result in impacts on competition and innovation, which
are explored in this policies standalone IA.
Small and micro business assessment (SaMBA)
The policies related to data sharing, provider selection and choice, medicines information
systems, Licensing of Cosmetic Procedures, information about payments, hospital food
standards, Food information for consumers: power to amend retained EU Law and reciprocal
healthcare arrangements for rest of world countries may have impacts on small or micro
businesses. It is not possible to provide a robust estimate of these costs, or give details of
exemptions, until the relevant powers are used. There is a commitment to examining these
impacts if and when secondary legislation is introduced. Further details are given in the
respective IA sections.
9
5. Post Implementation Review (PIR)
The PIR of the Health and Care Act 2022 is currently being commissioned through an NIHR
Policy Research Programme open-call. DHSC have invited proposals for a single primary
research project to provide evidence on the implementation and impact of the Act.
The primary interest of the PIR is to understand the different ways that Integrated Care Boards
and Integrated Care Partnerships, and system partners (at system, place, and neighbourhood
level) are coming together to design, commission and deliver services, and fulfil their duties,
and the potential impacts. The aim is to capture learning to identify how positive changes may
have been achieved, the obstacles to this (and how these can be avoided), and to disseminate
that learning across the system.
This evaluation will help to spread learning in a timely manner (e.g., learn and disseminate what
works in delivering quality integrated care and support). It will also support Ministers and
policymakers understand how the system is evolving following the legislative changes, how
DHSC can best support ICBs and ICPs in delivering better outcomes and inform future reforms
regarding integrated care.
This evaluation will be a mixed methods and multi-phased study, taking around 2-3 years to
complete. However, research outputs will be produced and shared before completion to be
disseminated with systems, facilitating the sharing of lessons learnt and best practice with
systems.
The PIR will be focused on the policies most directly related to the core themes of the Act of
increasing collaboration, reducing unnecessary bureaucracy and accountability. Policies outside
of this core focus will develop their own PIR plans as appropriate. For example, PIR plans
would be developed if it were decided to exercise particular powers described in the core IA and
additional measures IA. This is because for provisions which include enabling powers, the
details of the final policy will not be finalised until the implementation or secondary legislation
stage. This means that the specific plans for the PIR cannot be finalised until the final form of
the policy, and the specific outcomes it is likely to affect, are known.
Some policies which have standalone IAs, such as the provisions concerning advertising of
foods and drinks which are High in Fat, Salt and Sugar (HFSS), have committed to completing a
PIR. Details of PIR plans are outlined in these standalone IAs.
10
6. Impact assessments of additional
measures
The proceeding section is the impact assessment for several additional policies to support
public health, and quality and safety.
11
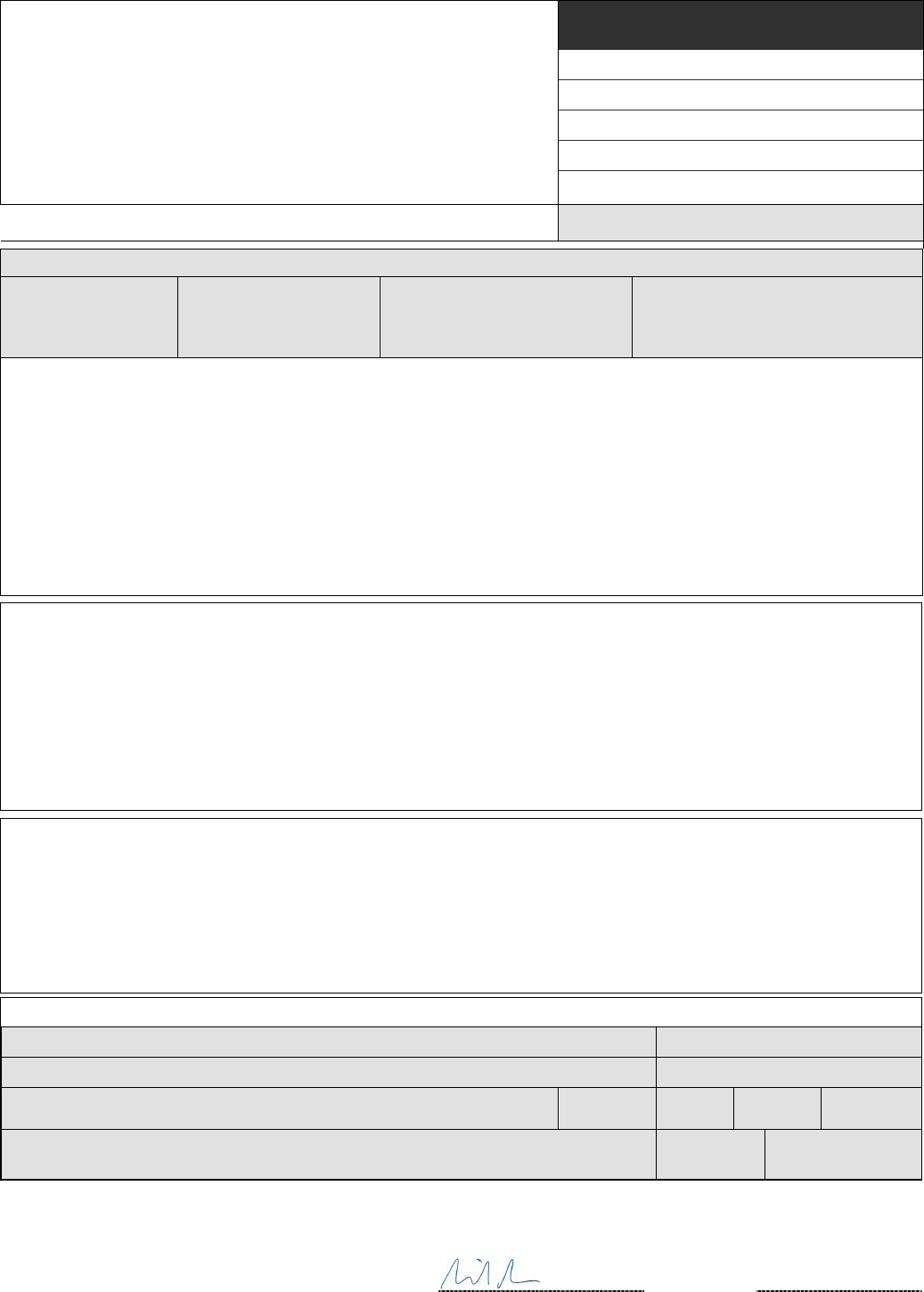
Title: Health and Care Act 2022
IA No: 9570
RPC Reference No: RPC-DHSC-5082(1)
Lead department or agency:
Department of Health and Social Care (DHSC)
Other departments or agencies:
NHS England and NHS Improvement
Impact Assessment (IA)
Date: 27/10/2022
Stage: Final
Source of intervention: Domestic
Type of measure: Primary legislation
Contact for enquiries: Not applicable
Summary: Intervention and Options
RPC Opinion: GREEN
Cost of Preferred (or more likely) Option (in 2019 prices)
Total Net Present
Social Value
Business Net Present
Value
Net cost to business per
year
Business Impact Target Status
Non qualifying provision
Unquantified Unquantified Unquantified
What is the problem under consideration? Why is government action or intervention necessary?
Demographic and social changes have, for a number of years, been changing the shape of the demands on the health
and care system. This Act implements the lessons learned from the evolution of the entire Health and Care System, as
well as the specific experience of responding to an unprecedented public health emergency during the Covid-19
pandemic. The measures considered in this impact assessment are targeted to address specific problems and remove
legislative barriers to allow front line staff and the government to deliver care more efficiently and maximise opportunities
for improvement. This is with the ultimate of aim of supporting the system in helping people to live healthier, more
independent lives for longer.
What are the policy objectives of the action or intervention and the intended effects?
Measures considered in this impact assessment relate most directly to the fourth principle of the Health and Care Act,
which have the aims of supporting social care, public health, and quality and safety. For example, the policies examined
in this impact assessment are targeted changes which will enable government to more effectively support the social care
system, and, implement comprehensive reciprocal healthcare arrangements with Rest of World countries (outside the
European Economic Area and Switzerland). The impact assessments for seven policies relating to the fourth principle
have been collated in this single document as they all entail small or unquantifiable impacts.
What policy options have been considered, including any alternatives to regulation? Please justify preferred
option (further details in Evidence Base)
This IA covers legislative changes developed by the Department of Health and Social Care, working with a breadth of
stakeholders including NHS England & NHS Improvement, and the Department for Levelling Up, Housing and
Communities. Given the complexity of the package of measures, this IA is focussed primarily on the leading options for
each of the policies and specific legislative changes. Impacts are by default compared against a ‘do-nothing’ option,
although in some cases alternative policy options are outlined.
Will the policy be reviewed? It will be reviewed.
If applicable, set review date: Not applicable
Does implementation go beyond minimum EU requirements? N/A
Is this measure likely to impact on international trade and investment? No
Are any of these organisations in scope?
Micro
Yes
Small
Yes
Medium
Yes
Large
Yes
What is the CO
2
equivalent change in greenhouse gas emissions?
(Million tonnes CO
2
equivalent)
Traded:
N/A
Non-traded:
N/A
I have read the Impact Assessment and I am satisfied that, given the available evidence, it represents a
reasonable view of the likely costs, benefits and impact of the leading options.
Signed by the responsible Minister:
Date:
27/10/2022
Summary: Analysis & Evidence Policy Option 1

12
Description:
FULL ECONOMIC ASSESSMENT
Price Base
Year N/A
PV Base
Year N/A
Time Period
Years N/A
Net Benefit (Present Value (PV)) (£m)
Low:
N/A
High:
N/A
Best Estimate:
N/A
COSTS (£m)
Total Transition
(Constant Price) Years
Average Annual
(excl. Transition) (Constant Price)
Total Cost
(Present Value)
Low
High
Best Estimate
N/A
N/A
N/A
Description and scale of key monetised costs by ‘main affected groups’
The policies set out in this IA are complex and to a significant extent consist of creating enabling powers which either lead to
practical but limited changes; require secondary legislation or consultation before practical changes can occur; and/or, require
system behavioural change before practical changes come into force. It is not possible to robustly estimate an overall cost
impact by affected groups, but despite this, costs which may be incurred following secondary legislation have been outlined as
best as possible at this stage. The medicines information systems section contains an illustrative example of monetised
impacts if those enabling powers were used. An assessment of impacts on businesses, including small or micro businesses,
and wider impacts such as those on the environment, trade and competition, will be completed where appropriate alongside
secondary legislation.
Other key non-monetised costs by ‘main affected groups’
The policies set out in this IA affect NHS providers, commissioners and arms’ length bodies, as well as local authorities, social
care providers, and independent organisations providing health and care service. However, as many of these provisions
introduce enabling powers, any costs will depend upon how those powers are exercised. If secondary legislation were to be
enacted, then an assessment of costs will be completed at that point if appropriate.
BENEFITS (£m)
Total Transition
(Constant Price)
Years
Average Annual
(excl. Transition) (Constant Price)
Total Benefit
(Present Value)
Low
High
Best Estimate
N/A
N/A
N/A
Description and scale of key monetised benefits by ‘main affected groups’
Benefits relating to these policies have not been monetised in this IA as a robust estimation of likely effects is not possible. This
is because the likely effects of, for example, an enabling power will depend upon how those powers are exercised. If
secondary legislation were to be enacted, then an assessment of benefits will be completed at that point if appropriate.
Other key non-monetised benefits by ‘main affected groups’
It is not possible to monetise the benefits of enabling powers, as the specific circumstances under which those powers may be
exercised will influence the potential costs and benefits. If secondary legislation were to be enacted, then an assessment of
benefits will be completed at that point if appropriate. However, examples of potential benefits from the policies in this IA
include reduced bureaucracy, and therefore, reduced burden on policymakers and providers, improved service provision to
patients, and, more informed patients.
Key assumptions/sensitivities/risks Discount rate (%)
N/A
It is difficult to fully determine the impact of these provisions quantitatively. There is a risk associated with any change
programme, even if intended to be limited, that resources are spent on implementing a new system to the detriment of output.
A further risk is that some provisions are enabling measures and do not contain substantive provisions. It is therefore difficult to
assess with any certainty what the impact of the measures will be, as the detail of those final provisions is not currently
available. Any policy that will be implemented using the regulation-making powers provided in these provisions in future will be
required to develop an impact assessment as appropriate.
BUSINESS ASSESSMENT (Option 1)
Direct impact on business (Equivalent Annual) £m: Score for Business Impact Target (qualifying
provisions only) £m: Not a qualifying provision
Costs: N/A
Benefits: N/A
Net: N/A

13
Health and Care Act: Evidence base for impact assessment
Background and overview
The Health and Care Act builds on the experience of previous reforms of the health and care
system, as well as the specific experience of responding to an unprecedented public health
emergency in the Covid-19 pandemic.
The measures considered in this impact assessment are targeted to address specific problems
and remove legislative barriers to allow front line staff and the Government to deliver care more
efficiently and maximise opportunities for improvement.
They are not intended to address all the challenges faced by the health and social care system.
Instead, these measures are targeted changes to allow the Government to support the social care
system, improve quality and safety in the NHS, grant the flexibility to take further public health
measures and to implement worldwide comprehensive reciprocal healthcare agreements.
The Government is undertaking broader reforms to social care and public health which will support
the system in helping people to live healthier, more independent lives for longer. As with the core
provisions impact assessment, many measures covered in this impact assessment will introduce
enabling powers and will require further secondary legislation in order to implement the policy.
Scope of the additional measures impact assessment
There are three guiding themes running through the core policies in the Health and Care Act.
These are: working together and supporting integration; reducing bureaucracy; and ensuring
accountability and enhancing public confidence. Alongside the core measures, there are additional
policies to make targeted changes to allow the Government to improve quality and safety in the
NHS, to grant the flexibility to take public health measures and to implement worldwide
comprehensive reciprocal healthcare agreements.
The 13 policies considered in this impact assessment relate most directly to additional policies to
support public health, and quality and safety. The analyses have been collated in this single
document as they all entail small or unquantifiable impacts. Several other additional
policies, such
as those relating to social care, have standalone IAs due to the size of the potential impact or
because the complexity of the analysis warranted a separate document. Readers should refer to
the impact assessments summary document for direction on where to find analysis on the other
policies in the Health and Care Act.
Post Implementation Review (PIR)
The exact details of the PIR for the
policies analysed in this IA will be set out once the provisions
are used. Please refer to the Impact Assessment Summary Document for further justification.
Summary of the costs, benefits, risks and mitigations of each
policy
This section provides details of each of the proposed changes to support the health and care
system.

14
1. Medicine information systems
Policy summary
Medicines registries provide a valuable resource for assessing and monitoring the safety and
effectiveness of medicines. The Independent Medicines and Medical Devices Safety Review
1
(IMMDSR) in 2020 made specific recommendations on the need for a national antiepileptics
registry. Following this, the Medicines and Healthcare products Regulatory Agency (MHRA) is
seeking regulation making powers to enable NHS Digital to establish and operate UK-wide
medicines information systems in order to ensure that comprehensive national registries can be
established and built in a sustainable way. This will require powers to be conferred on NHS Digital
to enable them to mandate relevant data collection, including from private healthcare providers and
devolved administrations, to build one or more comprehensive medicine information system(s).
The intention is that the information included in these systems will then be made available to the
MHRA to enable it to establish and operate UK-wide registries using existing powers contained in
the Human Medicines Regulations 2012.
This policy
only creates the power to make regulations to establish medicine information systems.
As such while there is no direct cost or impact associated with the clauses in this Act,
consideration as to how the regulations are likely to be laid out and their potential impact through
an illustrative example is appropriate. A more detailed assessment of costs and impacts can be
conducted when the regulations are made and exercised to develop a specific registry.
It is anticipated that, when there exists a need justified on public health grounds, the MHRA will
assess the option of introducing a particular national medicines registry when alternative
approaches to capturing sufficient data are not feasible. The proposal for establishment for a new
registry will be presented to the Commission on Human Medicines (CHM) who would need to issue
a formal registry-specific recommendation subject to the following criteria:
i. There are known risks associated with a medicine that can result in serious adverse health
outcomes and where adherence to effective risk minimisation measures is critical to ensuring
the benefits associated with the medicine outweigh the risks
ii. There is uncertainty about the safety or effectiveness of a medicine in a population in whom
prescribing may occur that means that urgent evidence is required to build the evidence base
on the benefit risk balance and inform the need for, and feasibility of, risk minimisation
measures
The CHM’s final advice on the need for a specific registry will be put to the appropriate authorities
to propose issue of a joint direction for NHS Digital to collect the appropriate information required
by the registry to be captured within the medicine information system.
A core register of all patients prescribed the specific medicine of interest will form the basis of a
bespoke registry. The aim is to use patient-level data already collected within the NHS to form this
core register, which should facilitate complete monitoring of patients prescribed specific medicine
where necessary. Therefore, powers are also sought to ensure that individual patient-level data
can be linked across different national datasets, held by NHS Digital and the devolved
administrations, according to the design specification agreed by the Registry Steering Committee
for a specific registry. The MHRA will work with the NHS to build and maintain these registries.
Rationale for intervention
1
First Do No Harm The report of the Independent Medicines and Medical Devices Safety Review, (2020).
[Online]. Available from: https://www.immdsreview.org.uk/downloads/IMMDSReview_Web.pdf
15
At present, there are no government funded UK wide registries for products that pose potential
health risks to certain patients. Either when a license is first granted for a medicinal product to be
placed on the market, or at a later stage should the need be identified, the MHRA can require a
Marketing Authorisation Holder (MAH) to establish a registry for that specific medicine to, for
example, identify or monitor adverse effects. This is an existing legal power of the MHRA acting as
the UK national competent authority for the regulation of medicine. The requirement for a registry is
defined in the terms of the license granted to the MAH. MAH-led medicine registries have had
mixed success in generating the strength of evidence required to make fully robust regulatory
decisions regarding the safe and effective use of medicine. This is in part because such stand-
alone registries are voluntary for clinicians, and full identification of eligible patients is also often
challenging due to a hesitance on the behalf of clinicians and their patients to enrol as part of an
MAH-sponsored registry due to data confidentiality concerns. Enrolment may also be affected as
registries can place additional burden on healthcare providers (HCPs) to supply data.
Academic led initiatives also exist and have demonstrated the value that evidence generated by
high quality registries can have in supporting regulatory, HCPs, and patient decision making. The
MHRA are increasingly using data from these larger disease registries led by clinical and academic
research groups, although these often have issues with sustainability. In addition, voluntary
participation means data is not comprehensive or representative, rarely including data from private
providers and with regional and clinical speciality variations in terms of coverage. NHS Digital and
the devolved administrations already collate extensive data on the use of medicines in the UK but
there are gaps in this which need to be addressed.
The key justification for this policy is that it will facilitate a better monitoring system of the use,
benefits and risks of medicines, leading to improved evidence bases for regulatory and clinical
decision-making and overall patient safety outcomes. The provisions make this possible. A central
UK wide medicine information system, or systems, run by the NHS, filling existing data gaps and
linking data from different sources will enable the initiation of high-quality inclusive registries
operated independently of industry.
Other policy options considered
This IA only presents the option to introduce statutory powers to enable NHS Digital to establish a
medicines information system. This system will enable MHRA to set up a comprehensive UK wide
registry for a product when CHM considers the criteria for such a registry is met. The baseline
status quo option involves the MHRA setting up registries without a medicine information system –
either by requiring MAHs to set up voluntary registries or trying to develop UK wide registries
without powers to mandate data collection.
Option 0 - Business as usual (Do nothing)
In the counterfactual, the MHRA would continue using existing powers to set up registries but
without statutory medicine information systems to support them. This could be through the
licensing process where MAHs could be asked to set up and maintain registries for specific
products or, for example, as is the case with antiepileptics, a national registry is being set up to
address an urgent safety concern as recommended by the IMMDSR, but this is reliant upon
existing data feeds and voluntary provision of additional data. Currently, there are gaps in data
from prescriptions in private practice and from the devolved administrations as well as a lack of
detail on clinical aspects that are vital in order for the registry to meet its objectives. This option
was not deemed feasible because the lack of robust, objective and comprehensive evidence poses
high risks for patients. Without a robust and complete medicine information system building a
comprehensive medicine registry, including all patients prescribed that medicine, independent from
industry, which can be necessary if public confidence is to be maintained, gaps in the data would
still remain meaning that the registry would not be able to support safe and effective use of
medicines in all patients.
16
Key impacts
There are no impacts resulting directly from this primary legislation as it only seeks the power to
make regulations to establish medicine information systems when the need for a registry is
identified.
If this power was exercised and a registry were to be set up utilising the medicine information
system, requirements would be placed on NHS Digital and potentially the devolved administrations
to capture and process the required data. Where individual health information for a medicine
information system is required from healthcare providers within Scotland or Wales it would be
collected via an intermediary organisation within those territories, unless an exception applies,
rather than, for example, collected directly from healthcare providers. The information would then
be shared onwards with NHS Digital. It is not considered that there will be an additional cost to the
provider of the data when an intermediary organisation is used as we expect that similar or the
same processes for providing the data straight to NHS Digital would be used. There may be small
costs for the intermediary organisation, likely to be a public body, in collecting this data, though it is
expected that this would be minimal as these bodies would already have infrastructure in place to
routinely collect, holds and process data in relation to medicines and health. In establishing each
new registry MHRA and NHS digital would work with the intermediary body or colleagues in Wales
or Scotland to identify who data is needed from and the best route for collection to minimise burden
and cost.
Costs of setting up and running a registry benefiting from a medicines information system are
unlikely to be very different from one that does not use a medicines information system. This is
because the design of a registry, and hence the requirements in terms of the types and volume of
data that would need to be captured, would be determined based on the scientific and regulatory
need which would be the same regardless of whether the regulations made based on the powers
being sought were in place. The purpose of the medicine information systems provisions is to
enable the appropriate authority to make regulations allowing them to direct the Information Centre
to collect the required data and to give the Information Centre a power to require provision of that
data from the relevant data holders. Regulations will also lay out the legal basis for collating and
sharing this data. The technology and supporting governance and documentation required to
deliver a medicines registry designed to meet its scientific and regulatory objectives would be the
same regardless of if it being underpinned by a medicines information system or not. However, the
potential benefits are likely to be greater as the powers provided to require submission of the
requested data will increase participation by HCPs and the availability of more comprehensive and
timely information. This added information would likely address risks to patient health and the
benefits would potentially extend to all patients treated with the medicine.
The provisions will enable NHS Digital to establish and operate UK-wide medicine information
system(s), the information from which will then be available to MHRA to establish comprehensive
national registries.
A figure for the Equivalent Annual Net Direct Cost to Business (EANDCB) has not been possible to
estimate as these provisions are enabling powers. The costs with regards to data provision are
largely expected to fall to the NHS and other public organisations. However, dependent on the
scope of a specific registry data may be sought from private HCPs for example, the potential
impact of data collection on business will depend upon the medicines of interest, remit, scope and
duration of the registry and hence the volume and complexity of the data that needs to be made
available to the information system. Therefore, at this stage it is not possible to estimate what the
potential cost on business may be. Any additional costs to HCPs from contributing to a medicine
registry would be examined as part of the business case process.
Furthermore, and for this same reason, a full small and micro businesses assessment (SaMBA)
has not been completed as part of the IA for the primary legislation. This can be included in future

17
assessments when the design of a specific registry, and therefore its impact on SMBs and other
potential data holders, is clearer. For context, in 2012 approximately 53% of NHS consultants
undertook some private practice, with an estimated 3,000 working entirely in the private sector
2
.
There are an estimated 515 private hospitals offering health care services in the UK, which are a
mixture of for-profit and non-profit. There are no comprehensive public data on the total number of
patients treated in private hospitals, but of the 285 hospitals that submitted data in 2017, 735,522
patients received treatment. By comparison, more than 8.5 million nonurgent patients were treated
by the NHS that year
3
. Again in 2012, an estimated 3% of GP consultations were private (~7
million consultations) although this may have increased since. Any impact on SMBs would be
around their need to submit data to the information system. This data would only consist of
information that they should already be capturing and recording as part of good clinical and
healthcare delivery practice to support individual patient management and safety. We believe there
may be two key categories of costs: i) familiarisation and training costs and ii) costs associated
with the data collection and submission processes. It is plausible that these costs may impact small
providers with less IT capacity more disproportionately. However, by working with the NHS to
deliver systems that integrate with local systems and capture the data into the information system
efficiently we can reduce the burden on businesses
. As described earlier, the number and types of
HCPs expected to contribute to an information system for a new medicine(s) will be unknown until
details of the needs for that medicine are finalised. It is therefore not yet possible to state whether
these businesses will be disproportionately affected or whether an exemption would be
appropriate. Again, potential costs to small and micro businesses will be considered as part of the
individual business case processes. There are no anticipated impacts on competition or
international trade.
There are potential impacts on the justice system. In particular, with regards to clauses on new
offences related to information disclosure from the medicine information system(s) and potential
identification in a specific registry of cases suitable for compensation. A Justice impact test to fully
assess the impacts will be completed in conjunction with the Ministry of Justice.
Indicative estimates of costs and benefits of a national registry
This analysis is an illustrative example as the provision relates to delegated powers to make
regulations about the establishment and operation of medicine information systems rather than the
actual establishment of specific registries. It examines the potential impacts of the use of this
power to enable NHS Digital to establish an information system, or systems to support a specific
registry. The overall aim would be to provide data from the system to MHRA to set up and maintain
such a national registry, using MHRA’s existing pharmacovigilance powers. The intention is to
provide an initial high-level assessment of the impact that the use of this power could have in the
future. This mirrors the approach used to assess the set-up of national registries for medical
devices in the MMD Act 2021 Impact Assessment
4
.
Each individual registry is likely to vary in design (size and function) as the specific risks relating to
the specific product are likely to be different and as a result so are the potential impacts. To
highlight this point, we present cost estimates using data from three existing registries that vary
significantly scope and size (Sodium Valproate
5
, National Joint Registry, Breast and Cosmetic
implants).
2
The Kings Fund, ‘The UK private health market’, 2014. [Online]. Available from:
https://www.kingsfund.org.uk/sites/default/files/media/commission-appendix-uk-private-health-market.pdf
3
The private healthcare information network, ‘Annual report 2016-17’, 2017. [Online]. Available from:
https://apicms.phin.org.uk/Content/Resource/158-PHIN_AR_2016-17.pdf
4
Medicines and Medical Devices Bill - IA No: 9556 - 2020
5
https://digital.nhs.uk/data-and-information/publications/statistical/mi-medicines-in-pregnancy-
registry/valproate-use-in-females-aged-0-to-54-in-england-april-2018-to-september-2020
18
Also presented are potential benefits of a national medicines’ registry using the England-only
Sodium Valproate registry example. Estimates of benefits must be treated with caution as they are
based on data available so far from the valproate example which is still being developed. These
are for illustrative purposes only. Specific costs and benefits of individual registries must be
considered as part of the business case process.
Costs
MHRA and NHS Digital – set up and running costs
MHRA would be responsible for establishing and running national registries, bringing in relevant
partners as required. This could be through a Registry Steering Committee to provide operational
support and clinical guidance, and oversight of the project’s set up, running, and translation of its
findings. There are likely to be both one off set up and ongoing opportunity costs of MHRA staff
time spent on these activities. It is not anticipated that there will be additional costs to Marketing
Authorisation Holders.
NHS Digital would collect the data needed for all medicine registries from various sources and hold
this in an information system. Data from the information system would be provided to the MHRA to
establish and operate/run medicine specific registries.
Table 1 outlines the cost of setting up three different medicines registries. The table demonstrates
that depending on specific circumstances such as size and scope, set up and running costs can
vary substantially. Similarly, the benefits of the registry may be expected to scale up according to
the size and scope of the registry, as for example a greater number of patients or treatments may
be covered, thus benefitting a larger patient population.
Table1: Indicative set up and running costs of national registries per annum
National registry example
Potential annual costs to MHRA and NHS Digital
(Set up / annual running cost)
Sodium Valproate
£1.014m / £183,000 (estimated)
Breast and Cosmetic Implant
registry
£83,000 / £183,000
National Joint Registry
£1.8m / £4.1m
Sodium Valproate - method to calculate set up and running costs
Based on the Sodium Valproate example, it is estimated that roughly that about 0.5 FTE hours of a
SEO, G6 and SCS costing approximately £164,000 in MHRA staff resources could be spent on a
registry annually
6
. This is an estimated average with likely slightly higher costs in the first 2-3
years, due to the need for more senior staff involvement while the registry is being developed,
balanced by slightly lower costs once it is established.
Total NHS Digital potential staff and non-staff costs on Sodium Valproate is estimated at about
£950,000per annum
based upon the costs for the delivery of the second phase of development
planned for 2021/22. This amounts to a potential total set up of £1.014million annually for the
Sodium Valproate registry. However, this estimate must be treated as indicative only as the
majority of the cost is to develop the registry and once established running costs will be
substantially lower. Beyond the initial set up which is likely to last 2-3 years, maintenance costs
could be estimated to be similar to the BCIR as described below given the comparative size. For
reference, the first year costs for NHS Digital were substantially lower at approximately £20,000.
6
Based on average salary data from MHRA Finance

19
National Joint Registry (NJR) and Breast and Cosmetic Implant registry (BCIR) - method
to calculate set up and running costs
The potential costs to NHS Digital below have been taken from the Medicines and Medical Devices
Bill IA June 2020
7
. The estimates are based on data from the National Joint Registry (NJR) and
Breast and Cosmetic Implant registry (BCIR). The size, scope and amount of activity undertaken
i.e. amount of information collected and how it is used would impact costs. The below is therefore
an indicative range of costs.
One off set up costs:
The MMD IA estimates that a large-scale registry such as the NJR (with over 225,000 procedures
reported to it in 2018) could require an initial set up cost of £1.8m. The BCIR (with just under
15,600 operations reported over a year July 2018-June2019) could involve set up costs of about
£83,000. The costs are likely to cover any IT systems set up, and staff resources to design the
registry and publish guidance for participating providers.
Ongoing costs:
The MMD IA reports ongoing costs of £4.1m per annum for the NJR and £183,000 per annum for
BCIR. Ongoing costs are likely to cover – auditing data collected, analysing and reporting on safety
alerts, communicating with HCPs, researchers, government and the public, IT systems
development as registry evolves.
Administrative costs to NHS and private healthcare providers
It will be mandatory for all HCPs to contribute to an information system. This could involve clinical
staff time spent on undergoing training on the new registry and on an ongoing basis, recording the
data. Some providers may already be providing this data voluntarily to existing MAH registries and
for them the additional costs are unlikely to be significant. The number of HCPs this will impact is
unknown as it will depend on each specific registry and the prescribing trends of each medicine. In
general, HCPs can refer to GPs, private and state hospitals but could also include nurses,
midwives, pharmacists for some registries. However, it is unlikely that the overall costs to HCPs
will be high, as most of the data required are likely to already be collected by HCPs as part of
clinical management.
In the case of Sodium Valproate, women should have annual appointments with a neurologist who
should review their treatment and ensure patients complete a signed annual risk acknowledgement
form (ARAF), which is part of the Pregnancy Prevention Plan. There are currently estimated to be
625 consultant neurologists
8
in England who might review a woman’s valproate treatment. Given
that they already have to undertake regular reviews with patients on their Sodium Valproate use,
the inclusion of an ARAF on the registry is unlikely to increase administrative burdens.
Benefits
More informed patients and greater public confidence in the health system:
Patients can directly report on safety issues and may be more informed on the risks and benefits of
their medicines. This is likely to enable patients to take more informed decisions about their health.
Improved regulatory system:
7
Medicines and Medical Devices Bill - IA No: 9556 - 2020
8
Association of British Neurologists, Neurology Workforce Survey, 2020. [Online]. Available from:
https://cdn.ymaws.com/www.theabn.org/resource/collection/219B4A48-4D25-4726-97AA-
0EB6090769BE/2020_ABN_Neurology_Workforce_Survey_2018-19_28_Jan_2020.pdf
20
Information from medicine information systems will support the establishment of national registries.
This would allow the MHRA to use registries to more widely support safe use of a medicine
through inclusion of it in regulatory information and prescribing guidelines, and to take swift
informed regulatory action, as it is likely to receive timely and more complete data on risks
associated with the specific medicines.
Improved service provision to patients:
Information from a medicine information system and the resulting medicines registry should allow
HCPs to analyse reports on data to evaluate outcomes. HCPs could recall/amend patient
treatment if necessary and offer more efficient and effective services.
Avoided patient harm:
Most importantly, information from medicine information systems and the resulting medicines
registry is likely to give healthcare professionals timely access to more complete information –
including at the individual patient level. This would enable them to take rapid action and avert
potential risks to patient health from adverse effects.
Illustrative example – avoided patient harm from Sodium Valproate registry:
Currently the Sodium Valproate registry, which is the basis of the planned antiepileptics
registry is not mandatory and coverage may not be comprehensive, particularly for women
treated in the private sector. One of the aims of introducing a comprehensive mandatory
Sodium Valproate registry is to increase coverage which should in turn further accelerate
the decline in prescribing and reduce the number of exposed pregnancies. Reducing the
number of exposed pregnancies was a key aim of the 2018 Pregnancy Prevention Plan
(2018). The proposed power would enable NHS Digital to collect data, subject to a
Direction, from private prescribers and from devolved administrations, which would then be
provided to MHRA to establish a comprehensive valproate/anti-epileptic drugs registry.
Therefore, the data illustrated below is a useful example of the possible impact of
enactment of the proposed enabling power.
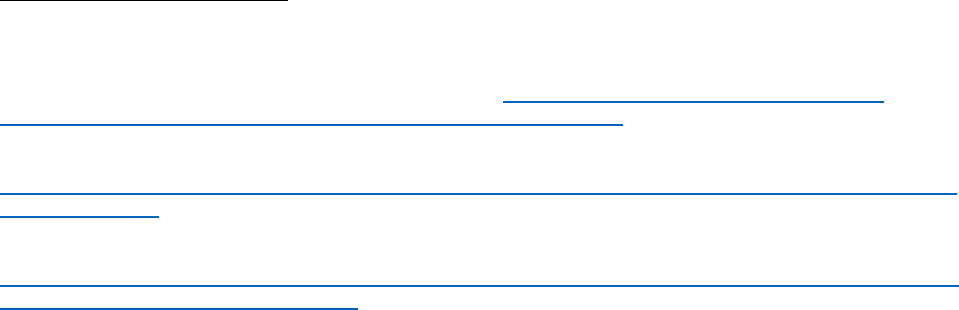
Figure 1 presents data published by the NHS Business Services Authority on the number of
women aged 14-45 in England prescribed Sodium Valproate over time. Figure 1 shows that
in the two years prior to the introduction of the PPP prescribing in women aged 14-45 was
falling by approximately 15%. Following the PPP, prescribing fell approximately by an
additional 10%.

21
Figure 1: The number of female patients aged 14-45 prescribed valproate over time
before and after introduction of the Pregnancy Prevention Plan
The first retrospective data from the non-mandatory Sodium Valproate registry suggest that
between April 2018 and Sept 2020, 181 pregnancies have been exposed to Sodium
Valproate (or approximately 70 per year). One hypothesis is that if a mandatory
comprehensive drug registry were in place, some of these exposures may have been
avoided as there would be more complete data on adherence by prescribers to best
prescribing practices and implementation of the Pregnancy Prevention Programme. Using
an arbitrary assumption of a further 20% reduction in the exposure to Sodium Valproate
following the introduction of a mandatory registry, it is estimated that this would reduce the
number of pregnancies exposed by a further 11 in the year September 2020-21.
Net benefits of the Sodium Valproate registry
This simple analysis examines what Quality Adjusted Life Year (QALY) gains are required
to compensate for the cost of setting up and running the Sodium Valproate registry. The
estimated cost for setting up and running the registry in the second year is £1.014million.
The best estimate for the value society places on a QALY is £60,000
9
. Therefore, it is
estimated that approximately 16.9 QALYs would have to be generated per annum through
development to account for the initial annual cost of the registry. If a 20% reduction in
exposure is achieved this would equate to 1.5 QALY per exposed pregnancy avoided.
However, in future years this would be lower at approximately 3 QALYS generated per
annum and a 0.3 QALY per exposed pregnancy avoided. Given valproate exposure during
pregnancy is associated with an approximately 50% risk of severe and lifelong physical and
neurological disorders this threshold would be reached. This demonstrates that with a very
modest reduction in exposure, only limited QALY gains are required for the net benefits of
the registry to break-even once the initial set up is complete.
9
HM Treasury: The Green Book: appraisal and evaluation in central government, (2020). [Online]. Available
from:
https://www.gov.uk/government/publications/the-green-book-appraisal-and-evaluation-in-central-
governent
0
5,000
10,000
15,000
20,000
25,000
Number prescribed valproate Pre-PPP estimated trend
Introduction
of PPP
22
Avoided costs to NHS from compensation / litigation and additional treatment:
Through early identification of risks and reducing scope for error (outlined above), harm to patients
could be prevented. This might in turn avoid potential claims and litigation costs against the NHS.
Costs to NHS of providing additional treatment to affected patients could also be avoided
10
.
Risks and Mitigations
The purpose of a medicine registry is to generate evidence. This evidence will be used by MHRA
and other organisations to inform regulations and guidance and to drive changes in clinical
practice. However, the economic benefits will only be fully realised if those changes actually
happen, and patient safety is improved. MHRA have an established role in leading within this area.
This is highlighted in the MHRA 2018-2023 Corporate plan
11
and the 2020/21 Business plan
12
,
which lay out the strategy to reshape post-market vigilance to run proactive life-cycle monitoring, of
which this policy is a component, and to increase MHRA influence on clinical practice through
further engagement with patients and key strategic healthcare partners.
2. Professional regulation
Policy summary
The powers in this Act form part of a wider programme to create a more flexible and proportionate
professional regulatory framework that is better able to protect patients and the public. These
powers will make it easier to ensure that professions protected in law are the right ones and that
the level of regulatory oversight is proportionate to the risks to the public.
Section 60 of the Health Act 1999 already provides powers to make changes to the professional
regulatory landscape through secondary legislation. The Act widens the scope of section 60 and
will enable us, where necessary, to make further changes in secondary legislation to ensure the
professional regulation system delivers public protection in a modern and effective way, and,
ensure professions are regulated in the most appropriate manner.
The new powers enable:
i. the abolition of an individual health and care professional regulatory body where the
professions concerned have been deregulated or are being regulated by another body;
ii. the removal of health care professions from regulation where regulation is no longer
required for the protection of the public;
iii. the delegation of certain functions to other regulatory bodies through legislation (which
was previously prohibited); and
iv. the regulation of groups of workers concerned with physical or mental health of
individuals, whether or not they are generally regarded as a profession i.e. senior
managers and leaders.
10
The literature review carried out by NICE estimates the percentage of hospital admissions due to ADRs in
the UK to be 6-7%. Of these ADRs, it is estimated that 1.6-3.7% were to be preventable. One review
estimated that the overall impact of ADRs in England was 4 out of 100 hospital bed stays with an equivalent
cost of about £380 million a year to the NHS in England.
https://cks.nice.org.uk/topics/adverse-drug-
reactions/background-information/health-financial-implications-of-adrs/
11
Medicines and Healthcare products Regulatory Agency (MHRA), ‘Corporate plan 2018-23’, 2018. [Online].
Available from:
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/702075/C
orporate_Plan.pdf
12
Medicines and Healthcare products Regulatory Agency (MHRA), ‘Business plan 2021-21’, 2020. [Online].
Available from:
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/889864/M
HRA_Business_Plan_2020_to_2021.pdf

23
The use of these additional powers will be subject to public consultation and the resulting
secondary legislation would be subject to the affirmative Parliamentary process. DHSC will work
with the regulatory bodies, the Professional Standards Authority and devolved administrations on
provisions to make further improvements to professional regulation through secondary legislation
in line with the other uses of the delegated powers in Section 60, Government will undertake wider
stakeholder engagement including with patient safety groups and the public prior to bringing
forward any legislation using the new powers.
The measures form part of a broader programme to modernise the regulatory system for health
and care professional in the UK. The programme is commencing with legislation that will reform the
legal framework for the General Medical Council and introduce two new professions, anaesthesia
associates and physician associates, into regulation by means of an Order in Council using powers
in the Health Act 1999. This will be followed by further legislation to extend these changes to all
regulated professions. The powers in the Act complement this transition to a modern and effective
professional regulatory system, by making it easier to deliver a reduction in the number of
healthcare professional regulators and make other changes which are being considered as part of
this work.
Rationale for intervention
The UK model of professional regulation for healthcare professionals has become increasingly
rigid and complex and needs to change to better protect patients, support the provision of health
services, and help the workforce better meet current and future challenges.
In 2017, the four UK governments consulted on high-level principles for reform of professional
regulation and set out their five objectives, to:
• improve the protection of the public from the risk of harm from poor professional practice;
• support the development of a flexible workforce that is better able to meet the challenges
of delivering healthcare in the future;
• deal with concerns about the performance of professionals in a more proportionate and
responsive fashion;
• provide greater support to regulated professionals in delivering high quality care; and
• increase the efficiency of the system.
The consultation Promoting professionalism, reforming regulation included questions relating to the
provisions in the Act. The link to the consultation can be found here
. The consultation set out the
proposals that were welcomed by key stakeholders, including professional organisations,
regulators and employers. The link to the consultation response can be found
here.
The consultation response also highlighted the case for broader changes to the regulatory
landscape including reducing the number of regulators. The Secretary of State for Health and
Social Care further committed to reviewing the number of health and care professional regulators
in the November 2020 Busting Bureaucracy policy paper and DHSC has commissioned an
independent review, led by KPMG, who submitted their report at the end of last year and which we
are now considering.
A further consultation Regulating healthcare professionals, protecting the public was published in
May 2021 which sets out reform proposals for all of the regulators. The implementation process
will start with changes to the General Medical Council’s legislation and the other regulators will
follow. A response to the consultation will be published in the next few months.

24
The Government has also consulted on the criteria for determining when statutory regulation of a
healthcare profession is appropriate. The consultation, Healthcare regulation: deciding when
statutory regulation is appropriate, ran from January to March 2022. While the Government
believes that there is no immediate case to change the groups that are regulated, the consultation
sought views on the proposed criteria to make decisions on which professions should be regulated;
whether there are regulated professions that no longer require statutory regulation; and whether
there are unregulated professions that should be brought into statutory regulation. The
consultation responses are currently being considered and a response will be published in due
course.
Additional wider reforms have also been considered such as the government response to the Law
Commission’s review of UK law relating to the regulation of healthcare professionals, and, the
recent review of the fit and proper persons test (further details are included in the fourth power
below).
The powers in the Act are:
i) the abolition of an individual health and care professional regulatory body where the
professions concerned have been deregulated or are being regulated by another body
There is the inevitable duplication in having nine regulatory bodies (10 including Social Work
England) that perform similar functions in relation to different professions. A reduction in the
number of regulators would deliver public protection in a more consistent way, while also delivering
financial and efficiency savings. Powers under section 60 already allow for the creation of new
regulators through secondary legislation. However, similar powers are not currently available to
close a regulator, and this can only be done via primary legislation.
This change would enable Parliament to use secondary legislation to abolish a regulator where its
regulatory functions have been merged or subsumed into another body or bodies, or where the
professions that it regulates have been removed from regulation.
The July 2019 Government response to the Promoting professionalism, reforming regulation
consultation set out the Government’s intention to consider reducing the number of regulators. The
Secretary of State for Health and Social Care further committed to reviewing the number of health
and care professional regulators in the November 2020
Busting Bureaucracy policy paper and
DHSC has commissioned an independent review, led by KPMG, who submitted their report at the
end of last year and which we are now considering. Use of these powers would be subject to
consultation and the affirmative parliamentary procedure.
ii) the removal of health care professions from regulation where regulation is no longer
required for the protection of the public
Statutory regulation should only be used where it is necessary for public protection. The level of
regulatory oversight for each profession should be proportionate to the activity carried out and the
risks to patients, service users and the public.
The landscape of the health and social care workforce is not static, meaning that the risks to the
public will change over time as practices, technology and roles develop. While statutory regulation
may be necessary now for a certain profession, over time the risk profile may change, such that
statutory regulation is no longer necessary. Clearly, in order to protect the public, professionals
such as doctors, nurses, dentists and paramedics will always be subject to statutory regulation.
The Government has recently consulted on the criteria for determining when statutory regulation of
a healthcare profession is appropriate. While the Government believes that there is no immediate
case to change the groups that are regulated, the consultation sought views on the proposed

25
criteria to make decisions on which professions should be regulated; whether there are regulated
professions that no longer require statutory regulation; and whether there are unregulated
professions that should be brought into statutory regulation. The consultation responses are
currently being considered and a response will be published in due course.
A provision to enable the removal of a profession from statutory regulation through secondary
legislation will make it easier to ensure that the protections and regulatory barriers that are in place
remain proportionate for all health and care professions. Any use of these powers would be
subject to consultation and parliamentary approval using the affirmative procedure.
iii) the delegation of certain functions to other regulatory bodies through legislation (which
was previously prohibited)
Currently, there are legal restrictions in place which limit the functions that professional regulators
can delegate to another body. This prohibits regulators from delegating the functions of the
keeping of a register of persons permitted to practise; determining standards of education and
training for admission to practice; giving advice about standards of conduct and performance; and
administering procedures relating to misconduct and unfitness to practise.
The removal of these restrictions would enable further collaboration in how regulation is delivered,
which could drive up quality, reduce costs and provide greater consistency. This would enable a
single regulator to take on the role of providing a regulatory function, such as the holding of a
register, the assessment of international applicants or the adjudication process for fitness to
practice, across some or all regulators. This will help to deliver public protection in a more
consistent fashion and may also increase efficiency. Where a function is delegated, a regulator
would retain responsibility for that function.
iv) the regulation of groups of workers concerned with physical or mental health of
individuals, whether or not they are generally regarded as a profession i.e. senior
managers and leaders
While the definition of those groups which can be included in regulation using the powers in
Section 60 of the Health Act 1999 is broad in relation to healthcare professionals, the proposed
changes allows for the regulation of groups of workers concerned with physical or mental health of
individuals, whether or not they are generally regarded as a profession, to be regulated. .For
example, those in senior management and leadership roles and other groups of workers are within
the scope of future regulation.
The 2019 Kark review of the fit and proper persons test recommended putting in place stronger
measures to ensure that NHS senior managers and leaders have the right skills, behaviours and
competencies. While it stopped short of recommending full statutory regulation, NHS
England/Improvement is currently considering how best to take forward the recommendations.
Extending the scope of professions who can be regulated using the powers in Section 60 of the
Health Act 1999 would provide additional flexibility to extend statutory regulation to, for example
NHS managers and leaders in the future, if further measures are needed.
Other policy options considered
Option 0 - Business as usual (Do nothing)
Not being able to expand the scope of Section 60 of the Health Act 1999 will restrict the extent of
reform can be made. Proposals are currently being developed using the powers available to
reform professional regulation in the areas of fitness to practise, governance and operating
26
framework, and the registration and education and training functions. However, the aim is to go
further to modernise professional regulation and the new powers will support this.
Option 1 - Seek fewer powers
If fewer powers were established through the Act, then it would be expected that primary legislation
would be pursued for the remaining powers in the near future. This is because all of the powers
proposed are expected to be required as part of our reform programme. This will delay completion
of our reform programme.
Costs
These provisions seek new powers to be taken forward through secondary legislation. There are
therefore no costs associated with these powers coming into force. An impact assessment which
calculates associated costs will be completed if the powers are put into effect.
Benefits
As mentioned above, these provisions seek new powers to be taken forward through secondary
legislation. Therefore, the benefits from all provisions are indirect and depend on the actions of the
Secretary of State. The potential benefits of these enabling powers, if put into effect through
secondary legislation, are outlined in the Rationale for Intervention section. An impact assessment
which calculates associated benefits will be completed if the powers are put into effect.
Important note
We are currently engaging with the devolved administrations, Treasury, Cabinet Office,
Department for Education and the Department for Business, Energy and Industrial Strategy
regarding the reform proposals.
3. Medical examiners
Policy summary
This policy allows NHS bodies in England and Welsh NHS bodies in Wales to appoint medical
examiners instead of local authorities and local health board respectively. This is so that medical
examiners employed in the NHS system will have access to information in the sensitive and urgent
timescales required to register a death. The following paragraphs of the policy summary section
set out the steps taken in the development of this policy.
The Department of Health and Social Care (DHSC) has developed policy over the past several
years which aims to provide a reformed system for certifying non-coronial deaths which improves
the quality and accuracy of Medical Certificate of Cause of Deaths (MCCDs) and provides
adequate scrutiny to identify and deter criminal activity or poor practice. The legal framework of this
system is set out in Part 1 of the Coroners and Justice Act 2009, but remains uncommenced at this
time, save for sections 18 and 21 as set out below.
As part of this work, DHSC ran a consultation from March to June 2016 seeking views on the detail
of the operation of the proposed reforms to the death certification process and draft regulations
setting out the system within which the services would operate. The consultation document
proposed the introduction of a unified system of scrutiny by independent medical examiners,
hosted by local authorities, of all deaths in England and Wales that are not investigated by a
coroner as set out in the Coroners and Justice Act 2009 and described how the Government saw
the new system working in practice.

27
The Department’s consultation response was published in June 2018 and set out an approach to
introduce a non-statutory medical examiner system by April 2019, where medical examiners were
to be appointed within the NHS without the introduction of a new fee at that time. An
Impact
Assessment was published alongside the June 2018 consultation response, outlining three policy
options and associated costings for England. Option 3 outlined the impact of introducing a Medical
Examiners system hosted within the NHS. Since the publication of the 2018 consultation response
and impact assessment, a non-statutory medical examiner system has been set up within the NHS
in England. To date, all NHS Trusts which require a medical examiner office (based on number of
deaths) under a statutory system have done so on a non-statutory basis. In terms of the impacts of
the amendment on Wales, an Impact Assessment has been published and can be found here:
Introduction of the medical examiner role and reforms to death certification | GOV.WALES
The consultation response also outlined the Government’s intention to commence sections 18 and
21 of the Coroners and Justice Act 2009. Section 18 was commenced in 2019 and section 21 in
2018. They make provisions for regulations to require medical practitioners to report deaths to the
coroner which the coroner has a duty to investigate, and, for the appointment of a National Medical
Examiner respectively.
The Health and Care Act includes provisions to amend the Coroners and Justice Act 2009 to allow
NHS bodies to appoint medical examiners instead of local authorities in England, and for Welsh
NHS bodies rather than only local health boards in Wales. The Secretary of State and Welsh
Ministers will have a duty to ensure that there are sufficient medical examiners and that they are
adequately funded, as well as a power to issue directions to ensure that the duty is met.
At the appropriate time the statutory provisions underpinning the medical examiner system set out
in the Coroners and Justice Act 2009 together with the amendments will be commenced.
Regulations will then also be laid for the Medical Certificate of Cause of Death (‘MCCD’) (under
s.20 of the Coroners and Justice Act 2009), Medical Examiners (under s.19) and the National
Medical Examiner (under s.21).
We had planned to provide an updated IA when laying regulations for the fee mechanism.
However, the medical examiner system will now be centrally funded, and a fee regulation is no
longer required. We are satisfied that the 2018 IA contains all relevant analysis for the three
regulations to be laid which put in place the centrally funded system.
Rationale for intervention
While the arrangements for scrutinising the cause of death have remained largely unchanged for
over 50 years, there are concerns about their efficacy and efficiency, particularly for those cases
which are not referred to a coroner. The statutory system was outlined in the Coroners and Justice
Act 2009 after the Shipman Inquiry concluded that it was no longer suitable to have different
certification processes for cremations and burials, and that all MCCDs should be subject to
independent medical scrutiny. As set out above, most of the provisions in the Coroners and
Justice Act have not yet been commenced. The rationale behind the provisions in the Health and
Care Act allowing NHS bodies in England and Welsh NHS bodies in Wales to appoint medical
examiners instead of local authorities and local health board respectively, is that medical
examiners employed in the NHS system will have access to information in the sensitive and urgent
timescales required to register a death.
Other policy options considered
An Impact Assessment was published on the gov.uk website alongside the June 2018 consultation
response, outlining three policy options and associated costings for England. This IA was cleared
across all departments (including HM Treasury) via write-round. The IA outlined in this paragraph
is England specific.

28
The preferred option (and that consistent with the consultation response) was option 3:
“Reform the current system for cremations and burials by introducing a new universal check by a
Medical Examiner (ME) applicable to all non-coronial deaths. The system will initially be funded
through cremation form fee revenues sourced from efficiencies in the system and DHSC. Following
the interim period, the ME system would be primarily funded through a fee for cremations and
burials.”
Costs and benefits
The impacts of the full statutory system for England are set out in the June 2018 impact
assessment, and for Wales in a separate impact assessment
which uses the same methodology,
where policy Option 3 set out the estimated costs and benefits of introducing a national statutory
system of medical examiners based in the NHS. The national statutory medical examiners system
set out at Option 3 in these IAs is an accurate representation of the system (apart from funding
solution) which we intend to be in place following the coming into force of the Coroners and Justice
Act 2009, the Health and Care Act and of the underlying regulations which set out operational
details.
The key policy change since 2018 is on funding. During the pandemic, cremation form 5 (the
cremation form completed by the second doctor) was suspended, and funding for the non-statutory
ME system was provided by central government. In March 2022, the suspension of cremation form
5 was made permanent through legislation, and from that point forward funding for the ME system
in England and Wales will be provided centrally, rather than from a public fee.
Costs to Business
As the June 2018 (England) IA sets out in para 77-79, it is anticipated that any new net cost to
business from implementing the statutory system would be minimal or zero. These included
potential familiarisation costs for doctors employed in both the NHS and private sector to
understand new procedures and establish contacts with new medical examiners, although those
costs ought to be mitigated to a large extent as DHSC is not proposing significant changes to the
MCCD itself.
In this previous version of this impact assessment and the 2018 impact assessment, we also
outlined that there may have been the potential for increased costs to funeral directors who must
collect cremation fees from the bereaved on the behalf of doctors. However, central funding for the
ME system removes this risk entirely. Minimal or zero costs to businesses are also expected in
Wales from policy option 3.
DHSC have further confidence that there will be no additional costs to businesses stemming from
this policy, as a non-statutory national system of medical examiner offices has been established
from 2019 within the NHS in England. This means that the ME system has moved away from the
‘Do Nothing’ option which was the baseline for the 2018 IA. Importantly, to date, all NHS Trusts
which require a medical examiner office under a statutory system have done so on a non-statutory
basis. Hence moving from the non-statutory system to the statutory system is unlikely to result in
further ME offices being established, thus resulting in minimal set up costs.
Updated Costs
The June 2018 IA for England outlines that the set up and running costs of the statutory system will
not fall on private businesses. The non-statutory arrangements currently in place in trusts reflect
the arrangements that would be in place in the statutory scheme.
For clarity, the June 2018 IA gave an estimated running cost (to DHSC and the public) of approx.
£34-£41 million per annum
14
in England (using 2018 prices). Based on more representative and

29
recent cost data, NHSE estimate that the cost of the medical examiner system will be £54m for
2022/23 (in 2022 prices), which is a public sector cost. The benefits of the scheme are not
monetised and remain the same as outlined in the 2018 IA (for England). These costs and benefits
are for the statutory system as a whole and relate to the set of primary and secondary legislation
which underpins it. Economic costs for Wales are outlined in the Welsh IA and have recently been
revised with more up-to-date data with a best estimate of £3.1 million per annum.
In summary, DHSC considers that our previous assessment of the costs and benefits of the
medical examiner system published in the 2018 English impact assessment, and Welsh impact
assessment, remain sufficiently accurate for the purposes of estimating the costs and benefits of
establishing a statutory scheme. As such, readers are referred to the previous impact assessments
for the purpose of understanding the impact of the statutory scheme once it is commenced.
4. Hospital food standards
Policy summary
The provision in the Act introduces a regulation making power afforded to the Secretary of State for
Health and Social Care to adopt secondary legislation mandating and enforcing NHS Hospital
Food Standards across the NHS in England. If introduced, these would be enforced by the Care
Quality Commission.
The Independent Review of NHS Hospital Food
13
made eight recommendations to improve the
standard of food across the NHS estate. NHSEI are leading on implementing recommendations
from the Food Review and are establishing an expert group to facilitate this. The new power in the
Act will allow the Secretary of State to make Food Standards mandatory. Granting this power will
allow the Government to deliver swiftly on its ambition to improve hospital food by putting it on a
statutory footing and ensuring its delivery via enforcement by the CQC and will send a clear
message that improving hospital food is a priority for the Government.
Rationale for intervention
The Food Review published on the 26th of October 2020 highlights that there is clear scope for
improvement in the provision of food in the NHS estate. Overall, patients in NHS hospitals are
satisfied with the quality of hospital food, with a 2019 survey
14
finding that 22% of NHS hospital
patients rated the food they received as very good, whilst 36% rated it as good. However, the
report also states that the public perception of hospital food is poor, and that 39% of NHS staff felt
that food and catering facilities offered in their workplaces were poor. The Food Review also
outlines detailed justification for the improvement of hospital foods. These include the role of food
and nutrition in the treatment of patients (“food as medicine”), the importance of food safety, and
the role of the food supply chain with respect to sustainability.
The Food Review recommended for improved NHS food and drink standards for patients, staff,
and visitors to be put on a statutory footing. Granting the Secretary of State for Health and Social
Care powers to adopt secondary legislation and provide for a mechanism to enforce failure to meet
13
Department of Health and Social Care, “Report of the Independent Review of NHS Hospital Food.”,
October 2020. [Online] Available:
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/929234/in
dependent-review-of-nhs-hospital-food-report.pdf
14
Care Quality Commission, “Adult inpatient survey 2019,” July 2020. [Online]. Available:
https://www.cqc.org.uk/publications/surveys/adult-inpatient-survey-2019
30
these required standards will allow Ministers to deliver swiftly on the Government’s ambitions to
improve hospital food standards via secondary legislation.
Other policy options considered
Option 0 - Business as usual (Do nothing)
Under the business as usual (do nothing) option, this statutory instrument would not be introduced.
At present, without the proposed reform, changes to food standards would be challenging to
implement and measure. Monitoring methods for food targets are already in place but do not go far
enough to ensure the highest quality of food standards are carried out and prioritised by all
organisations. Given the recommendations from the Food Review, this was not deemed a viable
option as it would restrict the extent of reform that can be made.
Costs
The provision brings forward a statutory instrument which in itself has no direct impact, An
Equivalent Annual Net Direct Cost to Business (EANDCB) has not been estimated, as any costs
would not be incurred until these powers are exercised. However, it is acknowledged that this
primary legislation does grant powers, if exercised, which could entail costs to businesses who
provide catering services in hospitals. This may result in increased costs to adhere to higher food
standards, through for example, requirements to serve more fresh produce in hospital meals. The
true extent of these costs or savings cannot be estimated until the use of the power is finalised. In
particular, there are additional steps to be completed, such as Public Consultation, which will
inform the food standards which may be put in place. A full examination of costs to businesses will
be completed at that stage.
Similarly, a Small and Micro Business Assessment (SaMBA) which quantifies the costs to SMBs is
not possible at this stage. Current high-operable data suggests that 659 contracts across 48
companies associated with the 216 NHS Trusts would be impacted by legislation; however, at this
point that impact cannot be clearly defined. An impact assessment would be produced before the
regulation making power is exercised. Any secondary legislation would take into account views and
needs of small and micro businesses, and potentially nutritional standards would not impact any
organisation, small, medium, or large. Any exemption for small or micro businesses from the
regulations would be investigated at the time of secondary legislation being developed, and at that
point a SaMBA will be produced.
If the Secretary of State uses the powers granted to him as part of the statutory instrument, then
there may be costs for trusts to train staff or to buy equipment to meet the food standards. These
costs will be considered as part of an impact assessment if these enabling powers are exercised
and secondary legislation is enacted.
Benefits
The benefits of this policy are indirect and will depend upon how the enabling powers are
exercised. Granting the Secretary of State for Health and Social Care powers to adopt secondary
legislation that will implement the national standards for food across the NHS will enable the
government to move more swiftly in acting and ensuring delivery of the recommendations outlined
in the Food Review.
Risks
It should be noted that this provision introduces enabling powers with a duty to consult
stakeholders prior to introducing legislation and does not in itself contain substantive provisions as
the standards will be detailed in secondary legislation made under the power. It is therefore difficult
to assess with any certainty what the impact of the measures will be. Any policy that will be
implemented using the regulation-making powers provided in future will be required to develop an

31
impact assessment for the settled policy at the point at which the government is ready to legislate.
Important note
Any future regulatory policies that intend to introduce secondary legislation via the enabling
provision in the Act will need to be consulted upon and will need to be accompanied by an impact
assessment.
5. Water fluoridation
Policy Summary
The provisions in the Act, once commenced, will transfer the current powers and duties of local
authorities (LAs) in respect of water fluoridation to the Secretary of State, including the power to
propose new, variations or terminations to fluoridation schemes, the responsibility for ensuring that
schemes are operable and efficient and the duty to consult.
The Secretary of State was already legally responsible for capital and revenue funding for water
fluoridation schemes, and exercised the statutory power to require LAs to meet (i.e. reimburse) the
revenue costs. Following the commencement of the clauses in the Act, revenue costs will be met
by central Government. The Act introduces future flexibility to seek contributions in respect of all
water fluoridation costs, though it is not currently intended to exercise this power. Arrangements
will continue to be held and managed by central Government. Water companies are funded under
these arrangements to install and maintain fluoridation schemes and their role is unchanged. They
are refunded for the revenue and capital costs they incur. Any proposed changes to the funding
arrangements will be subject to regulations, consultation, engagement, and assessment of
impacts.
There will be a requirement for the Secretary of State to consult on water fluoridation schemes,
except in certain circumstances, and the Secretary of State will continue to be responsible, as now,
for directly entering into arrangements with water undertakers. Central Government will also
continue to be responsible for managing contracts with water undertakers and for monitoring the
effects of water fluoridation schemes on the health of people living in the areas covered by these
arrangements, and to produce reports at no greater than four-yearly intervals will remain. This will
include the monitoring of health outcomes. There will be no significant operational changes to
existing schemes.
Rationale for intervention
Fluoride is widely agreed to be a clinically effective intervention for oral health
15
. Around 70% of
five-year-old children live in areas with naturally low levels of fluoride. If they were to drink
fluoridated water, then there would be between 17-28% fewer children with tooth decay. Research
has shown that drinking fluoridated water benefits children and adults so there could be a
significant public health benefit.
Fluoride mitigates the impact of poor diet and/or poor oral hygiene. It can be applied directly to
teeth via toothpaste (most toothpastes now contain fluoride) and mouthwash, or professionally
applied through varnishes and gels or added to the water supply (water fluoridation). All methods
of delivery are effective, but water fluoridation has the strong advantage that no action or change in
15
Public Health England, “Water Fluoridation: Health Monitoring Report for England 2018”, 2018. [Online].
Available:
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/692754/W
ater_Fluoridation_Health_monitoring_report_for_England_2018_final.pdf
32
behaviour is required by the individual or dentist and it has a greater impact on reducing oral health
inequalities.
Fluoride is naturally present in drinking water, but apart from a few areas in England, is at too low a
level to be effective against tooth decay. A community water fluoridation scheme involves raising
the level of fluoride in water to 1mg of fluoride per litre of water (mg/L), the level accepted in the UK
to be most effective in reducing tooth decay whilst minimising unwanted effects.
Current community water fluoridation schemes in England serve around 6 million people, resident
in 28 upper tier and unitary local authorities, including large areas of the North East and the
Midlands. This means about 10% of the population in England currently receive fluoridated water.
Whilst fluoride is naturally present in most water supplies, it is only present at an optimum level for
dental health in a small number of areas such as Hartlepool and Braintree.
From 2013 LAs were responsible for proposing new fluoridation schemes, and for variations and
terminations to existing schemes. However, the Secretary of State had a significant role in the
process, in confirming that the proposals were operable and efficient and responsibility for entering
into any resulting arrangements with the water companies. LAs also had responsibility for carrying
out and funding the actions needed to take forward a proposal. This included feasibility studies and
public consultations. However, in the last 7 years no new schemes were successfully implemented.
The transfer of responsibility for proposing schemes to the Secretary of State recognised that LAs
faced a number of specific barriers to proposing and leading discussion on new schemes:
• Water flows usually cross LA boundaries and all LAs affected must be invited to take part in
the decision-making process. Everyone who is affected by the proposal must be consulted-
even if their LA chooses not to take part in the decision-making process. Where multiple
LAs are involved in the process this adds procedural complexity and the challenge of
establishing consensus across multiple organisations.
• The structure of LAs can add further time and complexity. LAs coordinating the process
across more than one decision-making body face multiple committee stages which creates
problems of coordination, particularly if the LAs have different election cycles.
• Cost is another barrier. For an individual LA the cost of feasibility studies and public
consultations may be a significant deterrent particularly as LAs have no guarantees that the
proposal will be agreed and result in an operational scheme.
•
Overall, the existing framework had multiple complex processes built in which, taken
together, presented a significant barrier for LAs.
Other policy options considered
The prevention green paper Advancing our Health: Prevention in the 2020s, published in July
2019, set out the Government’s initial intention to explore the funding barriers to water fluoridation
expansion. Transferring LAs’ existing responsibilities to the centre was decided following
consideration by Ministers as the only way to effectively remove the entirety of barriers facing LAs.
The responsibilities of all current parties with a role in water fluoridation are set out in the WIA 1991
and these powers and duties can only be altered by primary legislation. The existing legislation
required amendment to allow:
• The Secretary of State to directly initiate, vary or terminate water fluoridation schemes.
• LAs’ powers to be removed.
• A duty for the Secretary of State to consult.
• Transitional arrangements to require water undertakers currently providing existing
fluoridation schemes to transition onto new contracts to enable them to comply with the
new legislative regime.
• To enable possible future cost sharing between the Secretary of State and other entities.

33
Costs
The costs of existing schemes will not be affected by the provisions. As part of the reforms,
responsibility for revenue costs (estimated to be £3.7m for 2021/22) will pass from LAs to DHSC.
This means a transfer of costs for existing schemes but no overall increase in costs. Capital costs
are already borne by central government (DHSC). The changes include flexibility to allow for future
cost sharing with water companies and/or public sector bodies, however, any firm proposals will be
subject to regulations, engagement, consultation, and assessment of impacts.
The Water Industry Act 1991 places a duty on the Secretary of State for Health to reimburse water
companies any costs incurred that are associated with water fluoridation. Therefore, the transfer of
responsibilities for existing water fluoridation schemes to the Secretary of State has no direct costs
for water companies, and so the Equivalent Annual Net Direct Cost to Business (EANDCB) is zero
for this particular aspect of the policy. However, the provisions allow for possible future cost
sharing with water companies which if exercised, may introduce costs on water companies to
fluoridate water. It is not possible to estimate what this cost would be until details of how the power
will be exercised are known. Any proposal will be subject to an impact assessment and an
EANDCB will be calculated at that stage.
There are 16 statutory water undertakers (i.e. regional monopolies) in England that provide either
water services, or both water and sewerage services
16
. There are a number of other regulated
companies, including: local companies providing either water or sewerage services or both; water
supply and sewerage licensees that offer water and sewerage retail services to business
customers; and infrastructure providers delivering large infrastructure projects. However, there are
currently no small or micro businesses which would be responsible now or in the future for
fluoridating water supplies. There is no current intention to exempt any small or micro businesses
who may provide water fluoridation from possible future cost sharing. However, were the enabling
powers to be exercised, any secondary legislation would take into account views and needs of
small and micro businesses, and any exemption for small or micro businesses from the regulations
would be investigated at the time of secondary legislation being developed. At that stage a SaMBA
would be produced, and as mentioned at the end of this section, DHSC will continue to engage, as
appropriate, with DEFRA and water companies as any proposals develop.
The transfer of responsibilities is intended to streamline and make the process of proposing and
consulting on new schemes less burdensome. Decisions on any future schemes will be taken in
the context of wider financial decisions on health and care, including through Spending Reviews.
Benefits
The Act, when commenced, will transfer the power to propose new schemes, variations or
terminations to central government and as part of this LAs will no longer be required to undertake
consultations or feasibility studies which may generate some savings in terms of reduced
resourcing costs. This is in order to reduce the burdens on local authorities and allow for the
process to be streamlined. The legislation has preserved the duty to consult on any proposed new
schemes or changes to schemes, except in certain circumstances.
Risks and mitigations
There is a potential risk that, in transferring these powers to the centre, the benefits of greater
autonomy are forgone. However, the barriers LAs face in effectively proposing new fluoridation
schemes, or in varying or terminating existing ones mean that no new schemes have been
established in the past seven years. Transferring these powers to the centre aims to break down
these barriers to implementing fluoridation schemes which will have positive public health benefits.
16
Drinking water 2020, The Chief Inspector’s report for drinking water in England, 2020, p13. [Online].
Available from: Drinking water 2020 - The Chief Inspector’s report for drinking water in England (dwi.gov.uk)
and confirmed by internal analysis from PHE.

34
Important note
The changes remove responsibilities and costs from LAs and are subject to DLUHCs reverse
burdens process.
DEFRA is the other government department with a direct interest (through the role of water
companies). The department has engaged the water companies and DEFRA on the provisions and
they were content that the provisions do not substantively affect the current relationship with the
water companies and the Secretary of State for Health and Social Care. However, any decisions to
alter the duty to reimburse water companies will alter the relationship between water companies
and the Secretary of State and will be subject to regulations, engagement, consultation and
assessment of impacts.
6. Food information for consumers: power to amend retained EU
Law
Policy summary
The retained Regulation (EU) No. 1169/2011 of the European Parliament and of the Council on the
provision of food information to consumers (‘Regulation (EU) No. 1169/2011’) was incorporated
into domestic law, carried forward and modified according to the EU (Withdrawal) Act 2018. It sets
out requirements on the provision of food information to consumers which includes the labelling of
prepacked food and drink in the UK. Due to its status as retained direct principal EU legislation,
primary legislation is often required to amend or otherwise, modify the provisions contained within
Regulation (EU) No. 1169/2011.
The Act confers a power on the Secretary of State and Ministers of Scotland and Wales to amend
and modify by regulations parts of the retained direct principal EU legislation, set out in Regulation
(EU) No. 1169/2011. The intention of the power is to broaden the reach for any modifications to
Regulation (EU) No. 1169/2011 to those matters that fall within the scope of section 16 (1) (e) of
the Food Safety Act 1990. Regulations made under the new power are subject to the affirmative
process.
The new power to amend retained direct principal EU legislation, Regulation (EU) No. 1169/2011
will enable the Secretary of State and Ministers in Scotland and Wales to implement new policies
for food information by amending food labelling requirements so they meet the needs of their
respective nations and territories. For example, the Government’s obesity strategy: ‘Tackling
obesity: empowering adults and children to live healthier lives’
17
included a commitment to consult
on front of pack nutrition labelling and whether to mandate alcohol calorie labelling to help support
consumers make healthier choices. If consultations indicate that changes to food and drink
labelling and/or presentation is required, this provision will enable Ministers to introduce key
policies, whilst retaining a level of scrutiny on any proposed changes. It will also support the
alignment of labelling policies across the three nations, by allowing each nation to make changes
applicable to their relevant territories.
Other policy options considered
Option 0 - Business as usual (Do nothing)
17
DHSC, ‘Tackling obesity government strategy’, 2020. [Online]. Available from:
https://www.gov.uk/government/publications/tackling-obesity-government-strategy/tackling-obesity-
empowering-adults-and-children-to-live-healthier-lives

35
Not taking forward these powers would restrict the extent that reform can be made to labelling and
food information if consultations indicate that changes to food and drink labelling and/or
presentation is required.
Costs
This Act provides enabling powers; no immediate impacts are expected as the exercise of powers
in the provisions are subject to any secondary legislation which may or may not be implemented in
future. An impact assessment would be undertaken prior to any regulations under the new power
being made.
Costs of any future secondary legislation using this power will likely be the costs associated with
businesses having to implement changes to labelling requirements, as well as burden placed on
local authorities and the justice system for enforcing it. DHSC will look to align its work on labelling
with other government departments, namely DEFRA, where possible.
An EANDCB figure has not been provided as the impact of the power will depend upon whether
and how the powers are exercised. Any exercising of this power is likely to affect a large number of
manufacturers and / or retailers. Depending on how the power is used, there may be familiarisation
costs to manufacturers who need to put the new labelling practices into place, as well as greater
administrative costs as companies are required to provide more information on their products. This
may disproportionately affect smaller businesses where administration costs may account for a
larger proportion of their overheads.
DEFRA report that “There were approximately 7,130 micro, small and medium sized enterprises
(SMEs) in the food and drink sector with turnover of around £21 billion and 135,000 employees in
2019. In the food sector (excluding beverages) SMEs accounted for 79% of businesses, 27% of
employment and 17% of turnover.”
18
This suggests that small and micro businesses, along with
medium sized enterprises, account for a significant proportion of food and drink market share. At
this stage it is not possible to state whether and the extent to which small and micro businesses
will be impacted by any secondary legislation introduced using these powers, as these proposals
have not yet been brought forward or finalised. Any secondary legislation would take into account
views and needs of small and micro businesses, and by extension any exemption for small or
micro businesses from the regulations would be investigated at the time of secondary legislation
being developed. At the secondary legislation stage an EANDCB will be calculated as part of an
impact assessment, and, a SaMBA will be produced.
Costs may be further influenced by factors such as the extent of the labelling change (major or
minor) and length of implementation period. These costs are illustrative and would depend upon if
and how the enabling power is used. Any potential wider costs and benefits (such as those on the
environment) of future secondary legislation would be covered in an impact assessment.
Benefits
The Health and Care Act grants the flexibility to act on the evidence, once final policy proposals
have been fully consulted on. Scientific evidence and consumer needs continue to evolve, and due
to the limitations and restrictions set out in Regulation (EU) No. 1169/2011 DHSC did not have the
legislative ability to respond to those changes as and when they occur. Having left the European
Union, this provision in the Health and Care Act will allow us to continue to meet consumers’ needs
in the future.
When secondary legislation is enacted using the enabling powers in this Act then this will be done
in circumstances where the government considers that there is sufficient evidence to support such
measures as necessary to improve food information for consumers, potentially resulting in
18
DEFRA, Foot statistics in your pocket: Food chain, 2020. Food Statistics in your pocket: Food Chain -
GOV.UK (www.gov.uk)

36
consumers making better informed dietary choices. This may have the spill-over effect of
preventing ill health, such as illnesses linked to poor diet such as diabetes or coronary heath
failure
19
, and by extension reduce cost to the NHS and public services further down the line
20
. This
may not be applicable for all possible regulations introduced using the power, and because the
details of possible secondary legislation have not yet been finalised, it is not possible to give
greater detail on the possible benefits of the regulations which may be enacted using this enabling
power.
Risks
It should be noted that this Act contains enabling powers and does not contain substantive
provisions as the specific details of any legislative requirements will be set out in any secondary
legislation made under these powers. At this stage, it is therefore difficult to assess with any
certainty what the impact of the measures will be. For example, the government has gathered
views and evidence on its current multiple traffic light label, new international examples and
whether FOPNL should reflect updated dietary advice for free sugar and fibre, and is considering
the results. The government also intends to publish a consultation on whether to introduce calorie
labelling requirements on alcoholic drinks. Since these policies are at consultation stage it is not
possible to assess the potential impacts until the evidence has been considered and any policy
options deemed necessary are finalised. At the point the government is ready to legislate using the
regulation-making powers provided in this provision, industry will be consulted, and a detailed
impact assessment will be produced for the settled proposals.
Important note
Any future regulatory policies that intend to introduce secondary legislation via the enabling
provision in the Act, will need to be consulted upon and will need to be accompanied by an impact
assessment. Since changes to Regulation (EU) No. 1169/2011 will require an affirmative process,
any policies using this power will be scrutinised and approved by both Houses of Parliament.
7. Reciprocal healthcare arrangements with Rest of World
countries
Policy summary
The Act will provide the Secretary of State with powers to make secondary legislation to fund and
implement reciprocal healthcare arrangements with countries around the world. Until these
provisions come into force the UK is limited to implementing comprehensive reciprocal healthcare
arrangements with the EEA and Switzerland.
The UK has multiple reciprocal healthcare agreements outside of the EEA with countries such as
Australia and New Zealand.
However, without financial reimbursement or data sharing
mechanisms, these agreements are limited in scope and reach and take the form of simple equal
treatment or waiver agreements.
The new powers will enable the government to strengthen
existing agreements and to implement new comprehensive reciprocal healthcare agreements with
Rest of World countries.
19
NHS, Obesity (web page). Available from: https://www.nhs.uk/conditions/obesity/
20
DHSC, Advancing our health: prevention in the 2020s – consultation document, (2019). [Online]. Available
from:
https://www.gov.uk/government/consultations/advancing-our-health-prevention-in-the-
2020s/advancing-our-health-prevention-in-the-2020s-consultation-document
37
These provisions in the Act have no immediate impacts as the exercise of powers are subject to
the negotiation of future reciprocal healthcare arrangements with Rest of World countries.
Rationale for intervention
Establishing reciprocal healthcare agreements with Rest of World countries is in line with the
government’s Global Britain strategy, looking to invest and strengthen the UK’s relationships with
countries across the globe and strengthen international healthcare cooperation.
Comprehensive reciprocal healthcare agreements with Rest of World countries could offer benefits
for UK residents when they travel abroad for tourism or short-term business purposes. Such
agreements make healthcare in other countries more accessible and can support individuals with
long-term conditions who usually pay higher travel insurance premia or face difficulties in getting
comprehensive insurance cover. They can also foster closer collaboration on healthcare with our
international partners, supporting improved health outcomes for all.
Other policy options considered
Option 0 - Business as usual (Do nothing)
If the Secretary of State does not use the regulation making powers the UK will only be able to
implement comprehensive reciprocal healthcare arrangements with the EEA and Switzerland. This
would restrict the government’s ability to strengthen existing agreements and to implement new
comprehensive reciprocal healthcare agreements with Rest of World countries. Agreements with
other countries would be limited to either: i) waiver agreements, where any fees associated with
accessing the healthcare system are waived at the point of access and there is no provision made
for the costs incurred to be reimbursed or ii) an agreement for equal treatment, whereby visitors to
a country have the same access to healthcare as the residents of that country, facing the same
fees and/or exemptions as regular users of the system.
Costs
As this provision introduces enabling powers, there are no costs associated with its introduction.
Any policy using the regulation-making powers provided as well as future agreements which will be
implemented under the proposed powers will be subject to a new impact assessment as
appropriate.
While the expected costs of implementing new Rest of World reciprocal healthcare agreements are
currently unknown, the following types of costs could occur depending on the content of future
agreements:
• Costs to the UK government to reimburse other countries’ governments for healthcare
provided to UK residents while travelling abroad and to administer the system.
• Costs to the NHS in terms of forgone income where the agreements result in lower tariff
charges in England for Rest of World residents receiving NHS treatment than currently.
There may also be increased demand for NHS services if visitors are entitled to treatment
when visiting the UK. This is because the likelihood may increase of visitors using the NHS
for needs-arising treatment during their visit compared to if they were directly charged for
treatment, although the impact is expected to be minimal.
• Until the details of the reciprocal healthcare agreements are finalised, it is not possible to
produce an Equivalent Annual Net Direct Cost to Business figure, nor is it possible to
identify whether small or micro businesses would be disproportionately affected. An
EANDCB and SaMBA will be completed when reciprocal healthcare arrangements are
implemented via secondary legislation.
• Were a reciprocal healthcare arrangement to be introduced, the types of businesses
impacted may be travel insurance companies as lower premiums and reduced income from
excess payments may result in forgone profit for insurance companies. Furthermore,
businesses who have staff that travel abroad may be affected, although the impact on
these firms is expected to be small as savings stem from cheaper travel insurance premia.

38
• There is the potential for reciprocal healthcare agreements to affect trade in goods and
services. The nature of these impacts will not be fully understood until details of the
reciprocal healthcare agreements are finalised. DHSC will engage with the Department for
International Trade when reciprocal healthcare arrangements are being agreed with rest of
world countries to fully examine these impacts in line with Better Regulation guidance.
Benefits
As noted above, as an enabling measure, there are no direct benefits arising from the power
coming into force. Any benefits associated with the power being used will be subject to future
analysis. As an indication, the types of benefits that could arise from reciprocal healthcare
agreements are:
• Cost recovery rates for the NHS may be improved due to the introduction of a
reimbursement mechanism which means that healthcare costs could be covered by
governments instead of direct charging. The UK government will therefore receive income
for the treatment of residents from other countries (though this may be offset against the
NHS costs of providing the treatment depending on the agreements).
• Incorporating reimbursement/data exchange mechanisms to facilitate reimbursement into
new or updated existing agreements will allow for improved monitoring and evaluation of
the cost-effectiveness of these agreements over time. Existing waiver agreements do not
routinely include accurate data exchange, limiting our ability to evaluate the effectiveness of
these arrangements.
• There may also be operational savings to the NHS from reduced administration costs
required to administer the current system of directly charging patients.
• Savings to individuals, including avoided costs of paying directly for healthcare treatment
abroad, lower travel insurance premiums and increased ease and convenience of travel.
• If agreements cover treatment for certain long-term conditions, it will be easier for these
groups of people to travel, improving equality of opportunity. Treatments such as kidney
dialysis, oxygen and antenatal care have been covered by the UK’s reciprocal agreement
with the EU.
• UK businesses, charities and the UK government may benefit from reduced costs when
providing travel insurance for business trips due to the likelihood that insurance premiums
might be reduced.
• Revisiting existing agreements would also support broader healthcare cooperation and
diplomacy, especially with our closest allies (e.g. British Overseas Territories, Crown
Dependencies, Commonwealth countries).
• Widening the scope of agreements could encompass other areas of strategic interest,
including on wider healthcare cooperation. This could build on existing relationships and
dialogues, including on COVID-19.
Risks
The relevant provisions in the Act are an enabling measure. They do not contain substantive
provisions in relation to the content of future reciprocal healthcare agreements with Rest of World
countries which will be subject to negotiations. It is therefore currently difficult to assess with any
certainty what the impact of the measures will be.
Important note
An impact assessment will be conducted for any new reciprocal healthcare agreement with Rest of
World countries.
Ahead of future agreements there would also be extensive engagement with stakeholders as
appropriate on the feasibility and impact of any proposed arrangements.
39
8. Powers allowing further products to be centrally stocked and
supplied free of charge to community pharmacies without the
need to reimburse them under the standard NHS
arrangements
Policy summary
This amendment adds to Section 164 of the NHS Act 2006 and Section 88 of the NHS (Wales) Act
2006 enabling regulations to be made that would allow no reimbursement under the standard NHS
arrangements for certain products centrally stocked and supplied free of charge to community
pharmacies. This adds to the pre-existing exemption introduced in 2017 for unlicensed medicines,
more commonly known as ‘specials’.
Rationale for Intervention
Without this provision, the only practical way to achieve this on a secure legal footing would be to
for the NHS to sell stock to wholesalers. These wholesalers would in turn sell to community
pharmacies who are reimbursed by the NHS. Such an approach would be inefficient compared to
supplying the products directly, as there is an additional step in the supply chain which may entail
costs (such as resource costs to community pharmacies purchasing products from wholesalers).
Therefore, where it is deemed appropriate this amendment proposes to allow further exemptions
from the obligation to reimburse pharmacies for products to centrally stocked and supplied free of
charge to community pharmacies. This provision is enabling, and the exact circumstances of when
the powers may be used (if any) have not been finalised.
Other policy options considered
Option 0 - Business as usual (Do nothing)
Not taking forward these powers would restrict the extent that reform can be made if it is identified
that changes to the provision of certain medical products is required.
Costs
This provision provides enabling powers; no immediate impacts are expected as the exercise of
powers are subject to any secondary legislation which may or may not be implemented in future.
Were the powers to be exercised, we anticipate that the main costs would fall on actors within the
medicines supply chain, for example, if the role of wholesalers in the supply chain changed from
the role of a purchaser to a purely logistical role. Due to the limited cases where these powers are
likely to be exercised, any potential costs are also likely to be limited.
To provide some additional context with a simplified model of the medicines supply chain,
medicines flow from manufacturers to pharmaceutical wholesalers to end points, such as hospitals
and pharmacies, who in turn supply to patients. There are approximately 1,500 registered
pharmaceutical wholesalers, but only a very small number are considered a ‘full line wholesaler’
(i.e. they sell nearly all medicines). There are also specialist wholesalers, for example those who
deal with hospital only medicines, unlicensed medicines, generics or appliances. Additionally,
some have a wholesaler dealer’s license because they are, for example, a pharmacy or a hospital
but do a small amount of wholesaling as part of their business. To give an idea of the size of the
market, in primary care alone, one of the main ’full-line’ wholesalers will normally make two
deliveries a day to each of the 11,200 community pharmacies, and the total spend in primary care
is approximately £5.2 billion of branded medicines, £3.2 billion of generic medicines and £1.2
billion of appliances annually. When the wholesalers sell to pharmacies, they sell at more than the
price they purchased them at to pay for the distribution and a profit margin. However, there are
some medicines (mainly brands) where the manufacturer sells directly to the pharmacy/hospital
and procure a logistic service from the pharmaceutical wholesalers.
40
This amendment is an addition to a pre-existing exemption with a legal precedent. The changes
are restricted to vaccinations and immunisations, medicinal products used for the prevention or
treatment of disease in a pandemic, and associated products such as diluents and syringes.
Although it is not possible to predict the future scenarios where we may consider this option, we do
not anticipate that this will be a significant number of products when compared to the total number
of products delivered by wholesalers (there are over 10,000 products listed in the NHS Business
Service Authority’s Dictionary of Medicines and Devices). The aim of the provision is not to
radically change NHS pharmaceutical service provision or payment mechanisms to community
pharmacies or the pharmaceutical supply chain that they use. The aim is to strengthen the legal
basis for scenarios when the usual supply routes are bypassed. As a result, the specific products
for which this power may be used have not been decided, meaning estimating a quantified impact
of the provision is not possible. For example, without knowing the particular medicine (or the level
of margin associated with it), it is not possible to state how many units of the product may be
provided centrally, and hence the scale of impacts on the wholesaler sector.
The impact on pharmaceutical wholesalers would vary according to the proposed scenario, in the
case of a new vaccine or treatment such as COVID vaccinations the stock, supply and associated
business is entirely new and therefore does not deprive or interfere with pre-existing market
conditions in the sector. In the case of an existing vaccine or treatment, depending on the exact
nature of the alternative arrangements, distributors will still be needed to deliver the product to
pharmacies. Pharmaceutical wholesalers may undertake this function although this will be under
the terms of contracts performing the role of logistics suppliers rather than purchasers. While
pharmaceutical wholesalers in general might not lose out, particular pharmaceutical wholesalers
may potentially lose out while others may benefit, dependant on which pharmaceutical wholesalers
might have bought the stock under the traditional model and which pharmaceutical wholesalers
perform the role of logistic suppliers for centrally secured stock.
To take a counterfactual example, one such approach could see DHSC putting out to tender to a
number of vaccine suppliers as a direct contract to supply seasonal influenza vaccines. The
vaccine would be paid for centrally by the government as a split agreement award with either a
small number of manufacturers or all manufacturers, according to the tendering process. The
annual NIC for flu vaccinations is valued at £27.9 million (2020/2021 flu season) wholesalers would
currently earn a margin out of this when they sell it to pharmacies. As outlined above, rather than
denying wholesalers the business this would probably be recalibrated as per the contracts agreed
with the government with the additional benefit to wholesalers of reduced risk due to wastage of
expired stock, particularly applicable to this example due to the seasonal nature of flu vaccinations.
Although it is not possible to determine at this stage the specific circumstances and therefore
number of products when this exemption may be used, it is anticipated that this would be small,
and a full impact assessment, including an assessment of costs to businesses (EANDCB) and
small and micro businesses (SaMBA), would be conducted to accompany each regulatory change
as they are required.
Benefits
Overall, it is anticipated that the proposed amendment would result in benefits to the NHS, as an
efficient alternative option to distribute vaccinations or treatments connected to a pandemic when
the usual supply routes need to be bypassed.
Risks
This amendment is enabling only and as such would require regulations to be made to allow any of
the proposed products to be supplied in this way. Therefore, aside from counterfactual examples it
is not possible to accurately predict what the impacts will be.

41
9. Increasing gamete and embryo storage limits to a maximum of
55 years for all
Policy summary
In the UK, laws and regulations of assisted reproduction, including fertility preservation, are
governed by the Human Fertilisation and Embryology Act 1990 (the 'HFE Act'). The HFE Act was
introduced in 1990 and was amended by the Human Fertilisation and Embryology Act 2008.
Today, family units and family formation are vastly different than they were in 2008. Many more
people are benefitting from assisted conception and fertility preservation; for example, same-sex
couples, individuals who are choosing to start their families later in life, those who become
prematurely infertile due to medical conditions, or less commonly, those who undergo gender re-
assignment.
Fertility preservation is achieved through the freezing and storage of gametes (eggs and sperm) or
embryos. The current maximum storage limit for frozen gametes and embryos is set out in the HFE
Act at 10 years. However, the Human Fertilisation and Embryology (Statutory Storage Period for
Embryos and Gametes) Regulations 2009 (the ‘2009 Regulations’) permit an extension of the
baseline storage limit for 10-year periods up to a maximum of 55 years for those who can
demonstrate a medical need.
Since the last review
of the legislation on statutory storage limits in 2008, when limitations to the
technology meant that egg freezing in particular was poor, cryopreservation techniques have
improved significantly. Today there are no scientific or technical barriers in place for the use of
frozen gametes and embryos. Frozen gametes and embryos lead to comparable IVF success rates
as using fresh gametes or embryos.
To seek views from the public about whether the current gamete and embryo storage limits are
appropriate, the Government launched a public consultation
on 11 February 2020. The
consultation ran for 12 weeks and closed on 5 May 2020.
The consultation explored whether the storage limit should be changed and if so to what, whether
there should be restrictions or additional conditions applied to gametes or embryos being stored
and if so, what these conditions should be, and whether gametes and embryos should have a
different limit placed on them.
The responses to the consultation
were overwhelmingly in favour of increasing the storage limits
for everyone, although there was not an obvious consensus on what the new limit should be.
Following careful consideration of the views expressed in the consultation, the Government
decided to offer 10-year renewable storage periods to everyone, regardless of medical need, up to
a maximum of 55 years. As part of this new settlement, there will be a statutory requirement for 10-
year review periods. Explicit written consent from the patient will be required to continue storage.
Introduction of the new maximum storage limit via the Health and Care Act 2022 will amend the
HFE Act to bring the legislation in line with both societal and technological advances.
Rationale for Intervention
Individuals across the UK are increasingly choosing to freeze and store their gametes (eggs and
sperm) and embryos. The HFE Act limits the storage of gametes and embryos to a maximum of 10
years.
The government has received representations from Parliamentarians and other interested parties
about the current provisions of the HFE Act concerning the storage of gametes and embryos and
whether they remain fit for purpose. The government recognises that current storage limits

42
people’s reproductive choices, and therefore launched a public consultation to seek views about
changing the statutory storage limits for gametes and embryos in 2020.
The results of the public consultation strongly supported increasing the limit from the current 10-
years, with 74% respondents agreeing that the limit should be increased. Many respondents
expressed a view that the current distinction between those who wish to freeze their gametes or
embryos for social vs medical purposes was unfair and should be ended.
In response to the COVID pandemic, the Department of Health and Social Care introduced the
Human Fertilisation and Embryology (Statutory Storage Period for Embryos and Gametes)
(Coronavirus) Regulations 2020 to allow individuals storing gametes or embryos an additional 2-
year extension so that they are not penalized, if their 10-year storage limit ran out during a time
when clinics could not treat patients. The effect of these regulations will end on the 30 June 2022.
Patients who are nearing the end of their storage limits would lose out on the offer of the new
scheme, if this legislative change is not introduced via the Health and Care Act.
Overall, without this intervention, the current unfair distinction would remain in place between
individuals who wish to freeze their gametes or embryos for social vs medical reasons.
Other policy options considered
Following response to the public consultation, the Government considered four options. These
were:
• Business as usual (do nothing)
• Increase the storage limit to 20 years
• Increase the storage limit to 55 years (with 10-year consent renewal periods)
• Increase the storage limit to the donor’s lifetime
Option 1: Business as usual (do nothing)
There was overwhelming support for an increase in the statutory limits of some kind. Leaving the
baseline at 10 years would perpetuate the unfairness, especially for women, for whom 10 years no
longer offers reasonable reproductive choice. It would also leave in place the current unfair
differentiation between individuals who wish to freeze their gametes or embryos for social vs
medical reasons. Finally, it would also be in direct opposition to the results of the public
consultation, where 74% of respondents expressed the view that the maximum storage limit should
be increased from the current 10 years.
Option 2: Increase the storage limit to 20 years
There was some support in the consultation for a new limit of 20 years, mostly from those with
ethical reservations. Such a limit would facilitate more reproductive choice for men and women
with non-medical needs but would still be a relatively short storage period in an individual’s fertility
lifetime and is less likely to be ‘futureproof’, as societal attitudes continue to move towards
accepting older parenthood. For those freezing their gametes at a very young age in order to
preserve fertility (e.g. children undergoing cancer treatment) and others with a medical need, a flat
rate of 20 years would not be sufficient and an extended offer to cover their medical needs, similar
to the existing 2009 Regulations of up to 55 years, would be required. The system would therefore
remain as complex to administer as now, and the current unfair differentiation between individuals
who wish to freeze their gametes or embryos for social vs medical reasons would remain in place.
Option 3: Increase the storage limit to 55 years (with 10-year consent renewal periods)
This option reflects the current maximum limit for prematurely infertile patients, which could then
apply the same limit to everyone, irrespective of medical need. This option would facilitate
reproductive choice for all patients equally and simplify administration. For extremely young

43
children storing their gametes (e.g. children undergoing cancer treatment), this limit would continue
to enable patients to use their gametes up to their 60s.
Option 4: Increase the storage limit to the donor’s lifetime
Some individuals and organisations supported a maximum storage limit of the donor’s lifetime. This
option could cause some disquiet for those with ethical issues around embryo freezing and
disposal. Without a maximum year limit, there may be an increased storage burden on clinics as
patients may defer indefinitely decisions to use or dispose of material.
Costs and Benefits
The 2021 Regulatory Triage Assessment provides rationale and analysis for the estimated impacts
of the increase in statutory storage limits for gametes and embryos from the current 10 years to 10-
year renewable periods up to a maximum of 55 years. Readers should refer to the 2021 RTA for
the full assessment of costs and benefits.
Direct and indirect impacts on business
Two main costs were identified to have a direct impact on businesses:
1. Familiarisation costs with the measure (which is estimated on a per-business basis)
2. Additional communication costs (the cost of contacting all patients, estimated on a per-
patient basis).
The breakdown of the calculation of familiarisation and additional communication costs are given in
the 2021 RTA, including a full list of indirect, negligible, or net zero costs (which include current
and additional storage costs which are passed on in full to patients).
As in the 2021 RTA under the costs to business paragraph, we anticipate that in the first-year total
direct costs to private business will be between £0.82 million and £1.64 million. This comprises of
£28,000 to £56,000 of one-off familiarisation costs, and £790,000 to £1.58 million in
communication and implementation costs per year. Familiarisation costs are estimated on a per
business basis. There are 113 licensed storage facilities in the UK, 44 of which are owned by 10
private clinic groups, leaving 69 as standalone. This means total familiarisation costs are
calculated on the basis on 79 businesses. Communication and implementation costs are
calculated on a per patient basis, at a cost of between £158 to £316 per patient based on medical
professionals’ time and administration costs.
Other direct and indirect costs and benefits
If current patients choose to continue to store their gametes or embryos beyond the initial ten-year
period, then the patient will be liable for the cost of storage (and not the business). The option of
destroying patient’s gametes or embryos still applies after the introduction of this policy. This
allows the estimated 11,000 to 20,000
21
patients who currently have gametes or embryos in
storage, and any future patients, the option to extend the storage period while retaining the option
to end the storage period at that point. Therefore, this does not constitute an additional cost to
patients unless they choose to opt-in and continue to store their gametes or embryos beyond the
ten-year period. This has the benefit of providing patients with a choice that was previously only
available to patients who could demonstrate a medical need. It is uncertain how many additional
samples the policy will lead to being stored, although these costs will be passed onto patients only
if they choose to opt-in.
21
Regulatory triage assessment: for increasing gamete and embryo storage limits to a maximum of 55 years
for all - GOV.UK (www.gov.uk)

44
Risks and Mitigations
There is a small risk that initial implementation of the new policy will be difficult for fertility clinics
and storage facilities, with the potential for mistakes. This is because patients will have to be
migrated from multiple old schemes to the new one, as their consents expire. To mitigate this,
there will be a two-year implementation period, from the 1 July 2022 until 30 June 2024, during
which time fertility clinics and storage facilities will be able to migrate patients storing gametes or
embryos onto the new scheme. The regulator, the Human Fertilisation and Embryology Authority,
will be supported by the DHSC during this period to ensure that the legislation is rapidly and
appropriately translated into guidance.
10. Commercial dealings in organs for transplantation: extra-
territorial offences
Policy summary
The UK has long been opposed to the commercialisation of organ transplantation and is a
signatory of the Council of Europe Convention Against Trafficking in Human Organs and supports
the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
The Human Tissue Act 2004 and the Human Tissue (Scotland) Act 2006 already prevent
commercial dealings in human material for transplantation in the UK, and overseas when a
substantial part of the offence takes place in the UK. The Modern Slavery Act 2015, the Human
Trafficking and Exploitation (Scotland) Act 2015, and the Human Trafficking and Exploitation
(Criminal Justice and Support for Victims) (Northern Ireland) Act 2015, also cover cases where a
UK national arranges or facilitates the travel of an organ donor who is exploited in any part of the
world.
There have been concerns that the existing legislation will not cover all scenarios in which a
person from this country might engage in the purchase and sale of organs, as it is possible that
there are commercial dealings in organs which involve UK nationals or residents but where no part
of that dealing takes place here and where the UK national is not involved in the travel of the
donor.
Left unchanged, there is a risk that people with a close connection to this country may be seen to
be complicit in perpetuating human rights abuses by purchasing organs overseas, and that the UK
may be seen to tolerate such abuses. Though no evidence that UK nationals seek to purchase
organs overseas at any scale is available, DHSC recognises the value of addressing the gap in the
existing legislation so as to deter any patients who might be considering purchasing an organ
abroad and to signal this country’s opposition to organ commercialisation. This provision should
lead to minimal adverse consequences to legitimate donors or recipients while also minimising
additional resource burdens.
This clause inserts new section 32A into the Human Tissue Act 2004 and new section 20A into the
Human Tissue (Scotland) Act 2006, to extend the prohibition of commercial dealings in human
material for transplantation to acts done outside of the UK when the material concerned is a human
organ and when the act is committed by a person who is habitually resident in England, Wales or
Scotland or is a UK national who is not habitually resident in Northern Ireland. It would make it an
offence, anywhere in the world, to pay for the supply of an organ, pay for an offer to supply an
organ, or seek somebody willing to supply an organ for payment. It would also make it an offence
to supply, or offer to supply, an organ for payment. This includes initiating or negotiating any
arrangement involving the giving of a reward for the supply of, or for an offer to supply, an organ,
and taking part in the management of a body that does so.

45
The Department is working with NHS Blood and Transplant to provide guidance to organ donation
and transplantation professionals on the change in the law and how to communicate it to their
patients.
Rationale for intervention
The new provisions should help to deter those who may be considering purchasing an organ
overseas. Once in place, transplant professionals will be able to inform patients most likely to
consider purchasing that transplant tourism is against the law in all circumstances.
We believe that transplant tourism among UK residents and nationals who need an organ donation
is likely to be rare. NHS Blood and Transplant collect data on patients who receive an organ
transplant overseas and return to the UK for follow up treatment. This data suggests there has
been a consistent downwards trend in the number of those who receive transplants abroad since
2006, where 72 patients received follow up treatment for organs received overseas, to seven
patients in 2019. This reduction is likely to reflect a better supply of organs for donation, through
improvements to technology, donor matching, and donation rates. During this period, the total
number of transplants in the UK rose from 3,087 in the 2006-07
22
reporting year to 4,761 in 2019-
20
23
. This indicates that not only has the absolute number of overseas donations to UK residents
decreased greatly, but the relative significance of the remaining overseas donations has
diminished.
This data may represent mostly legitimate donations, particularly given that a significant proportion
of UK residents have family overseas, though it is possible that some of the transplants recorded in
this data have been paid for. Additionally, there are no data recorded for patients going overseas
for a transplant and not subsequently returning.
Despite indications that the scale of transplant tourism is small, the new provisions represent
progress to the extent that, beyond the deterrence effect they create, they provide assurance to
those who are concerned about gaps in the existing legislation and signal to other countries that
people in this country cannot legally be complicit in the abuses associated with organ
commercialisation.
Other policy options considered
Option 0: Business as usual (do nothing)
Not taking forward this amendment would mean the existing legislative gaps would remain and
potential benefits of deterrent would not be realised.
Option 1: Ban the receipt of an organ overseas without consent or for payment
This option would have criminalised the receipt of an organ abroad without proof of consent or for
financial or comparable gains.
Though this option would have been effective in prohibiting transplant tourism, targeting the
recipient of an organ, rather than the trafficker or their customers, could have negative impacts on
vulnerable people. This proposal would have also captured patients taken overseas for a
transplant who are not made aware of how their organ was sourced – if it were sourced illicitly they
would have been targeted for prosecution when they return to the UK, despite having no
involvement in arranging the purchase or sale of the organs.
22
NHS, Blood and transplant activity in the UK, 2006-7. https://nhsbtdbe.blob.core.windows.net/umbraco-
assets-corp/1290/transplant_activity_uk_2006-2007.pdf
23
NHS, Organ donation and transplantation, Activity Report 2019/20:
https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/19481/activity-report-2019-2020.pdf
46
This option may have had a disproportionate impact on those who legitimately receive organs
overseas, who are more likely to be from minority ethnic backgrounds, as it would have been likely
to prevent some from seeking follow up treatment for fear of being treated like a criminal suspect.
Though this remains a concern for the preferred option, it mitigates this by not specifically targeting
recipients and not setting consent requirements.
Option 1 would have required officials to report on the state of every deemed consent system in
the world and on the public understanding of each system, every year. Though this would have
given the Department a nuanced picture of where donations made under deemed consent are
likely to be legitimate or illegitimate, this seems a disproportionate use of resources given the
comparatively small number of reported cases.
Finally, this option would have required specified persons to keep patient identifiable records for all
instances of UK citizens who have received transplant procedures performed outside the United
Kingdom and report these instances to NHS Blood and Transplant. This measure would have
improved record keeping. However, in doing so, it could have also place NHS staff, who are likely
to have been the specified persons obliged to keep patient-identifiable records, in an inappropriate
position. Their questioning of transplant patients could have led to a criminal prosecution, which we
did not want to burden professionals with as their primary focus should be on patient wellbeing.
Costs
We believe very few cases will be brought to trial, and therefore the costs of enforcement are likely
to be very low. The number of prosecutions under existing provisions supports this view. The
Crown Prosecution Service has confirmed that, from their records dating back to 2007, they have
made no prosecutions under the existing Section 32(1) of the Human Tissue Act, which covers the
most serious organ commercialisation offences and is being extended to actions made overseas
by this amendment. They made one prosecution under Section 32(2) of the Act which covers
publishing or distributing an advertisement related to the purchase or supply of an organ for
reward.
DHSC and NHS Blood and Transplant would need to invest resources into producing and
disseminating guidance for transplantation professionals. There are likely to be some
familiarisation costs for professionals who themselves may spend time understanding and
communicating the new legal implications of transplant tourism to their patients. Given the
relatively small number of people estimated to be receiving organs abroad and needing follow up
treatment in the NHS, we expect the cost of communication to be relatively low.
It is possible that in deterring patients from purchasing an organ for transplantation overseas we
would be incurring a health loss to those patients as they are likely to have to wait longer for a
legitimate organ transplant, which would also maintain a longer waiting time for others. However,
this potential cost is offset by the increased likelihood that an organ purchased overseas will not be
appropriate for the recipient, leading to worse health outcomes for the patient (including, possibly,
the need for another organ transplant) and associated costs for the health service on the patient’s
return to the UK. Again, these effects are likely to be extremely small or negligible, particularly as
only seven patients received follow up treatment for organs received overseas in 2019.
Benefits
It is difficult to assess the scale of the benefits, though it may deter some patients from seeking to
pay for an organ overseas and will signal the UK government’s opposition to human rights abuses.
If the legislation acts as a deterrent to illicit organ transplants abroad, there may also be benefits in
that the NHS may treat fewer individuals who receive inappropriate organs overseas. As with the
costs above, this benefit is likely to be extremely small or negligible, particularly as only seven
patients received follow up treatment for organs received overseas in 2019.

47
Risks and mitigations
There is a risk that efforts to communicate the change in legislation will inadvertently draw attention
to transplant tourism as an option for desperate patients who previously had not considered it. We
plan to mitigate this by directing information to transplant professionals, rather than patients
themselves, about the change in the law and guide them towards communicating this change with
those they deem already most at risk of seeking a transplant overseas.
This measure may cause concern among people who legitimately receive organs overseas, who
are also more likely to be from ethnic minority backgrounds, that they will be treated as a criminal
suspect. However, we believe our provision is the option which minimises these risks while
achieving the policy objectives.
There is a small risk that the exemption for UK nationals who are habitual residents of Northern
Ireland is exploited by patients from the rest of the UK who are considering purchasing an organ
abroad, as they may attempt to evade the law by moving to Northern Ireland before travelling
overseas to purchase their organ. Our assessment is that this is unlikely, especially as many
activities associated with transplant tourism are covered by existing legislation which does apply in
Northern Ireland.
11. Information about payments to persons in the healthcare
sector, enforcement and consent
Policy summary
Through the Health and Care Act of 2022, the Government has introduced enabling
powers which will allow the Secretary of State to make regulations requiring manufacturers
and commercial suppliers of healthcare products to make details of the payments or other
benefits they provide to healthcare providers public. Regulations will define the detailed
operation and scope of this new duty, ensuring that it is proportionate and effective in
reaching the main policy goals: to improve patient and public confidence in the healthcare
system and to strengthen patient safety by helping to ensure clinical decisions are made
independently of any conflicts of interest.
Rationale for intervention
There are professional, academic, and commercial links between healthcare providers and
manufacturers and commercial suppliers of healthcare products. However, it is not
mandatory for details of these to be made publicly available.
The Independent Medicines and Medical Devices Safety (IMMDS) Review
24
of July 2020
considered where improvements related to safety needed to be made by hearing
experiences of patients treated by three medical interventions (the hormone pregnancy
test Primodos, the anti-epileptic drug sodium valproate and pelvic mesh surgical implants).
The review made recommendations to improve the healthcare system and raised
concerns about perceived and real conflicts of interest in the provision of healthcare where
financial links and personal and/or professional interests are involved.
24
https://immdsreview.org.uk/index.html

48
For medicines manufacturers, a voluntary disclosure scheme already exists under the
Association of the British Pharmaceutical Industry’s (ABPI) Disclosure UK programme
25
,
where, in 2020, 126 ABPI members and non-members disclosed payments or transfers of
value made to healthcare professionals and organisations. Disclosure UK is seen as a
leading disclosure platform in Europe. However, there is currently no similar disclosure
scheme, voluntary or otherwise, for manufacturers of medical devices.
Even though ABPI’s voluntary scheme exists, only around 18%
26
of medicines
manufacturers disclose their payments via Disclosure UK. A further challenge has been
that clinicians’ consent to being named in relation to payments declared is sought
27
. To
incentivise reporting, the ABPI have been supporting their members to move towards a
‘legitimate interest’ model for the reporting year 2021.
The IMMDS Review found that several professional bodies and patient groups recognised
that “patients and the public were not satisfied with the lack of detail in voluntary
declarations”, arguing that “without legislative power, clarity about where responsibility lies,
and support of the profession” no major changes could occur. The Review therefore
suggested that the UK should enact an equivalent to the US Physician Payments
Sunshine Act, where manufacturers have a responsibility to disclose any payments or
transfers of value to physicians or teaching hospitals. In the US, this information is then
centrally published for the public to access.
The IMMDS review recommended “there should be mandatory reporting for
pharmaceutical and medical device industries of payments made to teaching hospitals,
research institutions and individual clinicians.” In its response to the Review, the
Government accepted this recommendation in principle and committed itself to exploring
options of making reporting mandatory through legislation.
The costs and benefits mentioned below are expected to arise as a result of the legislation
for mandatory reporting of industry payments. As the Health and Care Act of 2022 only
contains enabling powers, these will not arise until regulations are laid pursuant to the
powers in the Act.
Costs
The provision will generate direct ongoing administrative costs to in-scope businesses in
the form of the resource required to complete and publish payment declarations. In
addition, businesses and/or trade associations, depending on who sets up a payment
scheme or portal, will incur direct monitoring costs and initial set-up costs, including but not
limited to: web-page and/or web-portal set-up, software download, familiarisation, training,
and, potentially, recruitment and legal costs.
25
https://www.abpi.org.uk/reputation/disclosure-uk/
26
This is based on the 126 medicines manufacturers that disclosed payments as part of the Disclosure UK
scheme in 2020 out of the 695 manufacturers of basic pharmaceutical products and pharmaceutical
preparations as stated by the Office of National Statistics in 2021;
https://www.ons.gov.uk/businessindustryandtrade/business/activitysizeandlocation/datasets/ukbusinessactivi
tysizeandlocation
27
https://www.abpi.org.uk/media/news/2021/december/abpi-champions-use-of-legitimate-interests-to-boost-
transparency/

49
Where administrative costs of completing and publishing payment declarations will fall
completely on in-scope businesses, monitoring and initial set-up costs will fall more
significantly on the party setting up a scheme, whether that be in-scope businesses, trade
associations, or the government. Although, monitoring and initial set-up costs will be
significantly higher if multiple schemes are set-up under multiple in-scope businesses
and/or trade associations. Furthermore, costs to patients in accessing this information
would be significantly higher where information on payments is spread across multiple
locations through multiple schemes.
Furthermore, there may be indirect unquantified costs to manufacturers and healthcare
providers if the need to declare payments leads to a change in relationships. There may
be potential financial costs to healthcare providers currently receiving payments if the
number of payments being made by businesses and/or accepted by healthcare providers
falls. There may also be costs to businesses of reduced usage of their products and costs
to healthcare providers of fewer provisions for training, education and research. Although,
if these relationships inappropriately influence clinical decision-making, we anticipate that
there will be a net benefit to society. Furthermore, a change or reduction in these
relationships from less payments may discourage recruitment and retention in the
healthcare sector.
There may also be costs in the form of reduced foreign and domestic investment in the UK
Life Sciences Sector if regulations are too burdensome. This could have indirect costs to
in-scope businesses through less investment in production and expansion activities and
therefore further indirect costs to the healthcare sector receiving payments through less
provisions from in-scope businesses overall.
Benefits
The legislation to make reporting of industry payments mandatory will address the conflicts
of interest concerns mentioned in the IMMDS Review. The disclosure will increase the
transparency of the relationship between manufactures and suppliers of healthcare
products and a person or organisation who provides healthcare (e.g. a doctor or hospital)
and a person or organisation who carries out activities connected with health (i.e. one
degree away from that direct patient interface, e.g. charity arms of hospitals) .
With this will come increased accountability for healthcare professionals and
organisations, who are therefore likely to become more hesitant about accepting payments
that would have to be declared under the duty
28
. In turn, this could lead to a reduction in
any relationships that inappropriately influence clinical decision-making
29
or which could
28
Three experiments found that disclosures of conflicts of interest can deter acceptance of conflicts of
interest so there is nothing to disclose except for the absence of conflicts. Sah, S. and Loewenstein, G.
(2014). “Nothing to Declare: Mandatory and Voluntary Disclosure Leads Advisors to Avoid Conflicts of
Interest”, Psychological Science, 25(2), 575-84
29
Centers for Medicare & Medicaid Services, Department of Health and Human Services (2013). “Medicare,
Medicaid, Children’s Health Insurance Programs; transparency reports and reporting of physician ownership
or investment interests. Final rule”, Federal Register, 78(27): 9457-528

50
undermine public trust. This will positively impact patient safety by reducing the risk of
prescribing behaviour influenced by payments
30
’
31
.
Another benefit could be increased patient confidence and trust in the healthcare sector
and the treatment they receive as a result of the increased transparency of industry-doctor
relations. With increased confidence and knowledge
32
of these relations, patients will feel
more confident to make informed decisions on healthcare professionals, choice, and
treatment. Together, clinicians and patients may choose more cost-effective treatment
choices (see footnote 30), improving value for money for the NHS.
Increased transparency can also benefit industry as more information and a reduction of
use of payments may help drive competition or reduce the barriers to market entry,
increasing efficiency within the market. Increasing competition may also improve value for
money for the NHS as price competition leads to lower prices of healthcare products.
Although, we recognise that competition will have distributional impacts on industry.
The powers also allow companies to be exempt if they participate in an equivalent
scheme. There is currently insufficient data to say whether participation in these schemes
would result in a net cost or benefit compared to companies reporting separately.
Furthermore, this duty would support professional regulation bodies uphold their own
conflict of interest policies, providing a check and balance with declarations made by
professionals and NHS Trusts.
Risks and mitigations
There are risks to consider with the mandatory reporting of industry payments. There could
be a risk that in the change of some industry-doctor relationships, appropriate relationships
may change too due to fear of being wrongly perceived. This will result in a net loss to
society. Beyond that, this could have impacts on costs for the NHS if manufacturers for
generic medicines and medical devices increase product prices on the basis of a change
in relationships.
Furthermore, increasing competition could lead to an inflation of the value and number of
payments being made to healthcare providers.
Another risk is that some manufacturers may adapt how they deliver payments or change
their operating procedures to keep out of scope of the regulations, and so full disclosure
may not be achieved.
30
A study by ProPublica in 2016 concluded that doctors who received payments were two times as likely to
be high brand-name prescribers and two to three times as likely to have very high brand-name prescribing
rates than doctors who did not receive payments. Jones, R. G. and Ornstein, C. (2016). “Matching Industry
Payments to Medicare Prescribing Patterns: An Analysis”, ProPublica
31
A study on payments received by orthopaedic and non-orthopaedic surgeons found that mandatory
reporting in the US may be successfully mitigating some of the potential for undue influence in the healthcare
sector. The findings show that there has been an increase in the number of general payments received, but
a decrease in their median value, but there has been no change in research payments between 2014 and
2017. Rhee, T. G.; Stanic, T. and Ross, J. S. (2020). “Impact of US industry payment disclosure laws on
payments to surgeons: a natural experiment”, Research Integrity and Peer Review, 5(1)
32
A study found that there was a significant increase, of almost 10% points, in awareness that payments
information was publicly available in the US since the launch of the Open Payments programme as part of
the US Physician Sunshine Payments Act. Kanter, G. P.; Carpenter, D.; Lehmann, L. and Mello, M. M.
(2019). “Effect of the public disclosure of industry payments information on patients: results from a
population-based natural experiment”. BMJ Open, 9(2)

51
As this information on payments is published and the conflicts of interest awareness
increases, there is a political risk that public distrust could in fact increase
33
’
34
for the
healthcare sector. We will mitigate this by reinforcing existing norms set by professional
regulators that encourage discussions on conflicts of interest between the practitioner and
their patient. By normalising discussions about conflicts of interest, this will ensure that
doctor-patient relationships are strengthened and public trust in professionals is
maintained and improved.
There is a further cost risk that the government may need to spend additional money to
solve any limits to delivering the patient transparency objective if self-publication is the
preferred option over reporting into a central database. There may be practical problems
with disclosing payments where this information is not accessed or used by the public as
they may remain unaware of it
35
or it may be too complicated to access, in which case
patients may not be able to make well-informed choices.
Employing the same mitigation of encouraging disclosures of conflicts of interest in the
consultation room would help ensure that patients would have the option whilst not being
expected to access this information themselves. This would reduce overall reliance on
publication systems and instead embed disclosures in patient-practitioner conversations.
Finally, there are data limitations due to the lack of available data on the payments that are
currently made. We have considered data and studies from systems in the US and some
European countries.
12. Eradicating slavery and human trafficking in supply
chains
Proposal summary
Modern slavery encompasses the offences of slavery, servitude, forced and compulsory
labour and human trafficking. The NHS has a significant role to play in combatting it,
including through taking steps to ensure that NHS supply chains and business activities
are free from ethical and labour standards abuses. Government relies on its suppliers for
the delivery of many important public services, and we expect the highest standards of
business ethics from our suppliers and their agents.
33
A US study found that patients’ trust in their own physicians and medical profession decreased after the
release of the Open Payments programme. Kanter, G. P., Carpenter, D., Lehmann, L. S. and Mello, M. M.
(2019). “US Nationwide Disclosure of Industry Payments and Public Trust in Physicians”, JAMA Network
Open, 2(4)
34
A US study found that physicians receiving high payments were perceived to be less honest and less
committed to patients’ best interests than physicians who received no payments. However, there was no
effect on trust in the medical profession or pharmaceutical and medical device industry. Hwong, A. R., Sah,
S. and Lehmann, L. S. (2017). “The Effects of Public Disclosure of Industry Payments to Physicians on
Patient Trust: A Randomized Experiment”, Journal of General Internal Medicine, 32(11): 1186-92
35
A US study which used a survey found that since the launch of the Open Payments programme, only 13%
of respondents knew that information on payments was available and only 3% of respondents knew whether
their doctor had received payments. Reference the same as footnote 32.
52
The Health and Care Act 2022 (The Act) requires the Secretary of State to take two
actions towards the risk of Modern Slavery in NHS supply chains:
1. Review: Under Section 47, the Secretary of State must carry out a review into the
risk of slavery and human trafficking taking place in relation to people involved in
NHS supply chains.
2. Regulations: Under Section 81 of the Act (which inserts a new section 12ZC into
the National Health Service Act 2006), the Secretary of State must by regulations
make such provision as the Secretary of State thinks appropriate with a view to
eradicating the use in the health service in England of goods or services that are
tainted by slavery and human trafficking.
Review
The Secretary of State may determine which NHS supply chains to consider as part of the
review into the risk of slavery and human trafficking, or otherwise limit its scope – it does
not necessarily need to include all of the supply chains in the NHS.
The review must at least consider a significant proportion of NHS supply chains for cotton-
based products in relation to which companies formed under section 223 of the National
Health Service Act 2006 (taken as a whole) exercise functions. This is intended to ensure
that the review includes the supply chains of a significant proportion of cotton products
procured by the organisation NHS Supply Chain, which manages the sourcing, delivery
and supply of healthcare products, services and food for NHS trusts and healthcare
organisations across England and Wales.
The Secretary of State must publish and lay before Parliament a report on the outcome of
the review before the end of the period of 18 months beginning with the day on which this
section comes into force, which was 1st July 2022. This report must include the scope of
the review, and the methodology used in carrying it out. It must also include any views of
the Secretary of State as to steps that should be taken to mitigate the risk of slavery and
human trafficking taking place in relation to people involved in NHS supply chains.
Regulations
The regulations that must be made by the Secretary of State can, in particular, include
steps that the NHS should be taking to assess the level of risk associated with their supply
chains; provisions in relation to procurement processes, including the basis on which the
NHS should exclude suppliers from a tendering process; and measures that must be
included in contracts.
The regulations can apply to public bodies procuring goods or services for the health
service and will be in line with DHSC’s existing procurement approach as set out in the UK
Government Modern Slavery Statement, which includes a zero-tolerance approach to
modern slavery and a commitment to ensure that respect for human rights is built into all
contracts, self-assessments, audits, training and capacity-building opportunities. This
approach – and measures taken through regulations – will apply to public bodies
undertaking procurements of goods or services for use in the health service in England.
Existing modern slavery measures
NHSE also have a number of complementary internal policies that support the
commitment to eradicating Modern Slavery, such as:

53
• Freedom to Speak up Whistleblowing Policy
• Managing Safeguarding Allegations Policy
• Safeguarding Policy
• Procurement Policy
NHSE supports NHS organisations to use the UK Government’s Supplier Registration
Service to undertake both Modern Slavery and Labour Standard Assessments where
thorough risk assessments indicate if a category or country is high risk.
NHSE agreed their latest modern slavery and human trafficking statement in March 2022,
in which they committed to a number of policies to strengthen their approach, including in
onboarding suppliers and supply chain management: NHS England » NHS England
modern slavery and human trafficking statement
The costs and benefits below are provided for illustrative purposes.
Rationale for intervention
The UK is committed to taking steps to prevent and address human trafficking in
government procurement practices, recognising that the Government has significant
financial leverage and policy options at our disposal that can help to prevent human
trafficking in global supply chains.
As well as supporting the NHS to identify and mitigate risk with a view to resolving issues,
the intention is to send a signal to suppliers that the NHS will not tolerate human rights
abuses in its supply chains and to create a significant incentive for suppliers to review and
improve their practices, both through the review and the regulations.
Other policy options considered
Option 0: Business as usual (do nothing). Not taking forward this review and
making the regulations would restrict the extent to which reform can be made if
goods or services used within the NHS are tainted by slavery and human trafficking.
Costs
Procurement across the NHS takes place through a variety of routes with circa 80,000
suppliers. To illustrate the likely impact of this review and these regulations, see below a
few examples of routes through which procurement occurs.
i. NHS trusts, foundation trusts, and Clinical Commissioning Groups (CCGs)
procurement via compliant routes, including full procurements initiated by the trusts,
via frameworks established by NHSE, Crown Commercial Services (CCS), Shared
Business Services (SBS) and others. Integrated Care Boards will (from July 2022
replace CCGs and be able to enter into legal contracts for the provision of goods
and services.
ii. NHS England (including Commissioning Support Units (CSUs), and Commercial
Medicines Unit (CMU))

54
iii. NHS Supply Chain, or Supply Chain Coordinated Limited (SCCL)
For the purposes of this impact assessment, below is a summary of a proposed approach
to supply chain analysis in these example procurement routes that make up a large
majority of the total spend on goods and services by the NHS, to illustrate the impact of
the Act and the potential regulations made under it.
This impact assessment addresses NHSE and NHS Supply Chain spend. If further
investigation determines that changes are needed, further analysis and planning to include
all NHS spend, including trusts, will be completed at a later stage where appropriate, to
illustrate the impact on all areas of the system.
Overall, the total cost of these clauses is not yet know but has the potential to be
substantial.
Costs associated with the review clauses
NHS England
In response to the review requirement in the Health and Social Care Act 2022, NHS
England will nominate a National Director-Level sponsor to oversee the approach to the
mitigation of slavery and human trafficking in the supply chain. This work will require staff
time, some of which will be met within existing resources by re-allocating staff time (or re-
prioritisation) and, some additional resource which may require further spend, although
that is currently unknown.
NHSE will also lead the coordination of a focused and proportionate supply chain mapping
exercise (cost/resource associated with this exercise outlined below). This exercise will
include the supply chain mapping outlined below undertaken by NHS Supply Chain.
Additional resources will be required by NHS England and indicative costs are outlined
below:
• A team within responsible for programme management and assurance.
• Digital system to provide visibility of supply chain mapping, the cost of which will be
verified through procurement of services and dependent on the viability of the
Supplier Registration Service supply chain mapping tool. This is expected to be a
substantial investment which, in the first instance, will require a focused group of
suppliers to submit information (costs outlined below).
• Third-party auditing and auditing software in addition to existing processes to
assure compliance - costs to be verified through procurement of services. On-site
auditing typically costs £20k per audit. Without knowing the exact number of
suppliers identified for auditing as part of the analysis it is a challenge to calculate a
total cost for this activity.
• Legal and assurance (internal and external which will therefore incur additional
cost) support will be required.

55
• NHS wide procurement processes will need to build in the requirement for full
supply chain visibility and audit access to the NHS.
Existing staff time to update and assure Modern Slavery processes in the governance and
commercial process – costs and impact assessment to existing teams and processes will
need to be calculated in scoping phase of analysis.
Increased engagement from NHS England will be required to support NHS procurement
teams with guidance, training and support. This resource will require additional FTEs
although the exact number is currently unknown.
NHS Supply Chain
The scope of the review will be agreed with the Secretary of State, who will request NHSE
to assist in the carrying out of the review.
At a minimum, NHS Supply Chain, who are a company wholly owned by NHSE, will
complete an assessment of products identified as high risk by geography and / or material
assessment as part of the review, including cotton products. This will cover:
• country of origin information to understand the overarching risks
• establish the overall risk of labour standards abuse
• put in place a proportionate response to assess and establish the “on the ground”
reality
Any further scope is to be agreed with the Secretary of State.
NHS – trusts, foundation trusts, ICBs (from July 2022)
The outputs from the analysis outlined above will inform regulations, and guidance and
advice to the 219 trusts across England and the Integrated Care Boards (ICBs)
established by the Health and Care Act 2022.
Suppliers
Suppliers of goods and services to the NHS will incur costs from partaking in the review,
by submitting information to the digital system. The suppliers are typically private
businesses. The total additional cost of the review on suppliers will depend on the
complexity of supplier supply chains, and the level of maturity the suppliers are already
operating to uphold high ethical standards. The additional cost per supplier of taking part in
the review may also be influenced by the size of the supplier. There is an absence of data
and therefore a robust EANDCB figure cannot be provided. However, below sets out the
best possible evidence and illustrative costs on potential costs of the review clauses.
Number of suppliers affected
The number of suppliers affected is not currently known. The scope of the review will be
agreed in due course with the Secretary of State and included in the published report on
the outcome of the review. NHS England could take a risk-based approach to supply chain
mapping, focusing on detailed review of potential high-risk products. Were a risk-based

56
approach to be taken, the number of suppliers in scope will depend upon the supply chain
mapping and the number of suppliers judged as high risk, meaning it is currently uncertain
how many suppliers will be asked to partake.
There are circa. 80,000 suppliers to the NHS. A risk-based approach would reduce the
number of suppliers in scope because not all of these suppliers will be operating in high-
risk regions or providing products or services susceptible to slavery and human trafficking.
If all NHS suppliers were included within the scope of the review, for an illustrative
scenario, it may be determined that 10-15% of NHS suppliers would need to be mapped,
leading to 8,000-12,000 suppliers impacted.
The review will include the supply chains of a significant proportion of cotton products
procured by NHS Supply Chain. NHS Supply Chain is a wholly owned company of NHS
England, through which c.60% of NHS spend on products is procured via pre-agreed
frameworks.
Unit costs to suppliers
There may be costs to suppliers to submit information for the supply chain mapping digital
system being coordinated by NHSE. Although it is the intention to minimise the resource
burden, the information return is likely to require suppliers to undertake a supply chain
mapping, which mainly entails costs in terms of supplier staff time. Estimating a cost per
business is not possible owing to the absence of data and the lack of similar analyses. We
have however attempted to provide an illustrative example below.
Undertaking a supply chain mapping is a complex task with a range of potential costs. The
task for suppliers would be to trace their goods or services upstream through the
production steps in the supply chain. Taking the example of a cotton product, such as face
masks, the supply chain may have several tiers. An ultra-simplified supply chain is outlined
below:
Figure 1: Simplified cotton facemask supply chain
This is included to demonstrate potential complexity and length of supply chains, with the
reality that for each stage each supplier is likely to have multiple suppliers
Farming
Ginning
Spinning
Garment
manufacture
Dyeing
and
finishing
Weaving
Retail/suppliers
NHS
57
Figure 1 demonstrates that there may be several tiers in the production of cotton goods
before they arrive with suppliers (with one box representing one tier). These tiers may be
undertaken by different companies or subsidiaries and in different locations. The length of
the supply chain will also differ based on the nature and complexity of the goods produced,
which is particularly relevant as the NHS procures many hundreds of different goods and
services from private suppliers. There is no available data on the complexity and length of
supply chains for suppliers to the NHS, and much of this information will become apparent
once the review begins.
It is expected that the cost of the review for a particular supplier to be positively related to
the number of tiers being reviewed. The more tiers being reviewed (for example two tiers
below the supplier, as opposed to one), would increase the resource demands on the
supplier. Compared to a relatively ‘simple’ supply chain with very few manufacturing steps
and few input materials, a long, highly complex supply chain will take more resource to
fully understand. For example, a ‘complex’ supply chain may involve multiple input
materials and sources of labour, over a long time period and in multiple locations, making
the mapping more difficult. The depth of the supply chain mapping required (i.e. the
number of tiers being reviewed) is not currently known. Therefore, it is not possible to give
an indication of the potential complexity of the mapping task being set on suppliers.
Potential steps of a supply chain mapping for a supplier would be:
• Establish a documentation structure, and identify which products and materials are
in scope.
• Gather information and data on the 1
st
tier suppliers (e.g. manufacturers).
• Repeat the above step for all relevant tiers.
• For each relevant tier, gather information on material and labour inputs. This
involves tracing products back to their raw materials.
• Document all materials and transport routes for each tier.
The resource required for each of these steps will vary depending on the product. To
achieve a detailed mapping, engagement with upstream suppliers will be important, and
this collaboration may be ongoing to ensure the supplier provides the required information.
This engagement will entail a resource cost on the supplier to the NHS. Further costs may
be project management time and resources to track overall process of the mapping, along
with supplier staff time to check and verify the information provided by upstream suppliers.
We envisage the return to ask suppliers to set out the provenance of raw materials and
key manufacturing locations. The availability of this information, and the subsequent
reporting resource required from suppliers, will depend on the suppliers’ knowledge of its
supply chain and inherent complexity. It is also likely there will be indirect costs to
upstream suppliers for providing information. These costs will mainly entail time and
resource costs for completing information returns to their downstream supplier. There are
a wide range of scenarios for potential resource burdens on suppliers of sourcing this

58
information. We cannot accurately state the costs of this until the exercise itself takes
place, and therefore an EANDCB has not been provided.
The cost of the review may disproportionately affect smaller businesses, although the
extent to which this is the case is not known owing to the lack of data on the resource
burden of mapping supply chains. The Modern Slavery Act 2015 impact assessment sets
out that “There is not a strong rationale for requiring small businesses to disclose
information about supply chains over which they have little control.”
36
Therefore
consideration for the involvement of small businesses will be made when the review
commences. Without the visibility provided by the initial stages of the review, the
information required to expand on what businesses will be in scope is not available.
Illustrative examples are provided below to demonstrate how the size of the business
affect potential impacts. Assume that the three suppliers below all provide the NHS with
the same good (e.g. cotton masks), which have a relatively complex supply chain. The
burden on the smaller businesses may be disproportionately larger, although it is unclear
what proportion of total NHS suppliers each of these theoretical scenarios may apply to:
- Scenario 1: Large supplier (>250 employees) with large procurement team and an
existing deep knowledge of their supply chains. This supplier has a high level of
existing resource that can be shifted to work, for a short period, on the information
return or an audit. Large companies with a turnover of >£36m are also required to
produce a modern slavery transparency statement, as set out in the Modern
Slavery Act 2015. The impact assessment sets out a rationale that increased
transparency (of these large companies) may lead to them taking further action on
modern slavery in their supply chains, and therefore it may be the case that they
already have an in-depth understanding of their supply chain so can easily source
the information required for the information return, and experience in completing
such requests, resulting a low level of additional burden on the organisation.
- Scenario 2: Medium sized supplier (50-250 employees) but a lower level of existing
understanding. Resourcing the information return takes a small % of overall staff
time and no additional FTEs are recruited to complete the exercise, but it there is an
opportunity cost for the organisation to complete the return (i.e. staff are displaced
from other activities within the organisation).
- Scenario 3: A small supplier with <50 employees. The supply chain mapping
requires skills that the company do not possess, accessing information that is not
regularly collected and that the company has little leverage over their supply chain
to request. Collating this information would be notably more burdensome for smaller
companies and it would be more difficult for them to find and release useful
information about global supply chains
37
. There is also a risk they do not complete
the information return to the required level of detail.
36
https://www.legislation.gov.uk/ukia/2015/293/pdfs/ukia_20150293_en.pdf , p6
37
https://www.legislation.gov.uk/ukia/2015/293/pdfs/ukia_20150293_en.pdf, p6
59
As part of the analysis, some high-risk suppliers to the NHS will need to be audited and
undertake other transparency measures. This will increase workload for NHS procurement
teams and contract managers to ensure requirements are delivered effectively. There may
be costs to high-risk suppliers partaking in the audits, although we anticipate this being a
small number of all suppliers. These costs may involve staff time cooperating with auditors
and providing relevant information on supply chains. If there are costs to suppliers from
taking part in the audits or reviews, it is a risk that some of these may be passed onto
prices resulting in indirect impacts on the costs of goods and services.
Depending on the findings and recommendations of the supply chain risk analysis,
alternative routes of supply may be needed for certain products. The scale and impact of
this is not known until the analysis is conducted. See below for the potential risks of this
recommendation.
Existing requirements for suppliers
Suppliers of goods and services to the NHS are already expected to uphold high ethical
standards, reflected in the requirements of the NHS Standard Terms and Conditions.
Therefore, particularly owing to the requirements, it is plausible that the information
required for the review is already held by suppliers to the NHS, but there is no information
or data to verify whether this is the case. It should be noted that these terms are not
exclusively used within the NHS, and other terms and conditions are used, therefore this
may not apply to all contracts. The relevant clauses within the NHS Terms and Conditions
are included below:
• it shall: (i) comply with all relevant Law and Guidance and shall use Good Industry
Practice to ensure that there is no slavery or human trafficking in its supply chains;
and (ii) notify the Authority immediately if it becomes aware of any actual or
suspected incidents of slavery or human trafficking in its supply chains;
• it shall at all times conduct its business in a manner that is consistent with any
anti-slavery Policy of the Authority and shall provide to the Authority any reports or
other information that the Authority may request as evidence of the Supplier’s
compliance with this Clause 10.1.29 and/or as may be requested or otherwise
required by the Authority in accordance with its anti-slavery Policy;
• it will fully and promptly respond to all requests for information and/or requests for
answers to questions regarding this Contract, the Goods, the provision of the
Services, any complaints and any Disputes at the frequency, in the timeframes
and in the format as requested by the Authority from time to time (acting
reasonably).

60
Potential costs of regulations
Regulations created under this power will comprise of provisions with the overarching aim
to eradicate the use in the health service in England of goods or services that are tainted
by slavery and human trafficking. These will include:
• provisions in relation to processes for the procurement of goods and services for
the health service by public bodies, with a focus on the circumstances in which a
supplier is excluded for consideration for the award of a contract;
• steps that must be taken by public bodies for assessing and addressing the risk of
modern slavery in supply chains, ensuring that the risk is minimised with a view to
eradicating the use in the NHS of goods created with forced labour;
• matters for which provision must be made in contracts for goods or services
entered into by public bodies for the purposes of the health service.
The development of these regulations will include an evaluation of existing processes,
requirements under existing government policy, and NHSE policy, with a focus on areas of
risk of use of goods and services with an aim to eliminate all use over time.
The provisions to be created under the regulation-making power have not yet been
agreed, and therefore a detailed assessment of costs cannot be made at this stage. It is
plausible that if the review suggests, and results in, changes to procurement processes to
mitigate the risk of procuring goods tainted by slavery and human trafficking, then this will
result in time and resource costs for the NHS in complying with new rules. For example, if
additional assurance steps are included in the procurement process, this will increase the
amount of NHS staff time required to procure goods and services. There may be
subsequent indirect costs on suppliers (who may be private businesses) if their goods are
tainted by slavery and human trafficking, in that they may have to address issues in their
supply chain to comply with NHS procurement rules (although this ultimately will be
beneficial for the NHS and its commitment to eradicating modern slavery in supply chains).
These are illustrative costs and cannot be quantified at this stage.
A full impact assessment, including an assessment of costs to businesses (EANDCB) and
small and micro businesses (SaMBA), would be conducted to accompany any regulatory
change if appropriate.
Benefits
The NHS has a vast and complex supply chain, which through an appropriate,
proportionate, and targeted analysis of the supply chain, can have a beneficial impact,
although these benefits are not monetisable.
The supply chain review analysis will increase supply chain transparency, support wider
NHS supply chain resilience and may reduce risk of future modern slavery and labour
standard violations. This may ultimately benefit lower tier supply chain workers. Review
analysis may also support other NHS objectives, such as improved Social Value creation
(Modern Slavery is an NHS priority area under the Social Value Model theme of ‘Equal
Opportunity’).

61
Regulations will aim to eradicate the use of goods and services in the NHS tainted by
modern slavery, which will aim to ensure that government maintains the highest standards
of business ethics from our suppliers and their agents. Taxpayers expect that
government’s suppliers will behave in an ethical manner and this will further provide
assurance of the highest standards of business ethics from suppliers and their agents in
the supply of goods and services funded by the public purse.
The Modern Slavery Act 2015 impact assessment (p. 16) also outlines a more general
account of benefits to businesses. For example, business transparency on modern slavery
in supply chains could also reduce businesses’ exposure to risk and unforeseen costs,
retention and recruitment of employees, and improving brand image. See this impact
assessment for further detail.
Risks and mitigations
Modern Slavery and Human Rights issues often occur in the lowest levels of the supply
base, beyond the organisation that may be in contract with the NHS. This is also true in
the manufacturing of products where there are a number of entities between the NHS and
the manufacturing facilities or materials extraction points where the Modern Slavery and
Human Rights issues will most likely occur. Therefore, the resource required to complete
the analysis to this depth is not yet fully understood. Given the complexities and costs
involved, it is proportionate to take a risk-based approach to any supply chain analysis and
mapping exercise.
It is important to note that there are likely to be products under consideration (i.e. being
reviewed) that are essential goods and services required by the front line of the NHS and
this needs to be considered as part of the assessment. Should an alternative supply of a
product be recommended following the analysis, it should be determined that there is a
sustainable, alternative supply available, should improvement measures not be agreed
with existing suppliers.
The approach taken to modern slavery risk in supply chains, and to regulations made
under section 81 of the Act, needs to be consistent with continuing to ensure that the
burdens in public procurement remain as low as possible both for contracting bodies and
for suppliers, especially small businesses and VCSE organisations, to mitigate the risk of
barriers for small business to supplying public bodies.
13. Licensing of cosmetic procedures: Section 180 and
Schedule 19 of the Health and Care Act
Policy summary
This section makes provision for a licensing scheme to ensure that those who offer non-
surgical cosmetic procedures to the public are suitably trained and qualified, hold
appropriate indemnity cover and operate from premises which meet the necessary

62
standards of hygiene and cleanliness. The legislation permits the Secretary of State to
make regulations providing for a licensing scheme consisting of two interlinked
components: a practitioner licence and a premises licence:
• Regulations made under the provisions will ensure in respect of a practitioner license
that those who offer certain non-surgical cosmetic procedures to the public are
suitably trained and qualified, hold appropriate indemnity cover, and operate from
premises which meet the necessary standards of hygiene and cleanliness.
• Regulations made under the provisions will ensure that a premises licence will be
dependent on the premises being deemed a suitable environment for the procedures
being performed, through demonstrating compliance with hygiene standards and
infection control measures. Under the scheme, licensed practitioners must operate
only from premises which are themselves licenced.
The licensing scheme includes provision for regulations to be made that impose penalties
on those who fail to meet these standards and prevent them from operating. The licensing
scheme will be administered and enforced by Local Authorities.
This is a fast-changing industry, and the provisions in the Act mean that the licensing
scheme can be adapted as new treatments come to the market. This flexibility is essential
to ensure that the licensing scheme can continue to apply to the highest risk procedures.
This legislation provides powers to make regulations to establish a licensing scheme for
non-surgical cosmetic procedures. As such there is no direct cost or impact associated
with the clauses in this Act. Consideration as to how the regulations are likely to be laid out
and their potential impact through illustrative examples (see Annex A) is appropriate.
Another impact assessment, with a detailed assessment of costs, benefits and impacts
including costs to businesses and small and micro business impacts will be conducted
when the subsequent regulations are made, and stakeholders and the public have been
consulted. We will also conduct an equalities impact assessment.
How will the proposed licensing scheme operate?
If this power is exercised and a licensing scheme set up, the licensing scheme will consist
of two interlinked components: a practitioner licence and a premises licence. The
subsequent regulations will set out the detail of the licensing scheme, as well as the
standards that practitioners and premises will have to meet to be granted a license.
In addition, the powers provide for an offence to be created prohibiting an individual from
carrying out non-surgical cosmetic treatments without a license. Any regulations made
using the powers will be subject to public consultation (Section 180(4)) and to legislative
scrutiny through the affirmative parliamentary procedure (Section 183(4)).
The definitions in section 180 cover those procedures considered to have the highest
potential to cause harm, while also allowing flexibility for new procedures to be added in
the future. This future proofing is essential for ensuring that the licensing scheme can
continue to apply to the highest risk procedures. The definition of licensed procedures can
be further narrowed through the regulations. However, our initial intention is that the
following procedures will be licensed: Botulinum toxins, Dermal fillers, Laser, Intense

63
Pulsed Light (IPL) and Light Emitting Diode (LED), Chemical peels, PDO cog threads, and
cryolipolysis.
We will finalise the list of procedures in the regulations after stakeholders and the public
have been consulted. Once we have finalised the list of procedures in scope, we will also
be able to carry out a more detailed impact assessment and provide a comprehensive
assessment of costs and benefits. We will also conduct an equalities impact assessment.
Rationale for intervention
All cosmetic procedures have some risks and can lead to serious injury or harm if not
performed correctly. The provisions will provide regulations intended to replace the pre-
existing regulatory framework around non-surgical cosmetic interventions which is a
patchwork of different standards relating to either products, premises or practitioners,
overseen by a range of regulatory agencies. The pre-existing regulatory framework places
few restrictions on who may perform non-surgical cosmetic interventions. The provisions
also provide powers to legislate for nationally recognised requirements or standards
covering the education, training and qualifications required for the administration of these
treatments. Prior to these provisions, we did not have the ability to legislate to provide
assurance that the premises in which non-surgical cosmetic interventions are carried out
meet hygiene standards, or that individuals carrying out procedures have appropriate
indemnity and insurance arrangements in place.
There is growing evidence of the risk that non-surgical cosmetic procedures present to
members of the public. There is, however, no central collection of data on complications
following cosmetic interventions and hence no consistent or robust information on the type
or frequency of complications. This presents a partial barrier to making informed policy
decisions regarding the industry.
Recent reports and reviews on the cosmetic sector highlight how much there is for the
Government to do to ensure that non-surgical cosmetic procedures do not present a public
safety risk. These reports and reviews include:
• The Review of the Regulation of Cosmetic Interventions by Sir Bruce Keogh:
Review of the Regulation of Cosmetic Interventions - GOV.UK (www.gov.uk) 2013.
o The Keogh review covered both surgical and non-surgical cosmetic procedures.
The review highlighted that non-surgical interventions are almost entirely
unregulated.
o The report found that cosmetic surgery providers rely, in part, on the NHS to
treat clinical complications. There is limited data on the costs incurred by the
NHS in dealing with the clinical problems caused by cosmetic procedures.
However, data submitted to the Keogh Review by surgeons indicate that the
costs of dealing with adverse reactions to dermal fillers for one individual
resulted in costs of over £4,028 to the NHS.
o The report found that complications following botulinum toxin injections,
laser/IPL treatment, and dermal fillers were the most common issues upon
which patients consulted their GP.
o 57 plastic surgeons reported seeing 380 patients with complications of non-
surgical treatments. Nearly two-thirds of the complications reported were
irreversible.

64
o The review subsequently provided 40 recommendations to strengthen the
regulatory framework around cosmetic procedures.
• The Chartered Institute of Environmental Health (CIEH) reports on regulating
cosmetic treatments: A Fragmented Picture and The Ugly Side of Beauty, 2020.
o The CIEH reports highlight that whilst cosmetic treatments are rapidly growing in
popularity, the existing regulatory regime is poorly equipped to keep the public
safe.
o The data used in these reports show that out of 934 reports in 2018, common
complaints related to dermal fillers (66%) followed by ‘Botox’ or Botulinum
Toxins (24%). Of these complaints, 41% resulted in corrective procedures and
4% in visits to GPs and A&E.
o The reports also found the largest proportion of treatments that go wrong, are
carried out in domestic settings.
o The reports show significant support for legislative change among environmental
health and licensing practitioners, with 90% (out of 258 professionals surveyed)
agreeing that an England-wide licensing scheme could improve the regulatory
system.
• The Beauty, Aesthetics and Wellbeing (BAW) All-Party Parliamentary Group
(APPG) report: APPG-BAW-Report-on-aesthetic-non-surgical-cosmetic-treatments-
21.07.21-3.pdf (baw-appg.com). 2021
o In 2021 the Beauty, Aesthetics and Wellbeing (BAW) All-Party Parliamentary
Group (APPG) completed its inquiry into advanced aesthetic non-surgical
cosmetic treatments.
o The report found that practitioners (both medics and non-medics) can perform
treatments which they do not have enough training, knowledge or experience of,
thus putting the public at risk.
o The report provides illustrative examples from members of the public who have
suffered complications from non-surgical cosmetic procedures, including;
vascular occlusion (blockage of a blood vessel), and necrosis (death of tissue).
o The subsequent APPG report set out 17 recommendations, including three
relating to the introduction of a national licensing scheme for some non-surgical
cosmetic procedures.
In response to patient safety concerns and the expansion of the cosmetic interventions
market, there is increasing Parliamentary and stakeholder pressure to strengthen the
regulatory framework
In light of the above, in 2021, Parliament passed The Botulinum Toxin and Cosmetic
Fillers (Children) Act to prohibit the availability of ‘Botox’ and cosmetic fillers (commonly
known as ‘dermal fillers’) to under 18s for cosmetic purposes.
The Health and Care Act 2022 provisions will further increase public safety and confidence
by introducing regulations around who can carry out non-surgical cosmetic procedures, as
well as where such procedures can be carried out. This will reduce the risk of harm to
members of the public who choose to have a non-surgical cosmetic procedure.

65
Other policy options considered
The Department has assessed a range of options at a high level. This is set out below and
supports the amendment. This IA recommends the expansion of local authority oversight
of non-surgical cosmetic procedures as the recommended response to the issues raised.
Option 1: Business as usual (do nothing)
Non-surgical cosmetic procedures are not currently regulated. In recent years there has
been increasing parliamentary and stakeholder pressure to strengthen the regulatory
framework, citing poor practice in this area.
The risk to the public and to the NHS has been highlighted in the reviews and reports
listed above.
The ‘Do Nothing’ option will mean the public remains at risk from serious complications if
they choose to undergo a non-surgical procedure. This is a significant risk to public
safety.
This option could also burden the NHS who in some cases have to treat complications
from non-surgical cosmetic procedures if they arise.
Option 2: Voluntary register
There are currently two Professional Standards Authority accredited registers in
operation:
• Save Face: operates a voluntary register for doctors, nurses and dentists who
provide non-surgical cosmetic treatments. This register only covers regulated
healthcare professionals.
• Joint Council for Cosmetic Practitioners: operates a voluntary register open to all
practitioners working in the fields of cosmetic treatments. Practitioners must
demonstrate evidence of competence and proficiency to join the JCCP register.
Voluntary registers would not prevent risks to public safety. They are already in operation
and the vast majority of practitioners continue to operate outside of them.
The Save Face accredited register is only open to practitioners who are also registered
with a healthcare professional regulator. Pursuing this option would have significant
implications for many current practitioners/businesses, as it would prevent anyone who
was not a healthcare professional from registering.
A number of different voluntary registers, which have different requirements to join, is also
inconsistent and difficult for the public to navigate.
Option 3: Registration scheme
In England, under the Local Government Miscellaneous Provisions) Act 1982, local
authorities already have powers to register providers of acupuncture, tattooing, ear and
cosmetic piercing, electrolysis, skin colouring and sunbeds. These powers could be
expanded to cover other cosmetic procedures or services. This would require providers to
meet basic hygiene standards. Some local authorities have made byelaws to vary their
local requirements but the content of these is restricted to securing the cleanliness of
premises, fittings, persons, instruments, materials and equipment.
66
A registration scheme builds on a pre-existing regulatory framework and therefore could
support the development of an evidence base to determine whether more robust
regulation of cosmetic interventions is justifiable and proportionate.
A registration scheme does not allow Local Authorities to carry out robust checks on
practitioners or on premises. Local authorities also have few powers to refuse registration
or to place conditions on practitioners’ competence and qualifications.
Stakeholders have also raised that it is difficult for Local Authorities to remove practitioners
from the register once included, and that this presents a significant public safety risk.
Option 4: Regulation of activity – make cosmetic procedures CQC regulated
activities
This would extend the CQC’s remit to regulating providers of non-surgical cosmetic
interventions, in addition to its existing responsibilities for surgery.
There is currently no option to register with the CQC to provide non-surgical cosmetic
procedures, as they are not a CQC regulated activity. The CQC would, therefore, have to
expand its remit to include non-surgical cosmetic procedures.
An estimated 15,200 businesses provide injectable cosmetic treatments in England alone.
CQC enforcement would involve significant additional resources and increased costs to
Government
It would also be logistically difficult and financially burdensome for mobile and small/self-
employed businesses to register with the CQC. The fees for CQC registration are
significantly higher than the current licensing fees (as modelled on the Nottingham and
Croydon examples above).
The 2019/2020 annual fee for a health care single speciality services (i.e., a diagnostic
imaging centre) to register with the CQC ranged from £1,743 for 1 location, through to
£55,662 for more than 15 premises.
Option 5: Statutory Professional Regulation
Statutory regulation of practitioners carrying out cosmetic interventions would bring them in
line with regulated health and care professionals, such as doctors, nurses, pharmacists
and dentists.
This option would require Government funding to set up a new regulatory body. This would
be a significant financial investment. It is currently difficult to gather a strong evidence
base to indicate that this level of regulation is proportionate.
There is no pre-existing profession to regulate due to the diversity of practitioners who
currently perform cosmetic procedures in England.
Option 6: Licensing scheme
The proposed licensing scheme will consist of two interlinked components: a practitioner
licence and a premises licence.

67
Licences for both practitioners and for premises will be issued by Local Authorities who will
work with a range of partners to operate and enforce the scheme. The enforcement of
regulation will be at the discretion of each Local Authority. A licensing scheme builds on
existing models for ‘special treatments’ as evidenced through the illustrative examples.
We will finalise the list of non-surgical cosmetic procedures in the regulations after
stakeholders and the public have been consulted. However, as set out in the introduction,
it is essential that the licensing scheme applies to the highest risk procedures and at
present our intention is that the following procedures will be licensed: Botulinum toxins,
Dermal fillers, Laser, Intense Pulsed Light (IPL) and Light Emitting Diode (LED), Chemical
peels, PDO cog threads, and cryolipolysis
The introduction of a licensing scheme is supported by many key stakeholders. It has also
been recommended in a number of recent reports and inquiries on the cosmetic sector. In
2020, a report published by the Chartered Institute of Environmental Health found 90% of
the 258 professionals (Environmental Health Practitioners (EHPs) and Licensing
Practitioners (LPs)) surveyed agreed that an England-wide licensing scheme could
improve the regulatory system. The introduction of a licensing scheme was also a key
recommendation of the 2021, Beauty, Aesthetics and Wellbeing (BAW) All-Party
Parliamentary Group (APPG) report.
We would have to ensure the licensing scheme does not place unnecessary burden to
business, particularly those who are currently self-employed or who run or who are
employed by Small and Micro Enterprises (SME).
The Government’s preferred option is to take forward a licensing scheme
We will finalise the scope of the licensing regime following consultation with both
stakeholders and the public. We will then carry out a more detailed impact assessment
and provide a comprehensive assessment of costs and benefits.
Costs
There are no impacts resulting directly from this primary legislation as it only seeks the
power to make regulations to establish a licensing scheme.
If this power was exercised and a licensing scheme established, then we anticipate the
following costs to be incurred:
• Costs to practitioners/business (familiarisation costs, and ongoing
costs of licenses, costs of training/qualifications)
• Costs to Local Authorities (on going costs of licensing, enforcement,
and familiarisation)
• Cost to education and training bodies (familiarisation costs, on-going)
• Cost to criminal justice system (familiarisation, and ongoing costs)
• Costs to manufacturers/suppliers of cosmetic products (loss of profit)
A detailed overview of potential impact and costs can be found at Annex B.
Benefits
This legislation will support public safety by limiting the risk of injuries and complications
from non-surgical cosmetic procedures. It will do this by introducing regulations around

68
who can carry out non-surgical cosmetic procedures, as well as regulating where such
procedures can be carried out. This will reduce the risk of harm to members of the public
who choose to undergo a non-surgical cosmetic procedure.
The reviews and reports listed above, along with substantial anecdotal evidence, suggests
that the current model relies on the NHS being there to act as a safety net to treat clinical
complications. In some cases, it may be that the private provider does not have the
competence or equipment required to treat a specific condition. By making sure that
practitioners carrying out non-surgical procedures are properly trained and qualified and
have insurance if things do go wrong, the burden on the NHS will be reduced.
Risks and mitigations
We need to ensure that regulation of non-surgical cosmetic procedures is consistent
across England and that it is a mandatory requirement for all practitioners offering non-
surgical cosmetic procedures.
The purpose of this legislation is to provide the power to create regulations. We will now
work across government and with external stakeholders to develop the regulations and
guidance.
This means the economic impact can only be comprehensively assessed once we are
clear of the specific non-surgical procedures in scope, the costs to qualification bodies
training business around setting up and implementing a national curriculum and have
worked with local authorities to quantify the anticipated resources for
licensing/enforcement.
We are aware there may be a risk of businesses closing, particularly SMEs as a result of
the licensing scheme. This could also have an impact of the profit of cosmetic
manufacturers/suppliers of cosmetic products. However, the purpose of this amendment is
not to ban procedures, stifle innovation or close businesses down, but rather to ensure that
consumers who choose to undergo a cosmetic procedure can be confident that the
treatment they receive is safe and of a high standard.
To mitigate the risk to business, we will ensure that the regulations are proportionate to the
risk that the procedures present to the public. We will work with local authorities and
training bodies to ensure that the cost of qualifications and the cost of licensing fees are
not prohibitively expensive for sole practitioners and/or SMEs.
There is a risk that practitioners may continue to operate outside of the licensing scheme.
This presents a continued risk to patient safety. However, if practitioners operate outside
of the licensing scheme, they will be committing an offence. The licensing scheme will
provide members of the public with assurances, as they will be able to check if a
practitioner is licensed before choosing to undergo a procedure. We are, therefore, of the
view that the benefits of the licensing scheme outweigh the risks of the practitioners who
may choose to operate outside of it.

69
7. Annex A: Licensing of non-surgical
cosmetic procedures: Illustrative
examples
Existing licensing scheme for cosmetic treatments
A small number of Local Authorities in England have opted to introduce local licensing
schemes for certain procedures. This includes:
• Nottingham: introduced separate practitioner and premises licence for the
following procedures: cosmetic piercing; hair electrolysis; lasers; massage; saunas;
steam rooms; tanning; sunbeds; semi-permanent make-up; tattooing.
• The fees and requirements vary for each procedure. However, as an example, the
fees for cosmetic piercing are:
o Practitioner: £67.00
o Premises: £129.00
• Once the application form and fee are received, Nottingham Local Authority send
an Environmental Health Officer to inspect the premises for suitability. More
information can be found on the website: Health and Beauty Licences - Nottingham
City Council
• Croydon: introduced a ‘special treatments licence’ for a number of treatments. This
includes: Massage; acupuncture; manicure; piercing and tattooing; sauna and light;
electric or vapour treatments.
• All premises offering special treatments must comply with the special treatments
licence conditions. Practitioners also need to send a copy of relevant
qualifications.
• The total cost for a new licence is £439, annual renewal cost is £383, transfer cost
is £254, and variation cost is £260.
• The licence is valid for one calendar year.
• Members of some professional associations and organisations are exempt from
special treatment licensing.
• More information can be found on the website: Special treatments licence | Croydon
Council
The Animal Welfare (Licensing of Activities Involving Animals) (England)
Regulations
We anticipate the licensing scheme for nonsurgical cosmetic procedures will also be
comparable to the Animal Welfare (Licensing of Activities Involving Animals) (England)
Regulations 2018.
The Animal Welfare Regulations provide for the licensing of persons involved in England in
selling animals as pets, providing or arranging for the provision of boarding for cats or
dogs, hiring out horses, breeding dogs and keeping or training animals for exhibition.
The regulations provide for local authorities to be the licensing authorities; sets out how a
person may apply to the local authority for a licence; and sets out the conditions an

70
individual must meet to be granted a licence or have their licence renewed. It provides for
a local authority to charge fees to cover costs related to compliance with the regulations,
enforcement, and administration.
A person who undertakes licensed activities involving animals in England without a licence
commits an offence.

71
8. Annex B: Initial assessment of impact
and costs for a cosmetic licensing
scheme
Key impacts
There are no impacts resulting directly from this primary legislation as it only seeks the
power to make regulations to establish a licensing scheme.
If this power was exercised and a licensing scheme established requirements would be
placed on Local Authorities to ensure that businesses and practitioners met the required
standards.
Costs
The costs of licensing will fall on individual practitioners; public sector (e.g. local
authorities, courts); and private businesses who provide or supply non cosmetics
services.
This next section provides a narrative of the types of cost that the regulations will incur,
this includes transitional, set up, familiarisation and ongoing costs and how they impact
upon practitioners/business; Local Authorities; education and training bodies; and the
Criminal Justice System.
Costs to practitioners/business (familiarisation costs, and on going costs of
licenses, one off costs training/qualifications)
There would be a national agreed fee set out in regulations for both practitioner and
premises licenses, and licenses would be renewed on a yearly basis. Practitioners would
also be required to have indemnity insurance in place, which would be an additional
annual cost
The cost of the license may have a financial impact on small and micro business owners,
that represent around 97% of all businesses in the market in scope.
36
At present, there are also no regulations around mobile practitioners and the proposed
licensing scheme will also prevent mobile practitioners from operating. This impact is
justified for public safety reasons.
Impacts of policy on businesses
Reliable information about the number of businesses and/or practitioners who offer non-
surgical cosmetic procedures in England is difficult to determine. This is because the
industry operates through a complex variety of businesses involving manufacturers of
products, private hospitals and clinics, hotels and spas, beauty salons and self-employed
practitioners. Practitioners range from regulated healthcare professionals such as doctors,
dentists and nurses through to individuals who have no medical qualifications.
72
The Nuffield Council of Bioethics report “Cosmetic procedures: ethical issues” concluded
that most cosmetic procedures are provided within the private health sector and that
business models through which procedures are offered vary
37
. These include:
• self-employed health professionals who provide cosmetic procedures to private
patients on an individual basis, often as a complement to their work in the NHS
• private hospitals and clinics that offer cosmetic procedures alongside a range of
other procedures.
• large commercial ‘group’ providers who specialise in cosmetic procedures rather
than offering these alongside medical services
• beauty salons, spas, gyms and other parts of the beauty and ‘wellness’ sector
– providers in this sector are thought to be a significant supplier of non-surgical
cosmetic interventions, and they vary from single practitioners in stand-alone
beauty parlours to chains of salons; practitioners may be self-employed or
employed and may come from a variety of professional backgrounds.
Discussions with stakeholders, including the Joint Council for Cosmetic Practitioners
(JCCP), emphasised that this is a challenging industry to capture and describe.
Additionally, it was noted that many practitioners are mobile rather than being based in
one salon or clinic, and that self-employed practitioners typically provide services to
multiple businesses where they are not directly employed. Nevertheless, we have
estimated the number of businesses and practitioners we expect to be in scope of this
policy using a similar methodology to the modelling used for the Botox and Fillers Act.
We estimate that around 16,100 businesses will be in scope of the policy (as they are
involved in non-surgical cosmetic procedures), which consists of 11,500 unregistered
sole traders, 1,300 registered sole traders and 3,300 employers. This corresponds to
around 25,300 practitioners in total. “Registered” in this case refers to businesses that are
registered for VAT or PAYE.
These figures were estimated using the latest BPE and BRES data as follows.
The number of businesses was determined by the latest BIS Business Population
Estimates (BPE) publication.
38
In particular, the industries with the following SIC codes
were analysed:
• 86 Human health activities
• 96 Other personal service activities
LaingBuisson data (2018) suggests that the non-surgical cosmetic market is worth
£2.75bn in the UK. The total turnover of these industries (i.e., industries with SIC codes 86
and 96) amounts to £66.5bn, which was also provided in the BPE publication. When
adjusting both these figures to England only (using the proportion of the population of
England relative to the UK as a proxy), these figures are around £2.3bn and £56.1bn
respectively. This suggests that 4.1% of the wider turnover in these industries can be
attributed to the non-surgical cosmetic market. This was used as a proxy to determine the
number of businesses (including sole traders and employers) in the non-surgical cosmetic
market in England as all these figures are provided in the wider industries in the BPE
publication.
The number of practitioners was determined using the Business Register and Employment
Survey (BRES) data.
39
Reviewing the report on UK Standard Industrial Classification of

73
Economic Activities
40
and conducting a search on Companies House identified that known
providers of cosmetic procedures were recorded under the following Standard Industrial
Classification (SIC) codes:
• 8622 Specialist medical practice activities
• 8690 Other human health activities
• 9602 Hairdressing and other beauty treatment
• 9609 Other personal service activities N.E.C.
The total number of those employed under these SIC codes can be determined using the
BRES data. The 4.1% (i.e., estimated proportion of non-surgical cosmetic market relative
to wider industries) was again used as a proxy to determine the number of practitioners
expected to be in scope of the policy.
Impact on small and micro businesses
The preferred option is likely to impact businesses providing non-surgical cosmetic
procedures and practitioners who carry out non-surgical cosmetic interventions. The
majority of these are likely to be small and micro businesses.
The BIS Business Population Estimates (BPE) publication provides the most
comprehensive source on the number of businesses in the UK since it combines
information on registered businesses (i.e., those registered for VAT and/or PAYE) with an
estimate of the number of unregistered businesses in the UK
41
. It also breaks down the
number of businesses by employment size.
Similar to the process of estimating the total number of businesses in scope, the industries
with SIC codes 86 (human health activities) and 96 (other personal service activities) were
analysed.
Businesses by Employment Size Band
– England
Micro (0 to 9)
Small (10 to
49)
Medium-sized
(50 to 249)
Large (250+)
86 Human health activities
94.9%
4.6%
0.5%
0.1%
96 Other personal service
activities
98.1%
1.8%
0.1%
0.0%
Average
96.7%
3.0%
0.3%
0.0%
Using the estimated number of businesses calculated earlier (16,100) and applying the
average proportion of micro and small businesses calculated above, the estimated number
of businesses in these categories can be estimated. This results in approximately 15,600
micro and 490 small businesses
Given almost 100% of businesses are small and micro it is therefore unlikely that the
policy objectives will be met if they are exempt. However transitional arrangements will be
considered as part of the policy development and consultation which may help mitigate the
disproportionate impacts of this policy on small and micro businesses.
74
Costs to Local Authorities (on going costs of licensing, enforcement, and
familiarisation)
Licences for practitioners and for premises will be issued by local authorities who will work
with a range of partners to operate and enforce the scheme. These will include
enforcement partners including Environmental Health Officers, Trading Standards Officers
and the Health and Safety Executive.
The enforcement of regulation will be at the discretion of each Local Authority. In England,
the structure of local authorities varies between:
• Two-tier authorities: where the responsibility for services is split between
county and district councils; and,
• Unitary authorities: where the responsibility for all services in the area fall
to one council body.
In two-tier councils, Environmental Health operate in this space at district council level
whilst Trading Standards operate at county council level; some two-tier councils may
decide it is most appropriate to enforce through Trading Standards, others may feel it is
the responsibility of Environmental Health. Unitary authorities will likely operate through
Trading Standards.
We do not know which enforcement route will be preferred by local area. Costs estimated
may differ depending on how local authorities decide to license and enforce the scheme.
All local authorities will, however, need to appoint appropriate authorised officers to carry
out duties to secure compliance. Existing officers who are experienced in carrying out
enforcement duties in relation to businesses, such as environmental health officers and
Trading Standards Officers, could potentially carry out this work and could incorporate this
work into their other inspection activities. However, some local authorities will most likely
need to recruit additional officers.
We anticipate that the scheme will, therefore, require funding and resources for Local
Authorities. Our assumption is that the licensing regime will eventually be self-funded but
there will be initial start-up costs to support training and familiarisation.
Cost to education and training bodies (familiarisation costs, on-going)
We will need to work with stakeholders to develop a clear framework and standard
curriculum to cover the material that professionals providing non-surgical cosmetic
procedures must know.
The Joint Council of Cosmetic Practitioners (JCCP) has already developed a competency
framework covering high risk non-surgical cosmetic procedures. The JCCP has advised that
all treatments at level 6 and 7 in the JCCP/CPSA Competency Framework should be subject
to a system of licensing.
The power conferred through the amendment provides flexibility when developing the
regulations and the training and education required. This means that new procedures can
be added to the regulations, as and when they are developed. This flexibility does,
however, have an impact as education bodies will have to develop new education and
training courses to cover new procedures.
Stakeholder engagement suggests that the following training bodies are currently
operating:
75
• Four universities: UCL; Queen Mary University of London; Manchester; and
University of South Wales USW– (USW university provides a UK distance learning
programme which has been approved by JCCP) The University of Salford are also
in the process of developing a programme.
• FE Colleges: There are approximately 60 colleges providing programmes in
England. Most of these programmes focus on procedures out of scope of the
proposed licensing scheme. However, certain colleges, such as Birmingham
University College, is developing a programme that would link to further education
and provide training for procedures covered by the licensing scheme.
• Private training companies: There are over 1000 companies operating on the High
Street for lower-level beauty treatments. Of these approximately 250 provide
training for procedures that would be in scope of the licensing scheme.
• Ofqual approved qualifications: There are a range of Ofqual approved qualifications
that are delivered by recognised Ofqual awarding Bodies, such as VTCT, OTHM,
Qualifi, CIBTAC. The awarding bodies then approve private training companies to
deliver the approved qualifications. These include organisations such as Cosmetic
Courses and the Harley Academy.
Education and training costs will therefore impact on:
• Organisations developing the curriculum
• Businesses providing the training
• Practitioners who will have to pay for the training courses/qualification.
Cost to criminal justice system (familiarisation, and on going costs)
The regulations may create offences related to: (i) a breach of the prohibition in the clause
(ii)a breach of conditions related to the personal licence or the premises licence, and (iii)
the provision of false or misleading information to a local authority in connection with these
licences. Such an offence would be punishable on summary conviction with a fine.
Costs to manufacturers/suppliers of cosmetic products (loss of profit)
The licensing scheme may prevent businesses (particularly SMEs) from operating. This
may have a marginal impact on the volume of product sales by cosmetic
manufacturers/suppliers in the UK and therefore on their profit levels. As the licensing
scheme would cover a variety of non-surgical cosmetic procedures, the impact would be
spread across multiple manufacturers. Profit margins in the pharmaceutical industry are
commercially sensitive and it is a highly competitive market. As we will not know which
non-surgical cosmetic procedures will be licensed until after consultation with stakeholders
and the public, it is challenging to estimate the potential monetised losses that
manufacturers and suppliers will face as a result of the policy.
However, given the growth of the non-surgical cosmetic sector, any reduction in sales due
to the licensing scheme is likely to be overridden if the industry continues to grow at the
projected rate.

76
9. Glossary
CHM
Commission on Human Medicines
EANDCB
Equivalent Annual Net Direct Cost to Business
IMMDSR
Independent Medicines and Medical Devices Safety Review
LA
Local Authority
MAH
Marketing Authorisation Holders
MHRA
Medicines and Healthcare products Regulatory Agency
NHSEI
NHS England and NHS Improvement
SaMBA
Small and Micro Business Assessment

77
© Crown copyright 2018
Published to GOV.UK in pdf format only.
www.gov.uk/dhsc
This publication is licensed under the terms of the Open Government Licence v3.0 except
where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-
government-licence/version/3
Where we have identified any third party copyright information you will need to obtain
permission from the copyright holders concerned.
