Unit: Chemistry B – Water Properties
Science 21 Chem B – Water Properties C11
LESSON 1 - INVESTIGATING THE CHEMICAL
PROPERTIES OF WATER
Overview:
Students learn about the properties of water through a series of demonstrations, a reading and
question package, a Water Olympics activity, and a laboratory investigation.
Suggested Timeline: 3.5 hours
Materials:
• Chemistry: Our Liquid World – Unit Organizer (Teacher Support Material)
• blank unit organizer (1 per student)
• globe or map of the Earth
• The Word on Water (Student Handout)
• The Word on Water (Teacher Support Material)
• for ‘The Word on Water’ demonstrations:
- bowl of prepared JELL-O in a clear glass bowl
- flashlight
- chocolate syrup
- tablespoon
- glass of water
• Water Olympics (Teacher Support Material)
• Water Olympics (Student Handout)
• for ‘Water Olympics’:
- beaker with narrow spout - sheets of stiff cardboard
- yarn (soaking wet) - scissors
- container to hold water - soap chips (shaved from a bar of soap)
- colored water - large aluminum trays
- paper and pencils - stopwatch
- clear plastic cups - paper clips
- two dollars in pennies - fork
- eyedropper - magnifying glass
- several brands of paper towel - tall glasses
- tape - ruler
• The “Abnormal” Behavior of Water Lab (Student Handout)
• for ‘The “Abnormal” Behavior of Water Lab’
- large beaker (1000 mL) - thermometer (-10 to 110 degrees Celsius)
- crushed ice - 2-hole rubber stopper
- salt - fine glass tubing
- water - ruler
- 250 mL flask - ring stand
- transparent tape - adjustable clamp
• QUIZ – The Chemical Properties of Water (Student Handout)
Unit: Chemistry B – Water Properties
Science 21 Chem B – Water Properties C12
Method:
INDIVIDUAL FORMAT:
1. Have students preview the unit via an examination of a completed unit overview sheet.
2. Tell students that they will have the opportunity to learn about the unique properties of water
that make it well-suited to forming the basis of life on Earth.
3. Have students complete their vocabulary list, reading and questions on their handout ‘The
Word on Water’ (Student Handout). Review the questions with students.
4. If possible, introduce the ‘Water Olympics’ activity by the demonstration on ‘Water
Olympics’ (Teacher Support Material).
5. Have students move through the stations to complete the water activities and answer
questions on their worksheet. Have students submit their worksheet for assessment.
6. If possible, brainstorm with students about what they know about solids, liquids and gases.
Lead them to discuss the difference in density of these three states of matter. Key Q: How
is water different?
7. Facilitate students’ completion of ‘The “Abnormal” Behavior of Water Lab’ (Student
Handout). Have students hand in their lab for grading.
8. Announce a date for the quiz on material learned in this lesson.
GROUP FORMAT:
1. Preview the unit by having students fill in the unit organizer as you go through it with them.
2. Show students a globe or map of the world.
Key Q: What color is approximately 70% of the globe? (Blue for water)
Key Q: If one zooms in on a single cell of an organism on Earth, what % of that cell’s mass
do you think is water? (approx. 70%)
Key Q: Why do you think that water forms the majority of the Earth and the majority of
living things? Why not vinegar or mercury or oil?
3. Tell students that they will have the opportunity to learn about the unique properties of water
that make it well-suited to forming the basis of life on Earth.
4. Have students complete their vocabulary list, reading and questions on their handout ‘The
Word on Water’ (Student Handout).
5. Review the questions with students. Use the demonstrations on ‘The Word on Water’
(Teacher Support Material) to test further their understanding.
6. Introduce the ‘Water Olympics’ activity by the demonstration on ‘Water Olympics’ (Teacher
Support Material).
7. Have students move through the stations to complete the water activities and answer
questions on their worksheet. Have students submit their worksheet for assessment.
8. Brainstorm with students about what they know about solids, liquids and gases. Lead them
to discuss the difference in density of these three states of matter. Key Q: How is the
behavior of water different? Why is the behavior of water essential for life to exist in lakes
and ponds?
9. Facilitate students’ completion of ‘The “Abnormal” Behavior of Water Lab’ (Student
Handout). Have students hand in their lab for grading.
10. Announce a date for the quiz on material learned in this lesson.
Unit: Chemistry B – Water Properties
Science 21 Chem B – Water Properties C13
Assessment and Evaluation:
• Assessment of students’ understanding of solutes and solvents through questioning
• Assessment of students’ ‘Water Olympics’ handout
• Affective assessment of students’ ability to work in a group
• Student grade on lab
• Student grade on quiz

Unit: Chemistry B – Water Properties
Science 21 Chem B – Water Properties C14
ACTIVITY OVERVIEW:
Suggested Sequencing:
Activity Suggested
Timeline
Description Assignment
1 1 hour As a class or individually, preview unit using
unit overview.
Have students complete the vocabulary list,
reading and questions.
Review the questions. If in a group setting,
perform the demonstrations on solutions to
solidify students’ understanding.
The Word on Water
(SH) reading and
questions
2 1 hour Introduce the ‘Water Olympics’ activity by
performing a demonstration on capillary action.
Facilitate students’ completion of the ‘Water
Olympics’ activity as they work through
stations in the classroom.
Water Olympics (SH)
handout
3 1.5 hours Brainstorm properties of solids, liquids and
gases, discussing the usual changes in density
with changes of state. Intrigue students with
the opportunity to investigate the unusual
behavior of water.
Guide students in completing ‘The “Abnormal”
Behavior of Water Lab.’
The “Abnormal”
Behavior of Water
Lab (SH)

Teacher
Support
Material
1
Unit: Chemistry B – Water Properties
The Word on
Demonstrations – Are They All Solutions?
Prepare a package of JELL-O in a clear glass bowl and allow it to set overnight.
• Key Q – Think about all of the transparent things in your refrigerator – a bottle of 7-Up,
maple syrup, a container of apple juice. Are they all solutions?
• Place the bowl of JELL-O in front of the students and tell to observe it closely.
• Key Q – Is the JELL-O transparent? Is it a solution? (Hint: Can you see more than one
part?)
• Shine a flashlight through the bowl and have students observe the JELL-O closely. Ask the
last question again!
Have the following materials at the front of the class – a glass of water, a bottle of chocolate
syrup and a tablespoon
• Key Q – Do you think that chocolate syrup will form a solution in water?
• Add a tablespoon of chocolate syrup to a glass of water and stir until the syrup and the water
are thoroughly mixed.
• Key Q – Is the mixture homogeneous or heterogeneous? Is it a solution?
• Let the mixture stand for a while, then observe it again.
• Key Q – What do you notice? Can you think of any other mixtures that are like chocolate
syrup and water?
Science 21 Chem B – Water Properties C15

Teacher
Support
Material
2
Unit: Chemistry B – Water Properties
Water Olympics
Summary:
Students explore the unique properties of adhesion and cohesion of water through five activities.
Students can make connections to signs of water’s properties seen daily (e.g., beading on the
surface of a glass) which allows them to further explore the structure and behaviour of the water
molecule.
Duration:
Prep time: 40 minutes
Activity time: 50 minutes
Materials:
• Beaker or measuring cup with narrow spout
• Yarn (soaking wet)
• Container to hold water
• Coloured water
• Copies of Water Olympics Score Sheet (Student Handout)
• Water
• Paper and drawing materials
• For each event water is required in addition to the following materials:
Station 1
• Clear plastic cups
• Two dollars in pennies
Station 2
• Eyedropper
• Penny
Station 3
• Boat pattern
• Stiff cardboard
• Scissors
• Soap chips (shaved from a bar of soap)
• Large aluminum trays
• Stopwatch
Science 21 Chem B – Water Properties C16

Teacher
Support
Material
3
Unit: Chemistry B – Water Properties
Station 4
• Paper clips
• Fork
• Magnifying glass
• Clear plastic cups
Station 5
• Several brands of paper towels
• Tall glasses
• Tape
• Ruler
• Scissors
Key terms:
Adhesion
– the attraction of water molecules to other materials like glass or soil.
Cohesion – the attraction between water molecules.
Surface tension – the cohesive force between water molecules causes the water surface to behave
as though it is covered by an elastic sheet.
Capillary action – the movement of water up or along a surface which results from the cohesive
and adhesive properties of water.
Procedure:
Set: Water Walks a Tightrope
1. Setup a beaker partially filled with coloured water, an empty container, and the pre-soaked
yarn as described below as a demonstration for the students.
2. Hold the beaker with coloured water approximately 10cm off the table with the yarn
stretched across the mouth of the beaker to the empty container which is sitting on the
counter the length of the yarn away. The yarn should be held tight between the two
containers and should be allowed to drip into the empty container.
3. Slowly pour the water down the yarn.
4. Have students try to explain how water appears to be defying gravity by moving down the
yarn. Explain that the following activities will help them explore this and other unique
properties of water.
Activity:
1. Setup 5 stations around the classroom.
2. Divide the class into a maximum of 5 small groups.
3. Direct students to the stations. They can be completed in any order. Directions for each
station are provided on student handouts. Results may be recorded on their score sheets.
Science 21 Chem B – Water Properties C17

Teacher
Support
Material
4
Unit: Chemistry B – Water Properties
Conclusion:
1. Ask students to explain how each of the key terms (adhesion, cohesion, surface tension, and
capillary action) could be used to explain the various activities.
2. Have the students explain the motivational set: Water Walks a Tightrope
a. How does water’s cohesive forces work to keep the water from falling off the yarn?
b. What happens if the water is poured too quickly?
3. To extend this activity have students compare the unique properties of water to that of other
liquids (alcohol, hydrogen peroxide, oil). How do they differ? Can a paper clip be supported
on each of these liquids?
Adapted from H
2
Olympics, pp. 30-34 Project Wet Curriculum and Activity Guide
Science 21 Chem B – Water Properties C18

Unit: Chemistry B – Water Properties
Name: ____________________ Date: ____________ Period: ___
Student
Handout
The Word on
VOCABULARY
polar molecule –
adhesion –
cohesion –
surface tension –
capillary action –
mixture –
solution –
solvent –
solute –
homogeneous –
heterogeneous -
acid –
base –
pH –
Science 21 Chem B – Water Properties C19

Student
Handout
Unit: Chemistry B – Water Properties
After watching an insect walk on water or watching the droplets of water roll down the outside
of a glass, you may have some questions. Why doesn’t the insect sink? Why does water form
droplets? Many of the features of water that we experience are due to its unique properties.
The chemical formula for water is H
2
O. This means that it is made up of two hydrogen atoms
and one oxygen atom.
As can be seen in the diagram above, the oxygen end of the water molecule has a weak negative
charge and the hydrogen end of the molecule has a weak positive charge. In the water molecule,
the electrons (which have a negative charge) are more attracted to the larger oxygen atom,
causing the oxygen end to become negatively charged. Water is therefore a polar molecule – it
has oppositely charged regions. This is just like how a magnet has a north pole and a south pole.
As you may remember from other science classes, when the south pole of one magnet is brought
close to the north pole of another magnet, they attract. In the same way, when the positively
charged part of a polar molecule comes close to the negatively charged portion of another polar
molecule, they attract. In the case of water, a hydrogen bond forms between water molecules,
causing them to link up together. This is why water forms a stream when you pour it – the
molecules are clinging together!
Science 21 Chem B – Water Properties C20

Student
Handout
Unit: Chemistry B – Water Properties
Science 21 Chem B – Water Properties C21
The tendency of polar molecules to cling to one
another is called cohesion. This creates surface
tension, causing water to form droplets and allowing
objects to rest on the surface of water!
Adhesion is the attraction of water molecules to
other molecules, like glass or soil. When you see
water trickling down the side of a glass, it is due to
cohesion and adhesion. The water molecules are
attracted to each other, so they form a train
(cohesion). They are also attracted to unlike
molecules, in this case the glass, and stick to it
(adhesion). When water moves up or along a
surface like this, due to cohesion and adhesion, it is
called capillary action.
A spider resting on the
surface of water!
Many centuries ago, scientists were looking for a substance that they called the universal solvent.
If it existed, everything that was poured into the universal solvent would dissolve or mix so
thoroughly that if you looked at the substance, you couldn’t tell that two substances had been
mixed. The early scientists never found a universal solvent, but they came close! As you know,
many substances, from juice crystals to salt, dissolve in water!
A mixture is a combination of substances in which each substance keeps its individual
properties. When a mixture looks uniform or the substances are mixed so well that you can’t see
them individually, it is called a solution. In a solution, there are two parts – a solvent and a
solute. The solvent is the substance in which another substance is dissolved. The solute is the
substance that is dissolved. For example, if you were making iced tea, the water would be the
solvent and the iced tea powder would be the solute.
Some useful solutions:

Student
Handout
Unit: Chemistry B – Water Properties
Science 21 Chem B – Water Properties C22
soft drinks
solute: carbon dioxide (gas)
solvent: water (liquid)
solution type: gas-liquid
use: beverage
brass
solute: zinc (solid)
solvent: copper (solid)
solution type: solid-solid
use: decorative metal
maple tree sap
solute: sugar (solid)
solvent: water (liquid)
solution type: solid-liquid
use: feeds trees and pancake eaters!
air
solute: oxygen (gas)
solvent: nitrogen (gas)
solution type: gas-gas
use: breathing!
Remember that the parts of a solution that make it up cannot be seen. Solutions are therefore
described as being homogeneous (“homo” means same and “genos” means kind).
If a solute does not dissolve in a solvent or only partially dissolves, the mixture is a mechanical
mixture. Mechanical mixtures are not homogeneous. Since the two or more substances mixed
can be seen and have quite different properties, such a mixture is called heterogeneous (“hetero”
means different). For example, when you mix oil and water, the oil sits on top of the water. This
is a heterogeneous mixture.
Can you recall the burning feeling in your throat after throwing up? This feeling is due to the
acid from your stomach burning the soft tissues in your throat. All solutions can be classified as
acids or bases. Scientists have come up with a good way to measure how acidic or how basic a
solution is. It is called the pH of a solution. Acids have pH values lower than 7. Bases have pH
values higher than 7. Pure water is neutral and is said to have a pH = 7. Note the pH of
everyday solutions on the pH scale below.

Student
Handout
Unit: Chemistry B – Water Properties
QUESTIONS:
1. After a rain, you may notice that water forms droplets on the leaves of trees. What property
of water, a polar molecule, causes this to happen? Explain your answer.
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
2. As usual, you are studying hard for science and place a glass of water close to your notebook.
Some water that has condensed on the outside of the glass leaves a small puddle of water on
your desk. You accidentally place a piece of paper next to the wet spot and soon the whole
piece of paper is wet! The water seems to have moved across the paper, soaking it! What
property of water causes this to happen? Explain your answer.
_____________________________________________________________________
_____________________________________________________________________
Science 21 Chem B – Water Properties C23

Student
Handout
Unit: Chemistry B – Water Properties
_____________________________________________________________________
_____________________________________________________________________
3. You pour some bath salts into the bathtub as you get ready for an evening soak.
a) In this situation, the solute is the ______________________. The solvent is the
______________________.
b) Will the bathtub contain a solution or a mechanical mixture? Explain your answer.
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
4. How is a solution different from a mechanical mixture? In your answer, include the terms
homogeneous and heterogeneous.
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
5. Classify each of the following as a solution or a mechanical mixture. Explain each choice.
a) wood __________________________________________________________
b) tap water _______________________________________________________
c) orange juice _____________________________________________________
d) loonie coin ______________________________________________________
6. Make a list of 10 liquids found at home. For each liquid, do the following:
b) Examine the contents by reading the labels on the containers.
c) Identify liquids that meet your newly learned definition of a solution.
d) Write down the names of these liquids.
e) For each, list the solvent and solute that make up the solution.
Science 21 Chem B – Water Properties C24

Unit: Chemistry B – Water Properties
Water Olympics Score Sheet
Team Name: __________________________________ Date: _________________
Team member(s): _________________________________________________________
Be sure to read the directions all the way through before beginning each activity.
Station 1: Pushing the limits
Prediction: How many pennies can you add before the water spills over? ___________
Directions:
Using water fill the plastic cup until it is even with the rim. Add pennies one at a time,
continuing until water spills over the side. Keep track of the number of pennies added in the
space below. Repeat for each team member.
Team member Number of pennies added
Describe or draw the surface of the water:
Station 2: Here a drop, there a drop, everywhere a drop, drop.
Prediction: How many drops of water will you be able to fit on a penny? ___________
Directions:
Use an eyedropper to place as many individual droplets of water on the surface of a penny as
possible. Continue until the water drop collapses or the water spills over the edge of the penny.
Use the table below to record the number of drops added. Repeat for each team member.
Team member
Number of drops added
Student
Handout
Science 21 Chem B – Water Properties C25
Unit: Chemistry B – Water Properties
Student
Handout
Describe or draw how the water appeared on the penny before the drop collapsed:
Station 3: Cutting the tension
Directions:
Using the pattern provided cut out 2 boat shapes from a piece of cardboard. Place a soap chip in
the notch at the rear end of one of the boats. Place the boats in the tray of water and describe
what happens:
Place a drop of water on the table. What happens to it when you put a soap chip in it? What
caused the boat to move?
It is your turn – experiment to design a boat that will move faster! Adjust the boat shape,
placement of the soap chip, and the size of the soap chip. Choose your best design and time how
long it takes for your boat to travel from one end of the tray to the other. Record your time
below:
Team member Time
Science 21 Chem B – Water Properties C26

Student
Handout
Unit: Chemistry B – Water Properties
Station 4: Floating along
Prediction: How many paper clips can your team float on the surface of the water? _____
Directions: Use the prongs of a fork to lay the paper clips on the surface of the water. Record the
number of paper clips you could suspend in the table below. Repeat for each team member.
Team member Number of paper clips added
Observe the surface of the water where it comes in contact with the paper clip using a
magnifying glass. Draw a picture or describe what this looks like below:
Station 5: Bop, bop, bop. Reach for the top.
Prediction:
Which brand of paper towel will absorb the least water? _________________
Which brand of paper towel will absorb the most water? ________________
Explain your reasoning:
Directions:
Cut out strips of two of the brands of paper towel. Tape one end of each towel to the middle of a
pencil. Lay the pencil on top of a tall glass. Measure out enough water to immerse the ends of
the paper towel up to 1.5cm into the water (see the diagram below).
Science 21 Chem B – Water Properties C27

Student
Handout
Unit: Chemistry B – Water Properties
paper towel
immersed
in 1.5 cm
water
water
strip of
paper towel
glass
pencil
Remove the towels, fill the container to that level, and put the towels back in. Let the paper
towels absorb water until the water stops rising. Measure the height absorbed above the water
for each towel using a ruler. Record your data in the chart below:
Paper Towel Brand Height of water (cm)
Summary questions:
1. Place an (x) or (√) in the box that indicates which property of water was shown by each
activity:
Activities Adhesion Cohesion Surface tension Capillary action
1. Pushing the limits
2. Here a drop, there a drop,
everywhere a drop, drop!
3. Cutting the tension
4. Floating along
5. Bop, bop, bop. Reach for the
top.
Science 21 Chem B – Water Properties C28

Student
Handout
Unit: Chemistry B – Water Properties
2. For each term, choose one of the activities that demonstrated the property and explain how
the activity showed this property of water:
a. Cohesion
b. Adhesion
c. Surface tension
d. Capillary action
Adapted from H
2
Olympics, pp. 30-34 Project Wet Curriculum and Activity Guide
Science 21 Chem B – Water Properties C29

Student
Handout
Unit: Chemistry B – Water Properties
Name: __________________ Partner(s): ____________________ Date: _________
Period: ____
Science 21 Chem B – Water Properties C30
The “Abnormal” Behaviour of Water – Lab
The survival of much of the life in ponds and lakes depends on the abnormal or strange
behaviour of water. Most liquids become denser as they are cooled and become most dense at
their freezing point.
12
Problem: What is abnormal about the behaviour of water?
Materials:
• large beaker (1000 mL)
• thermometer (-10 to 110 degrees Celsius)
• crushed ice
• 2-hole rubber stopper
• salt
• fine glass tubing
• water
• ruler
• 250 mL flask
• ring stand
• transparent tape
• adjustable clamp
Procedure:
a) Copy out the following table in your notebook. (You will need to leave space for more time).
Completed table – 2 marks
Time (min) Level of water (mm)
1
2
3
4
.
.

Student
Handout
Unit: Chemistry B – Water Properties
b) Set up the apparatus as shown. Use transparent tape to attach the ruler to the tubing.
c) Every minute, note and record the level of the water in the tube.
d) Stir the ice-salt-water mixture from time to time to make it as cold as possible.
e) Continue to take readings until the temperature of the water is 0 degrees Celsius.
f) Remove the flask from the mixture. Let it warm up. Note and record the level of the water
in the tube every minute. Continue until the temperature of the water is back up near room
temperature.
Discussion:
1. Describe carefully what happens to the volume of water as the temperature drops to 0
degrees Celsius. 1 mark
2. What happens to the density of water as the temperature drops to 0 degrees Celsius? 1 mark
Science 21 Chem B – Water Properties C31
Student
Handout
Unit: Chemistry B – Water Properties
3. At what temperature does water have its smallest volume (and greatest density)? 1 mark
4. Why does ice float on water? 1 mark
5. What is abnormal about the behaviour of water? 2 marks
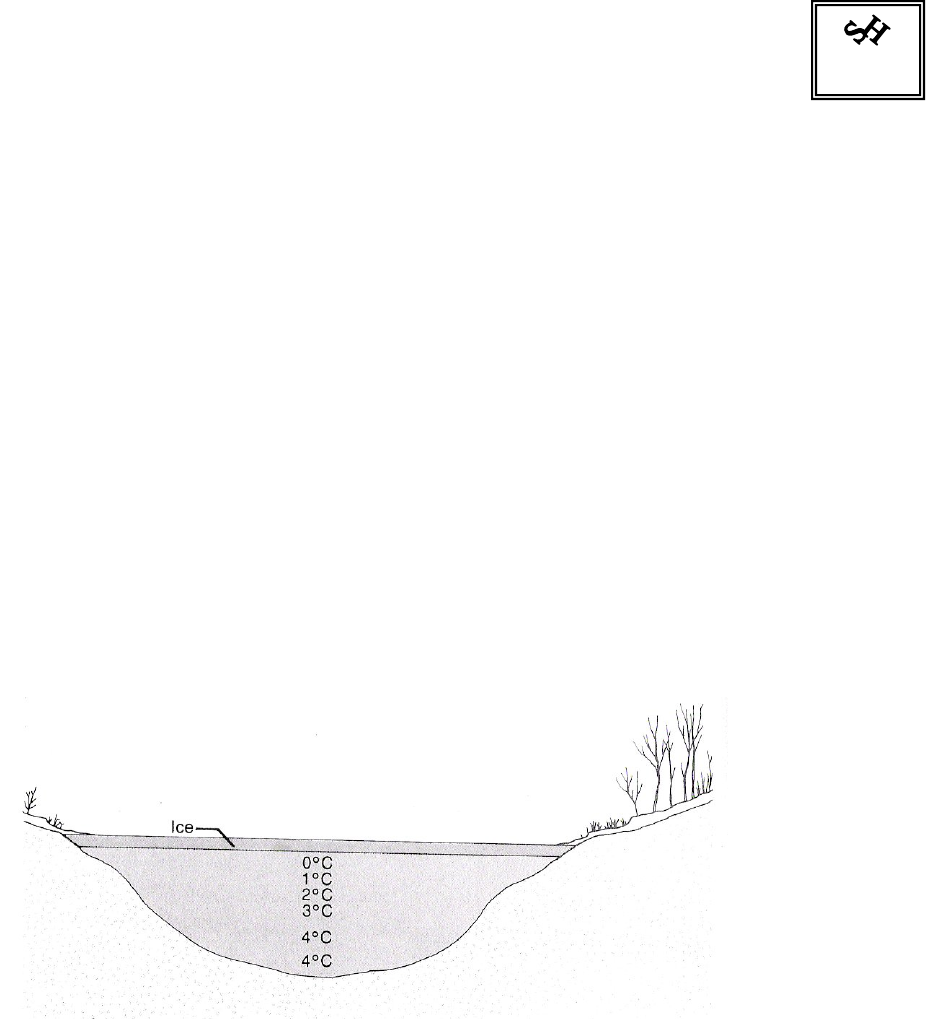
6. View the following diagram, showing a pond in mid-winter. Account for the various
temperature readings. 2 marks
7. If water did not have this abnormal behaviour, fish could not live in ponds and lakes in areas
where freezing occurs. Why? 2 marks
Adapted from Activity 4.2 – Investigating the Abnormal Behavior of Water, pp. 69-70
Investigating Aquatic Ecosystems
Science 21 Chem B – Water Properties C32

Student
Handout
Unit: Chemistry B – Water Properties
_____
10
Name: ______________________ Date: ___________ Period: ____
QUIZ – The Chemical Properties of Water
Instructions – Circle the correct answer.
1. The oxygen end of a water molecule has a slight negative charge. The hydrogen end has a
slight negative charge. For this reason, water is called a(n) ___________ molecule.
a) soluble b) cyclical c) non-linear d) polar
2.
Note how water forms droplets in the photo.
What term best explains this behaviour of
water?
a) cohesion
b) magnetism
c) adhesion
d) solubility
3. You are up late studying for science and decide to make a cup of hot chocolate for a quick
caffeine ‘pick-me-up’. You add the boiling water to the hot chocolate powder and mix up
the toasty drink. Which of the following statements about the mixture that you have created
is correct?
a) The hot chocolate powder is the solvent and the boiling water is the solute.
b) The hot chocolate powder is the solute and the boiling water is the solvent.
c) The mixed up hot chocolate and boiling water is heterogeneous.
4. Soft drinks have a pH less than 7 on the pH scale. Which of the following statements about
soft drinks is therefore correct?
a) They are basic b) They are neutral c) They are acidic
5. Water striders are insects that are capable of walking on the surface of water. The water
tends to bond to itself rather than wetting the insect’s feet and the insect is light enough to
remain on the surface of the water. What word or phrase best describes this behaviour of
water?
a) capillary action b) surface tension c) adhesion d) homogeneity
6. When water is a _________ it is the MOST dense.
a) liquid b) gas c) solid
Science 21 Chem B – Water Properties C33

Unit: Chemistry B – Water Properties
Science 21 Chem B – Water Properties C34
Student
Handout
For the next 4 questions, classify each as: a) homogeneous mixture or b) heterogeneous
mixture. Write a) or b) beside each question.
7. sandy water
8. coffee
9. soil
10. fruit salad
