
AP Biology AP Test Question – “Water” Name___________________________________________
The unique properties of water make life possible on Earth. Approximately three-quarters of the Earth’s surface is covered by
water. Cells are made up of around 70-95% water. Water comprises roughly 70% of the human body.
a) Describe the major physical/chemical properties of water that make it unique from other liquids.*
b) Explain the properties of water that enable it to travel up through the roots and stems of plants to reach the leaves.
c) Explain why the temperature of the oceans can remain relatively stable and support vast quantities of both plant and
animal life, when air temperature fluctuates so significantly throughout the day (especially year).
*There is an overlap between the first question and the subsequent two questions. You do not have to repeat information in your
answers as long as the answers refer to one essay question.
Directions: Answer all three parts in a 10 minute time frame. Answers must be in essay form and must be hand written (no
typing). Labeled diagrams may be used to supplement discussions, but in no case will a diagram alone suffice. It is important that
you read all three parts BEFORE you begin to write.
AP Biology AP Test Question – “Water” Name____________KEY____________________________
The unique properties of water make life possible on Earth. Approximately three-quarters of the Earth’s surface is covered by
water. Cells are made up of around 70-95% water. Water comprises roughly 70% of the human body.
a) Describe the major physical/chemical properties of water that make it unique from other liquids.
b) Explain the properties of water that enable it to travel up through the roots
and stems of plants to reach the leaves.
c) Explain why the temperature of the oceans can remain relatively stable and
support vast quantities of both plant and animal life, when air temperature
fluctuates so significantly throughout the day or over the seasons.
Directions: Answer all three parts in a 10 minute time frame. Answers must be in
essay form and must be hand written (no typing). Labeled diagrams may be used to
supplement discussions, but in no case will a diagram alone suffice. It is important
that you read all three parts BEFORE you begin to write.
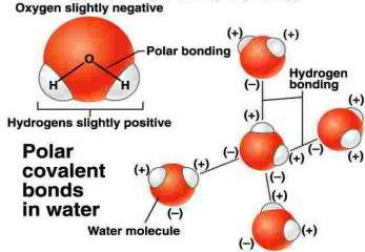
Due to the high electronegativity of oxygen compared to hydrogen, the hydrogen atoms in a water molecule have a partial
positive charge while the oxygen has a partial negative charge. The polar covalent bonds within the molecule, and resulting
polarity, influence the cohesive and adhesive properties of water. Hydrogen bonding between water molecules at the water
surface increases surface tension, enabling some organisms to ‘walk’ on water. Polarity also influences the ability of water to
act as an solvent for hydrophilic substances, and as an organizer for non-polar molecules. The high specific heat of water,
due to hydrogen bonding between water molecules, allows for moderation of coastal temperatures. The high heat of
vaporization, also due to hydrogen bonding, allows for the cooling of bodies and the ability of ice water to provide insulation
for bodies of water. Van der Waals forces can be mentioned or hydrophobic interactions (these are weak bonds, but there are
many within one molecular attraction – so, together, they are very strong).
Osmosis, cohesion, adhesion and transpiration of water allow for movement through plants. Osmosis is movement of water
from the soil to the roots (from high to low water concentration). Cohesion is the attraction of water molecules to each other
due to hydrogen bonding between the oxygen and hydrogen atoms of two water molecules. Adhesion is the attraction of
water molecules to other hydrophilic materials, such as the glass in test tubes (forming a meniscus), or along the cellulose
walls of the xylem tubes in vascular plants. Cohesion and adhesion form an attraction called capillary action. This allows for
movement of water from roots to the xylem tubes of plants, and up, against the force of gravity. Transpiration, or the
evaporation of water from plant leaves provides an additional pull of water up the plant from roots to leaves. As a result,
water and nutrients are transported from the roots, to the leaves.
Specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius. Due to liquid
water’s high specific heat, ( 1 calorie/gram
0
C = 4.186 joule/gram
0
C ) a considerable amount of energy must be absorbed to
increase the temperature of a body of water. Thus, even though the atmospheric temperature near water fluctuates, the
temperature of a large body of water will tend to remain stable, supporting the plant and animal life. This can also moderate
air temperature near a body of water. If the atmospheric temperature heats up, water will absorb the heat from the
atmosphere and hydrogen bonds between water molecules will break. This will cause the atmospheric temperature to drop.
Atmospheric temperatures near the ocean on a hot day will therefore be cooler compared to inland atmospheric
temperatures. If the atmospheric temperature cools, hydrogen bonds between water molecules will form and heat will be
released and move to the atmosphere. Thus, atmospheric temperatures near the ocean in the winter will tend to be warmer
than inland temperatures.
Heat of vaporization refers to the amount of heat that must be absorbed to break hydrogen bonds between water molecules
so that evaporation can take place. Water has a relatively high heat of vaporization (580 cal/gram). This allows for cooling of
the body on a hot day or moderation of climate temperature. When sweating takes place, heat from your body is absorbed
and hydrogen bonds break in the watery sweat on your arm. As a result, your body cools down. Likewise, if hot steam
condenses on your skin, it will transfer heat to your skin as hydrogen bonds form during condensation. This will cause a
significant burn.
(NOTE: The statements that are in italics are additional, and not needed to answer the question. You do not need to explain
what is happening to hydrogen bonds during heating and cooling but keep in mind that heat must be absorbed to break
hydrogen bonds and heat is released when hydrogen bonds form.)
