
PRF By The Numbers
Produced by Leslie B. Gordon, MD, PhD; MedicalDirector
Please Do Not Reproduce Children’s Photographs Without Express Permission From PRF March 31, 2021

Introduction and Collaborations
3
– 10
Overview Data
11
- 20
International Progeria Registry
21
- 24
PRF Diagnostics Program
25
- 28
PRF Cell & Tissue Bank
29
- 38
PRF Medical & Research Database
39
- 43
Weighing – In Program
44
- 47
Clinical Trials
48
- 56
PRF Grants Program
57
- 62
Scientific Meetings and Workshops
63
- 66
Publications
67
- 69
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
Table of Contents

PRF By The Numbers is a data sharing tool originating from
The Progeria Research Foundation’s programs and services.
We translate information collected within our programs and
services, and develop charts and graphs which track our
progress from year to year.
This allows you to assess where we’ve been, and the
improvements we’ve made for children with Progeria.
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
PRF By The Numbers: A Data Sharing Tool

Why Sharing Data Is Essential
According to the National Institutes of Health:
“data sharing is essential for expedited translation of research
results into knowledge, products, and procedures to improve
human health.”
http://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-032.html
In other words, everyone benefits by knowing and learning as
much as possible about Progeria - the scientific and medical
communities, the public, and the children.
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

PRF By The Numbers…Here’s How It Works
We take raw data collected through our programs and services,
remove any personal information to protect the participant, and
present it to you in a format that is engaging and informative.
PRF programs and services include:
The PRF International Registry
The PRF Diagnostics Program
The PRF Cell & Tissue Bank
The PRF Medical & Research Database
PRF Research Grants
Scientific Workshops
Clinical Trial Funding and Participation
As of March 31, 2021
© 2021 The Progeria Research Foundation. All Rights Reserved.

Our Target Audience
PRF By The Numbers is intended for a broad array of users
Families and children with Progeria
The general public and nonscientists of all ages
Scientists
Physicians
The media
This means that different types of slides will be of interest depending
on who is looking at the information. We have designed this slide
set so that you can pull out what is most important to you.
We love suggestions - if you don’t see some facts and figures here
that you think would be informative, please let us know at
info@progeriaresearch.org
As of March 31, 2021
© 2021 The Progeria Research Foundation. All Rights Reserved.

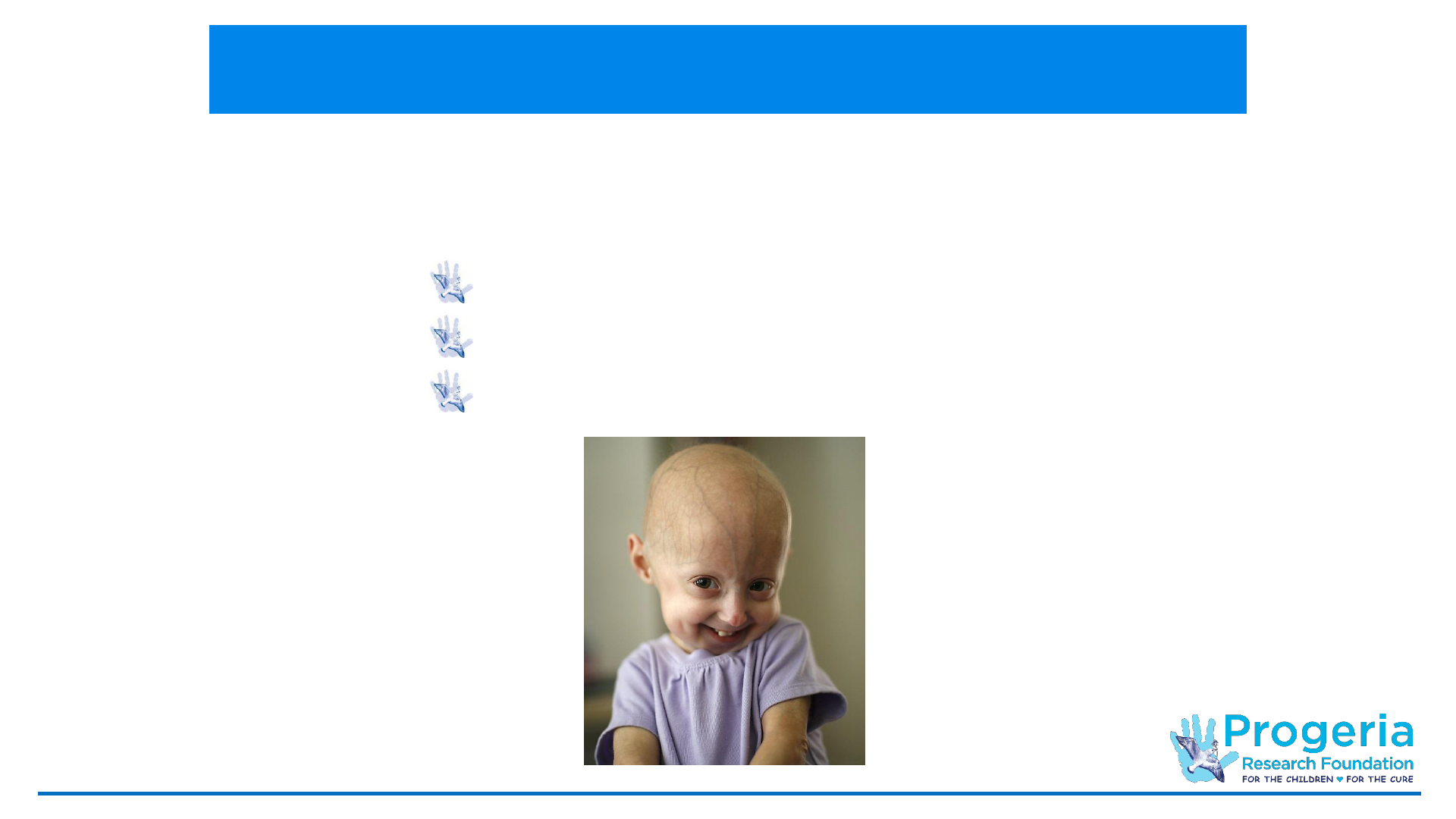
PRF Programs: It All Starts With The Children
Our participants
come from all over
the world. They find
us through our
outreach – the PRF
website, our
publications,
television
documentaries, their
doctors, neighbors,
friends and family.
Patient
Referral
Internation
al Progeria
Registry
Diagnostic
s Program
Cell &
Tissue
Bank
Preclinical
Research
Clinical Trials
Medical &
Research
Database
Weighing-
In Program
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
Program Collaborations For Success
PRF Cell & Tissue Bank Core
Laboratory
PRF Medical & ResearchDatabase
PRF Cell & TissueBank
PRF Diagnostics Program
PRF Diagnostics Program
Sequencing Laboratory
PRF Cell Bank Submission:
Immortalized Fibroblast CellLines
PRF Cell & Tissue Bank : iPS Cell
Line Generation
PRF Cell & Tissue Bank:
Lymphoblast Cell Line
Generation
PRF Clinical Trials
Non-HGPS Progeroid Patient
Diagnosis
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Our Program Collaborators
Our collaborating institutions are crucial to our ability to help children with Progeria.
We are extremely grateful for these ongoing partnerships:
Brown University
Location of The PRF Medical & Research Database
Program IRB approval
Hasbro Children’s Hospital
Location of The PRF Cell & Tissue Bank
Program IRB approval
PreventionGenetics
CLIA*-approved genetic sequence testing
Rutgers University Cell and DNA Repository
CLIA*-approved lymphoblast generation and distribution
University of Ottawa
Induced Pluripotent Stem Cell (iPSC)
CLIA*-approved generation and distribution
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Our Clinical Trial Collaborators
Our collaborating institutions are crucial to our ability
to help children with Progeria
Harvard University – Associated Hospitals:
Boston Children’s Hospital
Brigham and Women’s Hospital
Dana Farber Cancer Institute
NIH – funded Clinical and Translational
Study Unit at Boston Children’s Hospital
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

As of March 31, 2021:
HGPS
*
in the United States:
Progeroid Laminopathies
**
worldwide:
Number of Living PRF-Identified Cases
Total Number of Children with HGPS and PL** Worldwide:
131
19
60
Progeroid Laminopathies
**
in the United States: 13
195
*Children in the HGPS category have a progerin-producing mutation in the LMNA gene
** Those in the Progeroid Laminopathy category have a mutation in the lamin pathway
but don’t produce progerin
© 2021 The Progeria Research Foundation. All Rights Reserved.
HGPS
*
worldwide:
135

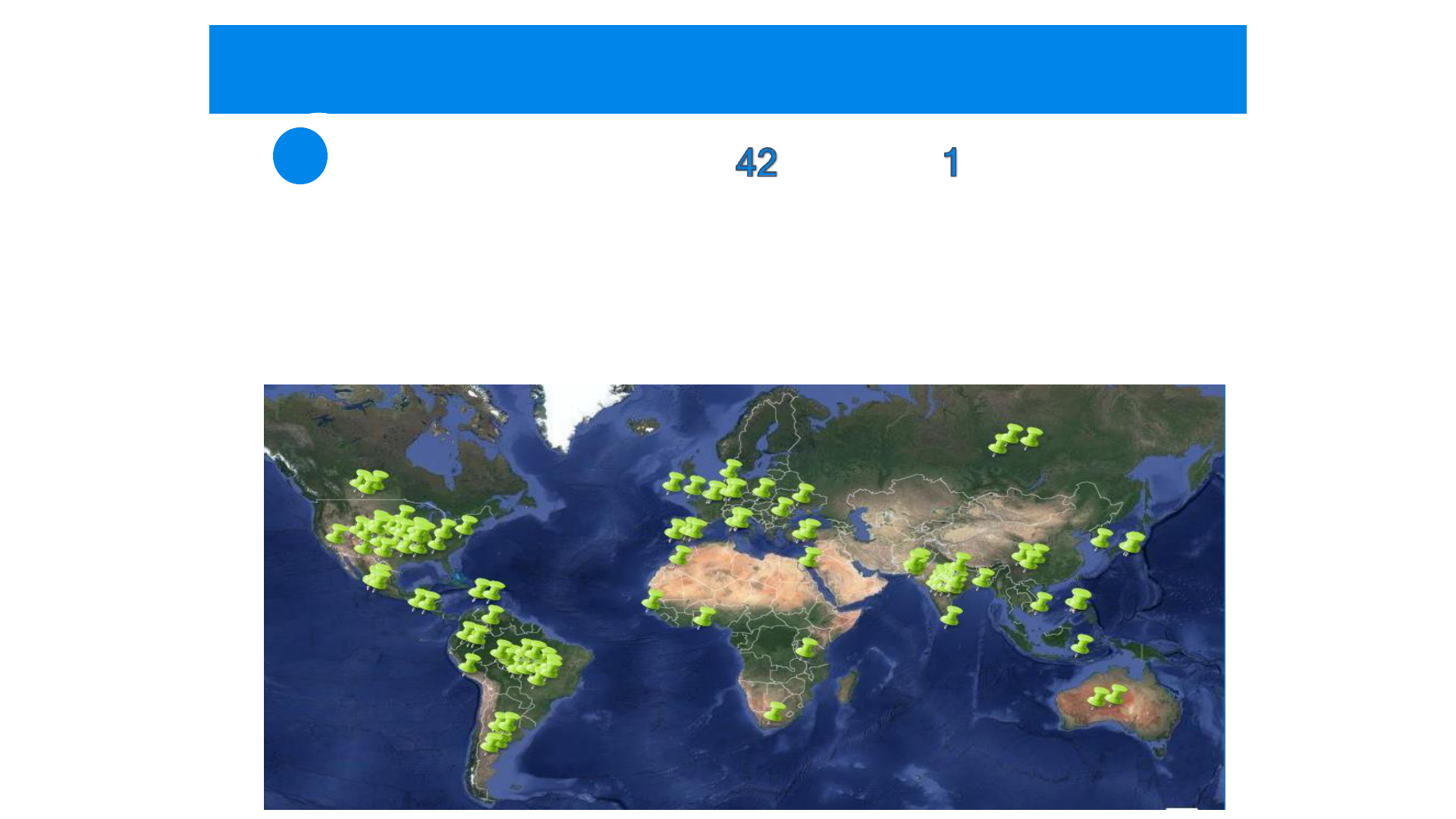
PRF-Identified Cases Reside In 52 Countries
Afghanistan Brazil Egypt Indonesia Japan Nepal Portugal Spain Togo
Algeria Canada England Iran Kazakhstan Oman Russia Sri Lanka Turkey
Argentina China France Iraq Libya Palestine-Gaza Saudi Arabia Suriname Ukraine
Australia Columbia Germany Ireland Luxembourg Pakistan Serbia Sweden USA
Bangladesh Denmark Honduras Israel Malaysia Philippines South Africa Tajikistan
Belgium Dominican India Italy Mexico Poland South Korea Taiwan
Children and Adults with HGPS
Children and Adults with PLs
135 Known Children and Adults with HGPS and
60 with PLs Living around the World as of
March 2021

…and Speak 32 Languages
شياخأبحاثمؤسسة
早衰症研究基金會
Progeria
早老症研究財団
조로증 연구 재단
బాలుడబాలికవయస్సముదరుకండానేవృద్ాాప్యరూప్ంలోనికి
వచ్చుటరీసెర్చుఫ ండేషన్
Progeria Araştırma Vakfı
прогерии исследовательский фонд
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
Arabic
English
Hindi
Marathi
Russian
Ukrainian
Bengali
Ewe
Indonesian
Malay
Serbian
Urdu
Cebuano
Filipino
Italian
Nepali
Spanish
Chinese
French
Japanese
Pashto
Tagalog
Danish
German
Kannada
Polish
Tamil
Dutch
Hebrew
Korean
Portuguese
Turkish

16
17 17
18
19 19
22
26
29
30
31
29
30
34
35
37
41
44
45
46
52
54
78
86
0
10
20
30
40
50
60
70
80
90
100
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011
Number of Children and Countries
Year
Countries
Progeria Cases*
Living Children PRF has identified with Progeria and the countries they reside in from 2000 - 2011
* Progeria cases: Total number of known cases include both HGPS & PL
* When a child passes away, numbers are decreased.
Every Year Our Numbers Grow Option
Every Year Our Numbers Grow

35
39
43
46
44
45
48
51
52 52
86
90
100
107
112 112
120
128
131
135
10
22
25
27
34
32
36
38
57
60
0
20
40
60
80
100
120
140
160
2012 2013 2014 2015 2016 2017 2018 2019 2020 Mar, 2021
Number of Children and Countries
Year
Countries
HGPS
PL
Living Children PRF has identified with Progeria and Progeroid Laminopathies and the countries they
reside in from 2012 – 2021*
* Numbers shown for living children as of Dec. 31 for any given year.
* When a child passes away, numbers are decreased.
Every Year Our Numbers Grow Option
Every Year Our Numbers Grow

Tracking Children with Progeria Through Prevalence
How does PRF estimate how many children we are searching for,
and in what countries? We use
population prevalence
.
Prevalence is the proportion of children with Progeria per total
population.
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

How Prevalence Is Estimated
At PRF, we use a formula based on the number of children
we’ve identified in the US. We then expand that out to the
world population.
We do this because we have the most complete reporting for
the US and since Progeria has no gender, ethnic, or other
biases, we assume that the prevalence in the US is the
same prevalence in other countries.
PRF estimates prevalence for years when the official US
census provides a reliable population number.
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

USA Prevalence of Progeria
March, 2021 population statistics:
The US population was:
Number of PRF-identified children with
HGPS in the US:
19
Prevalence of HGPS in the US:
19 in 331 million is about
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
*estimates routinely fall between 1 in 18 - 1 in 20 million people.
331,002,651 people
1 in 17 million people

Prevalence and World Population of Progeria
Given the world population as of March 31, 2021
.
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Using Prevalence To Find Children In A Certain Country
We can now use the total population estimates for any given country, in order to
understand whether we have found most or all children in a particular country.
For example, as of March, 2021:
Brazil’s population was estimated as
people
Using Prevalence, the number of children living
PRF has identified 7 of these 12 children, and is
searching for the 5 others
with Progeria in Brazil is 213,692,280/17,400,000 = 12
* Data based on the latest
United Nations Population Division
estimates
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
213,692,280

International Progeria Registry*
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
Program Goals:
Patient identification
Outreach to patient families and their physicians
A springboard for program enrollment
Registry forms available at
https://www.progeriaresearch.org/international-registry-2/
*PRF International Registry includes those with genetically confirmed or
clinically suspected Progeria, as well as those with other possible progeroid
syndromes

325 Children Have Registered With PRF
20 20
36
54
61
82
102
118
129
151
160
181
193
208
224
241
254
269
285
319
325
0
20
40
60
80
100
120
140
160
180
200
220
240
260
280
300
320
340
360
2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Mar,
2021
Number of Registrants
Year
© 2020 The Progeria Research Foundation. All Rights Reserved.
As of January, 2021

From 65 Countries and 1 Territory
Algeria
Bulgaria
Dominican Republic
Guatemala
Ireland
Mexico
Panama
Russia
Suriname
USA
Argentina
Canada
Ecuador
Honduras
Israel
Morocco
Peru
Saudi Arabia
Sweden
Venezuela
Australia
Chile
Egypt
Hong Kong
Italy
Nepal
Philippines
Serbia
Switzerland
Vietnam
Bangladesh
China
England
India
Japan
Netherlands
Poland
South Africa
Tanzania
Belgium
Colombia
Finland
Indonesia
Kazahkstan
Oman
Portugal
South Korea
Togo
Bolivia
Czech
Republic
France
Iran
Libya
Pakistan
Puerto Rico
Spain
Turkey
Brazil
Denmark
Germany
Iraq
Malaysia
Palestine
Romania
Sri Lanka
Ukraine
© 2021 The Progeria Research Foundation. All Rights Reserved.
Children Around the World Registered with PRF
As of March 31, 2021

South America
15.4%
N=50
Europe
15.7%
N=51
North America
25.5%
N=83
Asia
35.4%
N=115
Africa
6.5%
N=21
Australia
1.5%
N=5
…And All Continents
Participation (%) By Continent
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
Program Goal:
Genetic Sequence Testing for Progeria-causing mutations
Pre-requisites for Testing:
Registration with PRF International Registry
One or more of the following
Family history – proband, prenatal
Phenotypic presentation – proband, postnatal
Relative of positive proband
Testing information available at:
https://www.progeriaresearch.org/the-prf-diagnostic-testing-program/
PRF Diagnostics Program
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

As of March 31, 2021:
Total Number of Proband Tests Performed:
Exon 11 (HGPS) Mutations:
Other Progeroid Laminopathies (Exons 1 – 12):
Zmpste24 Mutations :
Average Number of Patients Tested Per Year :
Diagnostics Testing Summary
All tests are performed in a Clinical Laboratory Improvement Amendments (CLIA) certified facility.
105
13
2
8.4
154
© 2021 The Progeria Research Foundation. All Rights Reserved.

Mutations Identified Through PRF Diagnostics Program
DNA Mutation Amino Acid Effect Zygosity
Progerin
Producing?
Number
Diagnosed
Classic HGPS – LMNA Mutation
1824
C>T, exon 11 G608G heterozygous Yes 91
Non Classic HGPS– LMNA Mutation
1822
G>A, exon 11 G608S heterozygous Yes 4
1821
G>A, exon 11 V607V heterozygous Yes 2
1868
C>G, exon 11 T623S heterozygous Yes 1
1968+5 G>C, intron
11 --------- heterozygous Yes 2
1968+1 G>C, intron
11 --------- heterozygous Yes 3
1968+2 T>A, intron
11 heterozygous Yes 1
1968+1
G>A, intron 11 heterozygous Yes 1
Progeroid Laminopathy– LMNA Mutation
1579
C>T, exon 9 A527C heterozygous No 1
1579
C>T, exon 9 A527C homozygous No 6
1580G>T,
exon9 A527L Homozygous No 2
1619
T>C, exon 10 M540T homozygous No 3
331
G>A, exon 1 G111L heterozygous No 1
Progeroid Laminopathy– Zmpste24 Mutation
1274T>C, exon
10 L425P homozygous No 2
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

17
21
24 24
26
28
29 29 29 29
30
32
33
35
36 36
37
46
51
56
62
71
76
82
87
94
97
107
108
113
116
118
54
67
75
80
88
99
105
111
116
123
127
139
141
148
152
154
0
20
40
60
80
100
120
140
160
180
2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Mar,
2021
Number Tested
Year
Total Testing LMNA Negative Total Testing LMNA Positive Total Clinically Affected Tested by PRF
*Graph does not include Parents/Siblings tested
Number of Affected Children/Adults Tested and the Number Testing
Positive for
LMNA
Gene Mutation*
Longitudinal Testing Data for PRF Diagnostics Program
© 2020 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

PRF Cell & Tissue Bank
Program Goals:
Provide a resource for researchers
worldwide
Ensure the sufficient availability of
genetic and biological materials
essential for research aimed at
understanding the pathophysiology of
disease and the links between
Progeria, aging and heart disease
Obtain long-term clinical data
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
Resource information available at: https://www.progeriaresearch.org/cell-and-tissue-bank/
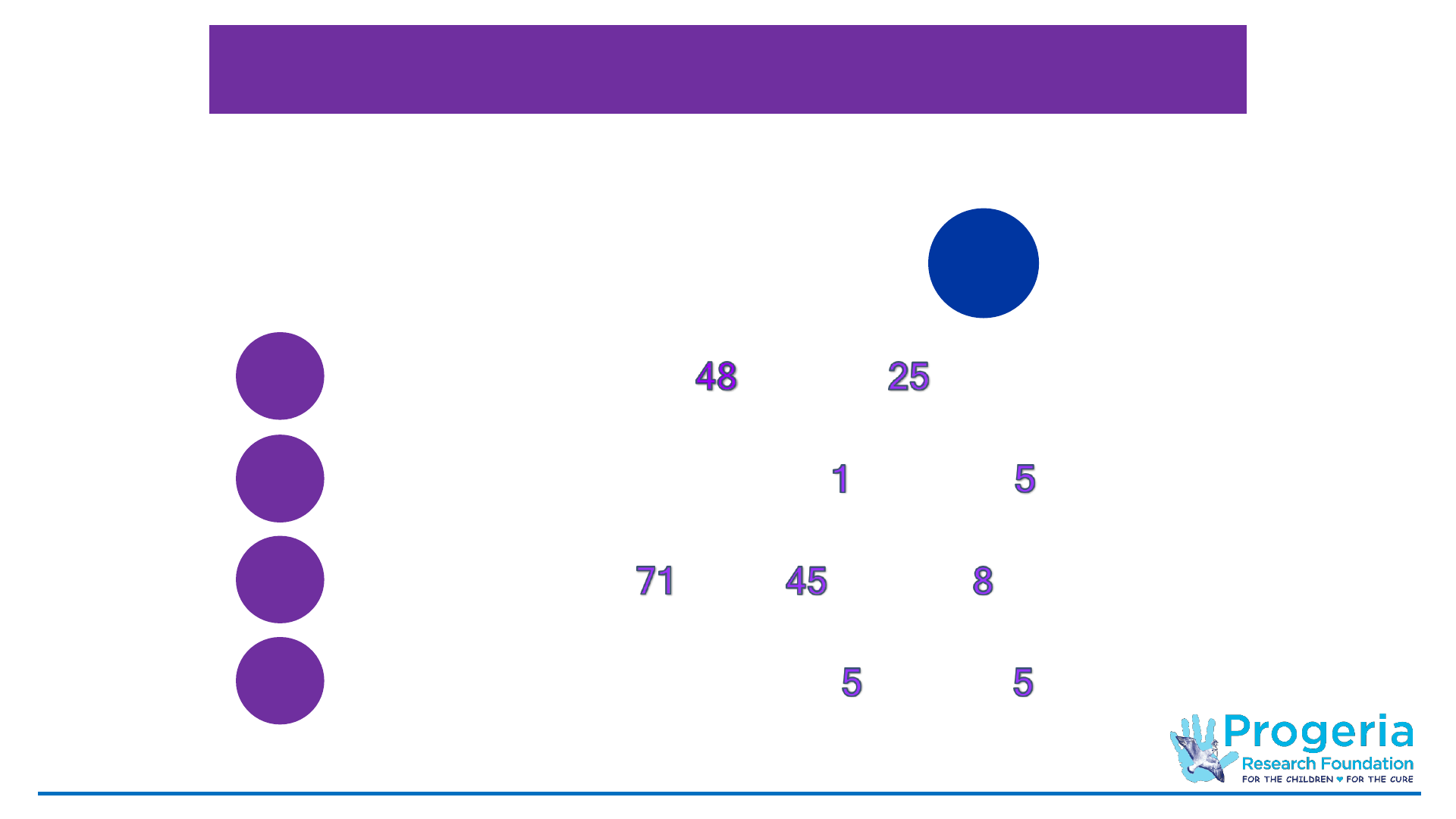
PRF Cell & Tissue Bank Holdings
73
Dermal Fibroblast Lines from
affected and parents
124
Lymphoblast Lines from
affected, parents and siblings
10
Induced Pluripotent Stem Cell Lines from
affected and parents
As of March 31, 2021:
Total Number of Participants:
286*
6 Immortalized Fibroblast Cell Lines from
affected and parents
* Participants may have donated multiple times
© 2021 The Progeria Research Foundation. All Rights Reserved.

© 2021 The Progeria Research Foundation. All RightsReserved.
As of March 31, 2021

Number Of Cell Lines By Year
20
33
35
36
36
39
42
46
61
65
78
78
78
79
82
86
88
88
88
88
17
29
44
54
62
69
74
80
92
100
112
116 116
118
121
123
124 124
125 125
37
62
79
90
98
108
116
126
153
165
190
194 194
197
203
209
212 212
213 213
0
50
100
150
200
250
Number of Cell Lines
Year
Total Cell Lines Parents/Siblings Cumulative
Total Cell Lines Affected Cumulative
Total Number of Cell Lines
© 2019 The Progeria Research Foundation. All Rights Reserved.

PRF Cell & Tissue Bank Distribution
Research Teams From Countries Have Received
As of March 31, 2021:
26
200
Cell Lines
DNA Samples
Tissue, plasma, serum
and other biological samples
Lonafarnib Samples
Senescent Progeria
Fibroblasts in Culture
© 2020 The Progeria Research Foundation. All Rights Reserved.

Biological Sample Distribution Over Time
20 20
16
32
17
12
92
24
29
27
95
62
108
122
168
178
135
219
86
20
0
50
100
150
200
250
2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Mar,
2021
Number of Samples Distributed
Year
Fibroblast Lines Lymphoblast Lines iPSC Lines
DNA Immortalized Cell Lines # = Total Distributed
© 2019 The Progeria Research Foundation. All Rights Reserved.

USA Cell & Tissue Bank Recipients
As of March 31, 2021
Recipient
Institution
Recipient
Institution
H. Erbil Abaci
Columbia
University Medical Center
Mansoor
Amiji
Northeastern
University
Martin
Dorf
Harvard Medical
School
Angelika
Amon
Massachusetts Institute
of Technology
Stephen
Doxsey
U. of Massachusetts
Medical School
Stelios
Andreadis
U. of
Buffalo
Jack
Elias
Brown
University School of Medicine
Samuel
Beck
MDI
Biolab
Mike
Erdos
National Institutes of
Health
Shelley
Berger
U of
Pennsylvania
Jed
Fahey
Johns Hopkins
University
Bruce
Blazer
U. of
Minnesota
Toren
Finkel
NIH
Joseph
Bonventre
Brigham and Women’s
Hospital
Shridar
Ganesan
Cancer Institute
of New Jersey
Demetrios
Braddock
Yale
University
Abhimanyu
Garg
U. of Texas Southwestern
Medical Center
Jonathan
Brown
Vanderbilt
University
Glenn
Gerhard
Temple
University
Ted
Brown
Institute for
Basic Research (IBR)
David
Gilbert
Florida State
University
Mark
Burkhard
University
of Wisconsin-Madison
Thomas
Glover
U.of Michigan
Medical School
Judy
Campisi
Buck
Institute
Robert
Goldman
Northwestern
University
Kan
Cao
U. of
Maryland
Susana Gonzalo
St. Louis School of
Medicine
Li
Chai
Harvard
University
Lilian
Grigorian
Cedars Sinai Medical
Center
Francis
Collins
National
Genome Research Institute
Gregg
Gundersen
Columbia University
Medical Center
Lucio
Comai
U. of Southern
California
Curtis
Harris
National Institutes of
Health
Daniel
Conway
Virginia Commonwealth
University
Martin
Hetzer
Salk
Institute
John
Cooke
Houston Methodist
Research Institute
Camila Hochman
-Mendez
Texas Heart Institute
Mauro
Costa-Mattioli
B
aylor College of Medicine
Liam Holt
NYU Institute for Systems Genetics
Adrienne
Cox
U. of
North Carolina at ChapelHill
Steve
Horvath
UCLA
Greg
Crawford
Duke
University Medical Center
Johnny
Huard
U. of Texas
Health Science Center at Houston
Antonei
Csoka
Howard
University
Jay Humphrey
Yale University
Kris
Dahl
Carnegie
MellonUniversity
Kohta
Ikegami
The University
of Chicago
George
Daley
Boston Children's
Hospital
Vishwanath
Iyer
U. of Texas
Austin
Channing
Der
U. of
North Carolina at ChapelHill
Jose Jalife
University of
Michigan
Mohanish
Deshmukh
U. of
North Carolina at ChapelHill
David
Kaplan
Tufts
University
Dennis
Discher
U. of
Pennsylvania
© 2021 The Progeria Research Foundation. All Rights Reserved.

Recipient
Institution
Recipient
Institution
Timothy
Kowalik
U. of Massachusetts
Medical School
Joseph Rabinowitz
Temple
University
Dmitri
Krainc
Massachusetts
General Hospital
Ana
Robles
National Cancer
Institute
Jan
Lammerding
Harvard
University
David
Sabatini
Whitehead
Institute
Dudley
Lamming
U of
Wisconsin-Madison
John
Sedivy
Brown
University
Jeanne
Lawrence
U.
of Massachusetts Medical School
Christian
Sell
Drexel University College
of Medicine
Joan
Lemire
Tufts University School of
Medicine
Jerry
Shay
UT Southwestern
Medical Center
Kam
Leong
Columbia
University
Jamila H
Siamwala
Brown
University
Jason
Lieb
U. of
North Carolina at ChapelHill
Andrew
Sonis
Boston Children's
Hospital
David
Liu
Harvard
University
Ronald
St-Louis
OVIBIO
Corporation, Inc.
Chengzu
Long
New
York University School of Medicine
Earl
Stadtman
National
Heart, Lung & BloodInstitute
Shigemi
Matsuyama
Case
Western Reserve University
Dylan
Taatjes
U. of
Colorado
Rachel
PattonMcCord
University
of Tennessee
Marc
Tatar
Brown
University
Andrew
Mendelsohn
Regenerative
Sciences Institute
Rajarajan
Amirthalingam Thandavarayan
Houston Methodist
Research Institute
Susan Michaelis
John Hopkins University School of Medicine
Eduardo Torres
U of Massachusetts Medical School
Jeffrey
Miner
Washington University
George
Truskey
Duke
University
Tom
Misteli
National Cancer
Institute
Tetsuro
Wakatsuki
InvivoSciences
, Inc
Ashby
Morrison
Stanford
University
Alan
Waldman
University
of South Carolina
Marsha
Moses
Boston Children’s
Hospital
Steve
Warren
Emory University School of
Medicine
Elizabeth
Nabel
National
Heart, Lung & BloodInstitute
Howard
Worman
Columbia
University
Timothy
Osborne
Sanford
Burnham Medical Research
Institute
Tom
Wight
Hope Heart
Institute
Junko
Oshima
U. of
Washington
Joseph
Wu
Stanford
University
Bryce
Paschal
U. of
Virginia
Feng Zhang
The
Broad Institute
Hamel
Patel
U.
Of California, San Diego
Alessandra
Zonari
OneSkin
Technologies
Mary
Patti
Joslin Diabetes
Center
You
Zou
East Tennessee
University
Taihao
Quan
University
of Michigan
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
USA Cell & Tissue Bank Recipients

International Cell & Tissue Bank Recipients
Recipient
Institution
Country
Recipient
Institution
Country
Andrea
Ablasser
Global Health
Institute
Switzerland
Christopher
Eskiw
Saskatchewan
University
Canada
Vicente
AndrésGarcia
Centro Nacional
de Investigaciones
Cardiovasculares
Spain
Gerardo
Ferbeyre
Université
de Montréal
Canada
Samuel
Benchimol
York
University
Canada
Lino
Ferreira
Center
for Neuroscience and Cell Biology (CNC)
Portugal
Martin
Bergö
Karolinska
Institutet
Sweden
Marco
Foiani
Instituto FIRC
di Oncologia Molecolare
ItalyMartin
Enrico
Bertini
Ospedale Pediatrico Bambino
Gesù
Italy
Alain
Garnier
Université
Laval
Canada
Michael
Blank
Bar
Ilan University
Israel
Yosef Gruenbaum
The Hebrew University
of Jerusalem
Israel
Antonio
Campos de
Carvalho
Federal University
of Rio de Janeiro
Brazil
Nady
El Hajj
Hamad
bin Khalifa University
Qatar
Ana
Carrera
Centro Nacional
de Biotecnologia
Spain
Robert
Hegele
University
of Western Ontario
Canada
Gordon
Chan
University
of Alberta
Canada
Andreas
Hermann
University
of Dresden
Germany
Mario D. Cordero
INEBIR
- Instituto par el estudio de la
Biologia de
la Reproduccion Human
Spain
Corinne
Hoesli
McGill
University
Canada
Lynne
Cox
University
of Oxford
England
Junho
K Hur
Kyung Hee
University
Republic
of
Korea
Thomas
Dechat
Medical
University of Vienna
Austria
Anthony
Hyman
Max
-Planck-Institute of Molecular Cell Biology and
Genetics
Germany
Annachiara
DeSandre-
Giovannoli
Laboratoire
de Génétique Moléculaire
France
Ulrich auf dem
Keller
Technical
University of Denmark
Denmark
Jerome
Dejardon
Institute
of Human Genetics
France
Jan
Korbel
European
Molecular Biology Laboratory
Germany
Karima
Djabali
TU
-Munich
Germany
Christian
Kubisch
Institute
of Human Genetics
Germany
Ma
Dongrui
Singapore General
Hospital
Singapore
Varun
Kumar
Uniklinikum
Heidelberg
Germany
J.
El Molto
Molecular World,
Inc
Canada
Kirsztian
Kvell
University
of Pecs
Hungary
Maria
Eriksson
Medicinsk
Naringslara
Sweden
Taejoon
Kwon
Ulsan National
Institute of Science & Technology
Korea
Chiara
Lanzuolo
CNR
Institute of Cellular Biology &
Neurobiology
Italy

International Cell & Tissue Bank Recipients
Recipient
Institution
Country
Recipient
Institution
Country
Caterina
La Porta
University
of Milan
Italy
Fiorella
Piemonte
Ospedale Pediatrico Bambino
Gesù
Italy
Delphine
Larrieu
University
of Cambridge
England
Neale
Ridgway
University
of Halifax
Canada
Lucia
Latella
National Research Council
(CNR)
Italy
Claudia
Ruebe
Saarland
University
Germany
Giovanna
Lattanzi
ITOI
-CNR Unit of Bologna
Italy
Kanaga
Sabapathy
National Cancer Centre
Singaport
Singapore
Jean
-Marc Lemaitre
Institute
of FunctionalGenomics
France
Isabella
Saggio
Sapienza
University of Rome
Italy
Nicolas
Levy
Génétique Médicale et
Développement
France
Kanda
Sangthongpitag
Experimental Therapeutics
Centre
Singapore
Baohua
Liu
Shenzhen
University
China
Yasuhiro
Shimoyima
Shinshu
University
Japan
Elsa
Logarinho
Instituto
de Biologia Molecular e Celular
Portugal
Ok Sarah
Shin
Korea
University Guro Hospital
Korea
Jun
Lu
Northeast
Normal University
China
Sanjay
Sinha
University
of Cambridge
England
Frank
Lyko
German
Cancer Research Institute
Germany
Michael
Speicher
Medical
University of Graz
Austria
Thorston
Marquart
University
of Münster
Germany
William
Stanford
University
of Toronto
Canada
Felipe Alonso
Massó
Rojas
National
Institute of Cardiology Ignacio
Chávez
Mexico
Michael
Walter
University
of Münster
Germany
Scott
Maynard
Danish Cancer Society
Research Institute
Denmark
Herbert
Waldman
Max
Planck Institute
Germany
Ohad
Medalia
University
of Zurich
Switzerland
Miguel
Weil
Tel Aviv
university
Israel
Denis
Mottet
University
of Liège
Belgium
Jesús
Vazquez Cobos
Haoyue Zhang
Spain
Silvia
Ortega-Gutiérrez
Universidad
de Complutense de Madrid
Spain
Ulrich auf dem Keller
Maastricht University
The Netherlands
Selma
Osmanagic-Myers
Max
Perutz Labs, Medical Universityof
Vienna
Austria
Haoyue Zhang
Shenzhen Bay Laboratory
China
Bum
-Joon Park
Pusan
National University
South
Korea
Alex
Zhavoronkov
Federal
Clinical Research Centre
Russia
Center for Neuroscience
and Cell
Zhongjun
Zhou
University of Hong Kong
China

PRF Medical & Research Database
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
Program Goals:
Collect the patient health records for living and
deceased children with Progeria
Obtain long-term clinical data
Abstract data for longitudinal and cross-
sectional analyses
Better understand the clinical disease process
in Progeria and aging related diseases
Develop treatment strategies and
recommendations for health care professionals
and families

Project staff obtain the patient’s medical records and film
studies from birth throughout the participant’s lifespan.
Medical records include visits to: primary care physicians,
specialty physicians, hospital emergency rooms, hospital
admissions, dentists, physical therapy, occupational therapy
and school health records.
Retrospective data abstraction protocol allows for
specifically targeted or broad spectrum of data.
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
How The PRF Medical & Research Database Works
Enrollment information available at: https://www.progeriaresearch.org/medical-
database/

Medical & Research Database Participation
191
Participants are enrolled from countries and US territory
Argentina China Germany Italy Netherlands PuertoRico
Sri Lanka USA
Australia Columbia Guatemala Japan Oman Romania
Suriname Venezuela
Bangladesh Denmark Honduras Kazakhstan Pakistan Russia
Sweden Vietnam
Belgium Dominica Republic India Libya Peru Senegal Tanzania
Brazil England Indonesia Mexico Philippines South Africa
Togo
Canada Egypt Ireland Morocco Poland SouthKorea
Turkey
Chile France Israel Nepal Portugal Spain
Ukraine
As of March 31, 2021© 2021 The Progeria Research Foundation. All Rights Reserved.

Database Longitudinal Enrollment
11
11
13 16
17
22
27 27
31
34
35
36
39
41
42
43
47
48
51 51 51
20 20
31
43
48
59
70
77
85
99
111
117
126
132
147
152
164
176
183
187
191
0
50
100
150
200
250
2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 Mar,
2021
Number of Participants and Countries
Year
Cumulative Number of Countries
Cumulative Number of Participants
Children Enrolled in The PRF Medical & Research Database
and the Countries of Residence
As of March 31, 2020
© 2021 The Progeria Research Foundation. All Rights Reserved.
Participants with Medical Records Reports:
Types Of Data Collected
Participants with Radiology Studies:
62
166
160°
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
PRF Weighing-In Program
A sub-program of The PRF Medical & Research Database
Collects weight-for-age data prospectively:
Home scale provided by PRF
Parents weigh child weekly or monthly
Report weights electronically
© 2021 The Progeria Research Foundation. All Rights Reserved.
Weighing-In Program Participation
Participants are enrolled from countries and US territory
122
Argentina
Canada
England
Indonesia Mexico
Puerto
Rico
South
Korea
Turkey
Australia
China
Germany
Ireland Morocco
Romania
Spain
Ukraine
Bangladesh
Colombia
Guatemala
Israel Nepal Russia Sri Lanka
USA
Belgium
Denmark
Honduras
Italy
Poland Senegal
Togo
Venezuela
Brazil
Dominion
Republic
India Japan
Portugal South
Africa
Tanzania
Vietnam
Weighing in Participants Around the World
As of April 1, 2019

Participants Enrolled In The PRF Weighing-In Program and
Countries of Residence
20
24
26
29
30
31
34
37
39
42
43
44
49
56
74
77
80
88
99
106
118
122
20
40
60
80
100
120
140
Number Enrolled
and
Number
of
Countries
(Cumulative)
0
2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018
Year
Number of Countries
Number Enrolled
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Data from this program were key in the development of
primary outcome measure for the first drug treatment trial for
Progeria.
As of December 1, 2018, children from The PRF
Weighing-In Program have entered clinical treatment trials
using this data.
Clinical Trials And The Weighing-In Program
Failure to Thrive Starts Towards End of Year One

PRF-Funded Clinical Treatment Trials
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Clinical Drug Treatment Trials
Goals:
To define the natural history of
HGPS in quantifiable terms
that will expand our ability to
measure treatment outcome
To assess the safety of new
treatments for HGPS
To measure effects of
treatments for children with
HGPS on disease status,
changes in health, and survival
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Current Therapeutic Intervention Strategies
Farnesyl-PP + Preprogerin 1
Preprogerin 2
Preprogerin 3
Progerin
Farnesyl
transferase
Zmpste24
ICMT
Autophagy
Everolimus
Key Properties of
Preprogerin/Progerin
not farnesylated;
terminal CaaXbox
farnesylated
farnesylated;
terminal aaX cleaved
farnesylated;
carboxymethylated
mTOR
Lonafarnib
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
Post-translational processing and medications currently under investigation in clinical treatment trials for
Progeria. Items in green = enzymes. Items in red = clinical trial medications that inhibit corresponding
enzymes. Lonafarnib is a farnesyltransferase inhibitor. Everolimus is a rapamycin analogue that inhibits
mTOR and promotes cellular autophagy. FT=farnesyltransferase.

Year Drug(s) Phase Location # Countries
2007-
2010
Lonafarnib 2 Boston 29 16
2009
Lonafarnib
Pravastatin
Zoledronate
Feasibility Boston 5 2
2009-
2013
Lonafarnib
Pravastatin
Zoledronate
2 Boston 45 24
2014-
present
Lonafarnib 2 Boston 71 32
2016 –
present
Lonafarnib
Everolimus
1/2 Boston 60 27
2018 -
present
Lonafarnib
2
Boston
43 from 23
countries enrolled
to date
PRF Funds Clinical Treatment Trials

Participation in PRF Clinical Trials
97
Children have participated in PRF Clinical Trials from
countries:
Argentina
China England Italy Pakistan Romania Sri Lanka Ukraine
Australia Colombia Germany Japan Peru Russia Sweden USA
Belgium Denmark Honduras Libya Philippines South Africa Tanzania Venezuela
Brazil
Dominican
Republic
India Mexico Poland South Korea Togo
Canada Egypt Israel Morocco Portugal Spain Turkey
© 2021 The Progeria Research Foundation. All Rights Reserved.

Treatment Trial Collaborations For Success
The children are seen by physicians from:
Boston Children’s Hospital
Dana-Farber Cancer Institute
Brigham and Women’s Hospital
Data were also generated by scientists from:
Alpert Medical School at Brown University
Brown University School of Public Health
University of California Los Angeles
National Human Genome Research Institute
Schering-Plough Research Institute
Lonafarnib generously provided by Eiger
Everolimus generously provided by Novartis
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Clinical Treatment Trial Efficacy Results
Lonafarnib, a type of farnesyltransferase inhibitor (FTI) is our
first treatment for Progeria.
Results showed improvement in:
Rate of weight gain
Increased vascular distensibility
Improved bone structure
Better neurosensory hearing
Increased Lifespan
Gordon et al, PNAS, 2011
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Clinical Trial Publications
Drug Effect:
Association of Lonafarnib Treatment vs No Treatment With Mortality Rate in Patients With Hutchinson-Gilford Progeria Syndrome.
Gordon et al,
JAMA
, 2018, 319(16):1687-1695.
Survey of Plasma Proteins in Children with Progeria Pre-therapy and On-Therapy with Lonafarnib. Gordon et al,
Pediatric Research
,
2018 Jan 17. Epub Ahead of Print.
Clinical Trial of the Protein Farnesylation Inhibitors Lonafarnib, Pravastatin, and Zoledronic Acid in Children With Hutchinson-Gilford
Progeria Syndrome. Gordon et al,
Circulation
, 2016 Jul 12;134(2):114-25.
Seeking a Cure for One of the Rarest Diseases: Progeria. Collins FS.
Circulation,
2016 Jul 12;134(2):126-9.
Impact of Farnesylation Inhibitors on Survival in Hutchinson-Gilford Progeria Syndrome. Gordon et al,
Circulation
, 2014 Jul 1;130(1):27-
34.
Moving from Gene Discovery to Clinical Trials in Hutchinson-Gilford Progeria Syndrome. King et al,
Neurology
, 2013 Jul 30;81(5):408-9.
Clinical Trial of a Farnesyltransferase Inhibitor in Children with Hutchinson-Gilford Progeria Syndrome. Gordon et al,
Proceedings ofthe
National Academy of Sciences
, 2012 Sep 24.
Neurologic Features of Hutchinson-Gilford Progeria Syndrome after Lonafarnib Treatment. Ullrich et al,
Neurology
, 2013, 81:427-430.
General:
Phenotype and Course of Hutchinson-Gilford Progeria Syndrome. Meredith et al,
New England Journal of Medicine
, 2008, 358(6): 592-
604.
Pubertal Progression in Adolescent Females with Progeria. Greer et al, Journal of Pediatric and Adolescent Gynecology, 2017 Dec 17.
Epub Ahead of Print.
Dermatology:
Initial Cutaneous Manifestations of Hutchinson-Gilford Progeria Syndrome. Rork et al,
Pediatric Dermatology
, 2014,1-7.
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Clinical Trial Publications Continued
Dental:
Hutchinson-Gilford Progeria Syndrome: Oral and Craniofacial Phenotypes. Domingo et al,
Oral Diseases
, 2009, 15(3):187-195.
Cerebrovascular:
Imaging Characteristics of Cerebrovascular Arteriopathy and Stroke in Hutchinson-Gilford Progeria Syndrome. Silvera et al,
American Journal of
Neuroradiology
, 2013 May;34(5):1091-7.
Cardiology:
Cardiac Abnormalities in Patients With Hutchinson-Gilford Progeria Syndrome. Prakask, et al,
JAMA Cardiology
, 2018, Apr 17;115(16):4206-4211.
Mechanisms of Premature Vascular Aging in Children with Hutchinson-Gilford Progeria Syndrome. Gerhard-Herman M, et al.,
Hypertension
.2012
Jan;59(1):92-97; Epub 2011 Nov 14.
Skeletal:
Hutchinson-Gilford progeria is a skeletal dysplasia. Gordon,et al.,
Journal of Bone and Mineral Research
. 2011 Jul;26(7):1670-9.
A Prospective Study of Radiographic Manifestations in Hutchinson-Gilford Progeria Syndrome. Cleveland et al,
Pediatric Radiology,
2012 Sep;42(9):1089-
98. Epub 2012 Jul 1.
Craniofacial Abnormalities in Hutchinson-Gilford Progeria Syndrome. Ullrich et al,
American Journal of Neuroradiology
. 2012 Sep;33(8):1512-8.
Extraskeletal Calcifications in Hutchinson-Gilford Progeria Syndrome. Gordon, CM et al.
Bone.
2019 Aug;125:103-111. Epub 2019 May 8.
. Skeletal maturation and long-bone growth patterns of patients with Progeria: a retrospective study. Tsai, A et al,
The Lancet. Child and Adolescent Health
.
2021. ePub 2021 Feb 28.
Ophthalmology:
Ophthalmologic Features of Progeria. Mantagos et al.,
American Journal of Ophthalmology,
2017 Jul 27.
Audiology:
Otologic and Audiologic Manifestations of Hutchinson-Gilford Progeria Syndrome. Guardiani et al,
The Laryngscope
, 2011, 121(10): 2250-2255.
Microbiome at Sites of Gingival Recession in Children with Hutchinson-Gilford Progeria Syndrome. Bassir et al.
Journal of Periodontology
. 2018, 89(6): 635-644.
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Program Goals:
Attract high level researchers to the field of Progeria, and
provide the ability for them to thrive in the field
Foster researchers of interest to PRF’s mission
Encourage high quality publications
Stimulate novel research that will lead to larger grants from
other resources such as NIH, Ellison Foundation, and others
Projects that are likely to lead to clinical treatment trials
within 5 years
Development of gene and cell based therapies to treat
Progeria
Grants program information available at
https://www.progeriaresearch.org/research-funding-opportunities/
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
PRF Grants Program

Back Row (L to R): Tom Glover PhD, Vicente Andrés Garcia PhD, Tom Mistelli PhD, Maria
Eriksson PhD, W Ted Brown MD, PhD, Frank Rothman PhD (emeritus), Bryan Toole PhD(chair)
Front Row (L to R): Monica Kleinman MD, Christine Harling-Berg PhD, Judy Campisi PhD,
Leslie Gordon MD, PhD, Marsha Moses PhD
PRF Medical Research Committee
Volunteer MRC Reviews Grant Applications Semi-annually
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

PRF Granting Structure
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
PRF’s research focus is highly translational. Topics must fall within the following research
priorities:
Projects that are likely to lead to clinical treatment trials within 5 years. This includes the
discovery and/or testing of candidate treatment compounds in cell-based or animal models
of HGPS. Only proposals that test compounds in a progerin-producing animal or cell model
will normally be considered. Analyses in non progerin-producing models are acceptable,
but only as a comparison to progerin-producing models and with strongjustification.
Development of gene-and cell-based therapies to treat Progeria
Assessment of natural history of disease that may be important to developing outcome
measures in treatment trials (preclinical or clinical)
Phase I Proposals: Awards are typically for 1-2 years in the range of $75,000/year. PRF will
conduct a thorough cost analysis for each project during evaluations of submissions.
Required Qualifications. Principal investigators must hold a faculty appointment or equivalent.
Awards will be granted only to applicants affiliated with institutions with 501(c)3 tax-exempt
status, or the equivalent for foreign institutions.
Letter of Intent (LOI). A letter of intent is required and must be approved before a full application
will be considered. Instructions to submit a Letter of Intent and grant application information, can
be found at https://www.progeriaresearch.org/grant-application/.

As of March 31, 2021, The PRF funding rate is 31%
funded Since inception, grant applications received and
PRF has funded principal investigators from institutions
in countries
Lamina A, progerin, Lamin B in HGPS and aging
Genetics and nuclear function
Preclinical Drug Therapy
Molecular Abnormalities and Therapies
Vascular Pathology
Mouse Models
Stem Cell Investigations and Therapy
Clinical Trials
Grant Funding Rates And Topics
© 2021 The Progeria Research Foundation. All Rights Reserved.
* Submissions include Letters of Intent and Full Grants
USA PRF Grantees
GRANTEE
NAME
INSTITUTION
GRANTEE
NAME
INSTITUTION
Richard
Assoian
University of
Pennsylvania
Joan
Lemire
Tufts University of
Medicine
Jemima
Barrowman
Johns
Hopkins University
Jason
Lieb
University of North
Carolina
Juan
Carlos
Belmonte
Salk Institute for
Biological Studies
Monica
Mallampalli
The Johns
Hopkins School of Medicine
Ted
Brown
The
Institute for Basic Research in
Developmental
Disabilities
Susan
Michaelis
The Johns
Hopkins School of Medicine
Abigail
Buchwalter
University
of California, San Francisco
Thomas
Misteli
National
Cancer Institute
Kan
Cao
NIH; University of
Maryland
Marsha
Moses
Harvard Medical
School; Boston Children’s
Hospital
Christopher
Carroll
Yale
University
Junko
Oshima
University of
Washington
Francis
Collins
National
Institute of Health
Bryce
Paschal
University of
Virginia
Lucio
Comai
University of Southern
California
Joseph
Rabinowitz
Temple
Medical School
John P.
Cooke
Houston Methodist Research
Institute
John
M. Sedivy
Brown
University
Kris
Dahl
Carnegie Mellon
University
Dale
Shumaker
Northwestern
University
Jed
W. Fahey
Johns
Hopkins School of Medicine
Michael
Sinensky
East
Tennessee State University
Toren
Finkel
NIH
Brian
Snyder
Beth
Israel Hospital
Loren
Fong
UCLA
Dylan
Taatjes
University of
Colorado
Michael
Gimbrone
Brigham & Women's
Hospital
Jakub
Tolar
University of
Minnesota
Thomas
W. Glover
University of
Michigan
Katherine
Ullman
University of
Utah
Robert
Goldman
Northwestern
University
Thomas
Wight
Benaroya Research
Institute
Leslie B.
Gordon
Tufts University
School of Medicine; Brown U.
Katherine
Wilson
Johns
Hopkins University
John
Graziotto
Massachusetts General
Hospital
Stephen
Young
UCLA
Brian
Kennedy
Buck Institute for Research on
Aging
Yue
Zou
East
Tennessee State University
Jan
Lammerding
Cornell
University
Dudley
Lamming
University of
Wisconsin Madison
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021
International PRF Grantees
GRANTEE
NAME
INSTITUTION
COUNTRY
Vicente
Andrés Garcia
Centro
Nacional de InvestigacionesCardiovasculares
Spain
Samuel
Benchimol
York University,
Toronto
Canada
Martin
Bergö
Karolinska
Institute
Sweden
Claudia
Cavadas
University
of Coimbra
Portugal
Jesús
VázquezCobos
Centro
Nacional de InvestigacionesCardiovasculares
Spain
Thomas
Dechat
Medical
University of Vienna
Austria
Karima
Djabali
Technical University
of Munich
Germany
Maria
Eriksson
Karolinska
Institute
Sweden
Gerardo
Ferbeyre
Université
de Montreal
Canada
Célia Ferreira de Oliveira
Aveleira
University
of Coimbra
Portugal
Roland
Foisner
Medical
University of Vienna
Austria
Giovanna
Lattanzi
University
of Bologna
Italy
Elsa
Logarinho
University
of Porto
Portugal
Evgeny
Makarov
Brunel
University
England
Silvia
Ortega-Gutiérrez
Universidad Complutense
de Madrid
Spain
Bum
-Joon Park
Pusan National
University
Korea
Isabella
Saggio
Sapienza
University of Rome
Italy
Charlotte
Sorenson
Centro
Nacional de InvestigacionesCardiovasculares
Spain
William
Stanford
University
of Toronto
Canada
Colin
Stewart
Institute
of Medical Biology
Singapore
Ricardo
Villa-Bellosta
Instituto
de Investigación Sanitaria - Fundación Jiménez Díaz
Spain
Anthony
Weiss
University
of Sydney
Australia
Zhongjun
Zhou
University of
Hong Kong
China
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

Meeting Goals:
To promote
collaboration
between basic and
clinical scientists
toward progress in
Progeria,
cardiovascular, and
aging research PRF
has held 13
international
scientific meetings.
PRF Scientific Meetings
13
© 2020 The Progeria Research Foundation. All Rights Reserved.
2020 PRF Workshop
Webinar

These are large multi-day workshops open to all scientists. Clinical and basic
researchers spend intense days sharing data and planning new
collaborations for progress towards treatments and cure.
Various NIH Institutes have funded all international workshops through R13
and other granting mechanisms
Other organizations have also generously sponsored workshops
International Workshops Promoting Global Interest In Progeria,
Cardiovascular Disease And Aging
© 2021 The Progeria Research Foundation. All Rights Reserved.

Growth of Global Interest In PRF Workshops
20
30
36
56
46
52
3
5
10 10 10
18
14 14
50
46
56
90
100
140
180
173
156
377
0
50
100
150
200
250
300
350
400
2001 2003 2005 2007 2010 2013 2016 2018 2020
Number
PRF Workshop Year
Number of Posters
Registrant Countries
Registrant Number
© 2020 The Progeria Research Foundation. All Rights Reserved.
As of January, 2021
2020 was a webinar.
Posters N/A

Subspecialty Scientific Meetings
Small, focused meetings designed to promote and support work in areas of high
interest for Progeria
First Genetics Consortium Meeting – “Searching
for the Progeria Gene”, August 23, 2002, Brown
University, Providence, RI
Second Genetics Consortium Meeting – “Post-
gene Discovery”, July 30, 2003, Bethesda, MD
Bone Marrow Transplant Meeting – “Forging
Ahead by Exploring Potential Treatments”, April
25-26, 2004, National Institutes of Health,
Bethesda, MD
New Frontiers in Progeria Research (2012),
Boston, MA
© 2021 The Progeria Research Foundation. All Rights Reserved.
As of March 31, 2021

As of March 31, 2021
Scientific articles have been published citing The Progeria Research
Foundation Grants Funding Program
Scientific articles have been published citing PRF Cell & Tissue Bank
resources
Scientific articles have been published citing The PRF Medical & Research
Database
Scientific articles have been published from clinical trial data
See slide #55 and #56
Scientific Publications
© 2021 The Progeria Research Foundation. All Rights Reserved.
163
97
28
23
Publication list available at: www.progeriaresearch.org/prfprp/

Progeria Clinical Care Handbook
768
The Progeria Handbook 2
nd
Edition. A
Guide for Families & Health Care
Providers of Children with Progeria.
The
Progeria Research Foundation.
Leslie B. Gordon
MD, PhD; Medical Director (editor) 2019.
Provided in English, Spanish
and Japanese
Expert contributors from Boston
Children’s Hospital
Number of Progeria Care Handbooks distributed to
families of those with Progeria and their care givers:
© 2021 The Progeria Research Foundation. All Rights Reserved. As of March 31, 2021

