
STATE BOARD OF EDUCATION
CASANDRA E. ULBRICH – PRESIDENT
•
PAMELA PUGH – VICE PRESIDENT
TIFFANY D. TILLEY – SECRETARY
•
TOM MCMILLIN – TREASURER
JUDITH PRITCHETT – NASBE DELEGATE
•
ELLEN COGEN LIPTON
NIKKI SNYDER
•
JASON STRAYHORN
608 WEST ALLEGAN STREET
•
P.O. BOX 30008
•
LANSING, MICHIGAN 48909
www.michigan.gov/mde
•
833-633-5788
GRETCHEN WHITMER
G
OVERNOR
S
TATE OF
M
ICHIGAN
DEPARTMENT OF EDUCATION
L
ANSING
MICHAEL F. RICE, Ph.D.
S
TATE
S
UPERINTENDENT
MEMORANDUM
DATE: September 28, 2021
TO: State Board of Education
FROM: Michael F. Rice, Ph.D., Chairperson
SUBJECT: Presentation of the Proposed Standards for the Preparation of Middle
Grades and High School Science Teachers
In pursuit of Michigan’s Strategic Education Plan Guiding Principles that “all
students have access to high quality instruction” and “all educators have the
resources, support, and training needed to educate students,” this proposal is
presented to the State Board of Education (SBE) for the revision and adoption of
new Standards for the Preparation of Middle Grades (5-9) and High School (7-12)
Science Teachers. These standards would replace Michigan’s current preparation
standards for teachers in the areas of integrated science, biology, chemistry, earth
and space science, physical science, and physics. This update would inform
program development and continuous improvement efforts at Michigan's educator
preparation institutions.
In November 2018 and August 2020, the SBE adopted new Standards for the
Preparation of Teachers of Lower Elementary (grades PK-3) and Upper Elementary
(grades 3-6) and Middle Grades (grades 5-9) and High School (grades 7-12)
English Language Arts and Mathematics. Following development of these standards,
stakeholder groups representing PK-12 science teachers, science curriculum and
instruction experts, college and university teacher educators, and college and
university science educators began meeting to revise Michigan’s teacher
preparation standards in science, which included standards for integrated science,
biology, chemistry, earth and space science, and physics. Stakeholders included
experts in adolescent learning and development and professional teacher
preparation, and science instruction and content, including the above-named
sub-disciplines of science. These groups met consistently from September 2019
through July 2021 to develop new sets of preparation standards in science for the
Middle Grades (5-9) and High School (7-12) grade bands.

Page 2
September 28, 2021
2
Additionally, feedback was provided throughout the process from the Michigan
Science Teachers Association, Michigan Mathematics and Science Learning Network,
the Michigan Science Professional Learning Network, and the Confederation of
Michigan Tribal Education Directors. Additional feedback was solicited from selected
stakeholders representing PK-12 schools and districts, intermediate school districts,
college and university teacher education programs, the education research
community, and teacher and administrator professional organizations. All feedback
was reviewed by the original stakeholder groups for refinement of the draft
standards.
Attachment A presents an introduction to the standards, including the development
of each set of standards, background, framework development, and stakeholders
involved in the drafting and reviewing process. Attachment B provides the
Standards for the Preparation of Middle Grades and High School Science Teachers.
Attachment C provides the Middle Grades Standards for Optional Disciplinary
Specializations. Attachment D provides the High School Standards for Optional
Disciplinary Specializations. Attachment E describes the framework used to develop
the proposed standards.
This presentation will be followed by a period of public comment and a request for
approval at the February 2022 SBE meeting.

Attachment A
3
Introduction to Standards for
the Preparation of Middle Grades (5-9) and
High School (7-12) Science Teachers

4
Development of the Proposed Standards
Since 2015, the Michigan Department of Education (MDE), in collaboration with
Michigan’s stakeholders, has been working to revise Michigan’s teacher certification
structure and improve the preparation of the educator workforce in Michigan. This
is in direct alignment with Michigan’s Top 10 Strategic Education Plan.
This collaboration has led to the design of a structure that places students at the
heart of the system. A key goal of this structure is deeper preparation of teachers
to meet the unique learning, developmental, and social-emotional needs
of children at each grade level. This structure includes focused grade bands to
provide new teachers with specialized knowledge about the students and content
they will teach and defined clinical experiences and foundational coursework for
each grade band.
The purpose of the Standards for the Preparation of Middle Grades (5-9) and High
School (7-12) Science Teachers is to establish a shared vision for the core
knowledge, skills, and dispositions that well-prepared beginning science teachers of
middle level and high school students in Michigan should possess and demonstrate
in their teaching. The standards reflect a vision of a well-prepared beginning
teacher who is prepared to enact high-quality science instruction; address the
needs of the whole child; use relevant, research-based criteria to establish a
supportive, engaging environment that fosters learning; and use practices that
meet the individual adolescent’s needs. These standards are intended to support
the preparation of classroom educators who will have deep understanding of
science content for teaching, enact best instructional practices, and be ethically
guided and prepared to address the state standards for student science learning.
Building on the work of the certification restructuring and the revision and adoption
of teacher preparation standards for the Early Elementary (PK-3) and Upper
Elementary (3-6) grade bands, as well as English Language Arts, Mathematics, and
Professional Knowledge and Skills for the Middle Grades (5-9) and High School
(7-12) grade bands, a stakeholder committee was convened to develop preparation
standards in science for the 5-9 and 7-12 grade bands. This group, representing
PK-12 science teachers, science curriculum and instruction experts, college and
university teacher educators, and college and university science educators began
meeting to revise Michigan’s teacher preparation standards in science, which
included standards for integrated science, biology, chemistry, earth and space
science, physical science, and physics. Stakeholders included experts in adolescent
learning and development and professional teacher preparation, and science
instruction and content, including the above-named sub-disciplines of science.
The stakeholder committee began its work by reviewing Michigan’s current
Standards for the Preparation of Teachers of Integrated Science–Secondary
,
adopted by the State Board of Education (SBE) in 2002, to determine whether they
provided adequate guidance to prepare teachers to support students in the 5-9 and
7-12 grade bands in achieving the Michigan K-12 Science Standards. The
committee considered whether to reaffirm existing Michigan teacher preparation
standards, compose new standards, or adopt a national set of standards as
Michigan’s standards. They unanimously recommended that new science
5
preparation standards be composed for two reasons: 1) to integrate science and
engineering practices and crosscutting concepts with science knowledge, and 2) to
align with both the National Science Teachers Association’s (NSTA) Teacher
Preparation Standards and Michigan’s K-12 Science Standards. The following were
used as source material and guidance:
• NSTA Teacher Preparation Standards and
NSTA Middle School Content
Analysis and NSTA Secondary Content Analysis
• MDE Core Teaching Practices
• MDE Clinical Experiences Requirements
• Upper Elementary (Grades 3-6) Teacher Preparation Standards for Science
• Michigan K-12 Science Standards
• A Vision for NSF Earth Sciences 2020-2030: Earth in Time
• Equity and Family Engagement
• MDE Focus on Whole Child
• MDE Strategic Plan Guiding Principles
At the start, the committee determined that because of the three-dimensional
nature of the Michigan K-12 Science Standards, a similar framework would be
required to guide the writing of the new preparation standards to ensure they align
well with the K-12 standards. The stakeholders determined that this framework
should both mirror the Michigan K-12 Science Standards’ three-dimensional
framework and allow for integration of and alignment with all of the key MDE
initiatives and essential aspects identified by these source documents for science
teacher preparation.
The three components of the framework stakeholders developed to draft these
proposed standards are termed facets. The first facet, core knowledge, defines the
knowledge needed to teach science, including both science content and knowledge
of learners and learning, planning and instruction, and safety in science instruction.
The second facet, science teaching practices, contains the skills and craft of science
teaching and describes what well-prepared beginning teachers of science need to
be able to do with the core knowledge to teach students. Finally, the third facet,
guiding principles for teaching science, are intellectual tools and critical dispositions
which serve as a mindful lens for decision-making in a variety of situations to
promote science learning for all students. These three facets were then braided
together to draft these proposed standards in such a way that each standard has an
element of each facet. Further explanation of the stakeholders’ rationale in
developing a framework with three facets can be found in the Science Teacher
Preparation Standards Framework in Attachment E.
The stakeholder committee met twice monthly beginning in September 2019
through July 2021. The framework was developed by January 2021, and the
standards for all science endorsements drafted by June 2021. The committee
solicited feedback from additional stakeholders with expertise in science content,
instruction, and teacher preparation for middle grades and high school and met
several times in July 2021 to refine the draft standards in response to this feedback
and to ensure alignment between the standards and research on effective science
instruction.

6
Resulting Shifts
These proposed standards represent several shifts from the current science teacher
preparation standards:
1. Multi-faceted – The proposed standards shift to a clear focus on the
multi-faceted understandings well-prepared beginning teachers need to teach
science and include an essential balance between core knowledge aspects of
pedagogy and content knowledge, direct alignment with K-12 science
standards, and contextualized knowledge within principled practice.
2. Equity – The proposed standards have a deep focus on equity, shifting the
vision of a well-prepared beginning teacher at the secondary level from an
emphasis on possessing decontextualized content knowledge and toward an
emphasis on classroom practices and contextualized understandings of
science instruction that address the diverse social, emotional, developmental,
and learning needs of the whole child.
3. Performance-based – The proposed standards are written in such a way as to
enable the shift toward practice-based teacher preparation programs to
ensure candidates have had multiple opportunities to practice the craft of
teaching and strengthen it based on specific feedback about their enactment
of science teaching practices.
Structure of the Endorsements
The stakeholders recommended that all teacher candidates pursuing certification
with an endorsement to teach science earn a broad Science endorsement as their
baseline credential. Candidates then have the option, either as part of initial
preparation or as an additional endorsement, to stack an endorsement in a
specialized science discipline onto the broad endorsement. As a result of this
endorsement structure, these standards were intentionally developed in such a way
that the broad Science endorsement contains all of the necessary knowledge and
skills that a well-prepared beginning middle grades or high school science teacher
needs to teach coursework addressing Michigan K-12 Science Standards for the
respective grade band. This allows for the specialized disciplinary endorsements to
tightly focus on additional content knowledge needed to teach that sub-discipline in
areas that are beyond the Michigan K-12 Science Standards for that grade band.
The proposed standards encompass the following areas:
Table 1: Science Endorsement Structure
Middle Grades (5-9)
High School (7-12)
ALL Science Teachers
will earn one of these.
Middle Grades Science
Endorsement
High School Science
Endorsement
Optional Disciplinary
Specializations may be
added.
• Biology
• Earth and Space Science
• Physical Science
• Biology
• Chemistry
• Earth and Space
Science
• Physics
7
Only teacher candidates earning or possessing a middle grades Science (5-9)
endorsement may add a middle grades biology, earth and space science, or
physical science specialized disciplinary endorsement.
Only teacher candidates earning or possessing a high school Science (7-12)
endorsement may add a high school biology, chemistry, earth and space science, or
physics specialized disciplinary endorsement.
Placement Considerations
A middle grades science teacher will be well-prepared to teach science courses
targeting grades 5 and middle school level Michigan Science Standards.
A middle grades science teacher with an optional disciplinary specialized
endorsement will be well-prepared to teach science courses targeting grades 5 and
middle school level Michigan science standards and science courses in that
discipline at the introductory high school level.
A high school science teacher will be well-prepared to teach science courses
targeting high school level Michigan Science Standards.
A high school science teacher with an optional disciplinary specialized endorsement
will be well-prepared to teach science courses targeting high school level Michigan
Science Standards and science courses in that discipline at the advanced high
school level, just beyond the Michigan Science Standards and Michigan Merit
Curriculum.
Table 2: The following chart is intended to clarify the placement considerations:
Endorsement or
Combination
This teacher is well-prepared to teach:
Middle Grades
Science only
Courses with grades 5-9 students that target grade 5 or
Middle School Michigan Science Standards.
Middle Grades
Science + MG
Biology
Courses with grades 5-9 students that target grade 5 or
Middle School Michigan Science Standards AND courses that
target beginning level life science standards in the High
School Michigan Science Standards.
Middle Grades
Science + MG Earth
and Space Science
Courses with grades 5-9 students that target grade 5 or
Middle School Michigan Science Standards AND courses that
target beginning level earth and space standards in the High
School Michigan Science Standards.
Middle Grades
Science + MG
Physical Science
Courses with grades 5-9 students that target grade 5 or
Middle School Michigan Science Standards AND courses that
target beginning level physical science standards in the High
School Michigan Science Standards.
High School
Science only
Courses with grades 7-12 students that target High School
Michigan Science Standards.
High School Science
+ HS Biology
Courses with grades 7-12 students that target High School
Michigan Science Standards AND courses that target
advanced level life science standards beyond the High
School Michigan Science Standards.

8
High School Science
+ HS Chemistry
Courses with grades 7-12 students that target High School
Michigan Science Standards AND courses that target
advanced level chemistry area physical science standards
beyond the High School Michigan Science Standards.
High School Science
+ HS Earth and
Space Science
Courses with grades 7-12 students that target High School
Michigan Science Standards AND courses that target
advanced level earth and space science standards beyond
the High School Michigan Science Standards.
High School Science
+ HS Physics
Courses with grades 7-12 students that target High School
Michigan Science Standards AND courses that target
advanced level physics area physical science standards
beyond the High School Michigan Science Standards.

9
Participants in Science Teacher Preparation Standards Development
Joseph Austin, Virtual Academy Teacher and K-12 Science Curriculum Consultant,
Waterford School District
Richard Bacalor, Science Consultant, Wayne Regional Educational Service Agency
Rachel Badinowski, Adjunct Professor, School of Education, Wayne State University
Rebecca Brewer, High School Biology Teacher, Troy School District; Textbook
Author, Biology Now; Teacher Ambassador, National Center for Science
Education
Dr. Diane S. Brown, Immaculate Heart of Mary, Associate Professor of Education,
Siena Heights University
Wanda Bryant, Middle School Science Teacher, Detroit Public Schools Community
District
Mary Burke, Science Instructional Consultant, Kalamazoo Regional Educational
Service Agency
Kristy Butler, High School Science Teacher, Forest Hills Public Schools; Michigan
Science Teachers Association Board Member
Sarah Cartwright, Science Teacher, Berkley Public Schools
Betty Crowder, Teacher Educator, Oakland University; Executive Director, Michigan
Science Teachers Association
Amy Deller, Math and Science Coordinator, Ann Arbor Public Schools
Melanie Dever, Science Teacher, Dexter Schools
James Emmerling, Science Consultant, Oakland Schools
Dr. Marcia Fetters, Associate Dean and Director of Teacher Education, Western
Michigan University
Jacob Foster, Founder, STEM (Science, Technology, Engineering, and Mathematics)
Learning Designs
Michael Gallagher, MiSTEM Regional Director, Oakland Schools and MiSTEM Network
Linnea Gibson, K-12 Science Consultant, Northern Michigan Learning Consortium,
Charlevoix-Emmet Intermediate School District
Mark Granger, Science Teacher, Jackson Public Schools
Mark Hackbarth, Science Teacher, Midland Public Schools; President, Midland City
Education Association
Dr. Brian Hancock, Assistant Professor of Education, Alma College
10
Tamara Heck, Science Education Research Consultant, Michigan Department of
Education
Dr. Charles Henderson, Professor of Physics, Western Michigan University
Holly Hereau, Science Educator, BSCS (Biological Sciences Curriculum Study);
Adjunct Faculty College of Education, Grand Valley State University; Adjunct
Faculty Biology, Macomb Community College
Dr. Deborah Herrington, Professor of Chemistry and Integrated Science Program,
Grand Valley State University
Kelly Hodges, Associate Director of Teacher Preparation and Accreditation, Michigan
State University
Dr. James Holly, Jr., Assistant Professor, Mechanical Engineering, University of
Michigan College of Engineering, University of Michigan
Dr. Jennifer Hicks, Director of K-12 STEM (Science, Technology, Engineering, and
Mathematics) Engagement, Purdue University
Dr. Jacqueline E Huntoon, Provost and Senior Vice President, Michigan
Technological University
Brian Hugo, 9-12 Science Department Chair and Physics Instructor, Grand Blanc
Community Schools
Dr. Thomas E. Keller, Former State Science Supervisor; Co-Leader of Framework
for K-12 Science Education; Founder and Director STEM Education Strategies
LLC
Dr. Sarah Keenan-Lechel, Regional Director, MiSTEM Network
Dr. Mitchell Klett, Professor, School of Education, Northern Michigan University
Angela Kolonich, Research, Create 4 STEM, Michigan State University
Dr. Sean Kottke, Educator Preparation Unit Manager, Michigan Department of
Education
Dr. Beth Kubitskey, Associate Dean of Students and Curriculum, College of
Education, Eastern Michigan University
Nancy Karre, Outreach Science Education Consultant, Battle Creek Math and
Science Center
Tina M. Larson, Science Education Consultant
Dr. Debra Linton, Professor, Biology; Chair, Interdisciplinary Science Education
Council, Central Michigan University
Dr. Stephen Mattox, Professor of Geology, Grand Valley State University
Rae McEntyre, Science Consultant, Kentucky Department of Education
11
Darcy McMahon, Higher Education Consultant, Michigan Department of Education
Julie Milewski, Curriculum Director, Central Montcalm Public Schools
Russ Miller, Science Teacher and Assistant Principal, Lakewood Public Schools
Lisa Ogiemwonyi, Science Education Consultant, Wayne Regional Educational
Service Agency
Dr. Mark Olson, Associate Professor, School of Education, Oakland University;
Faculty Associate, School of Education, University of Wisconsin-Madison
Keith Piccard, Science Teacher, Allendale Public Schools; Biology Lecturer, Grand
Valley State University
Michael Pillay, MiSTEM/Science Coordinator, Genesee Intermediate School District
Emily Pohlonski, Science Teacher/Content Area Leader, NBT Teacher, Novi
Community School District
Dr. Gail Richmond, Associate Dean for Students and Curriculum, Associate
Professor of Physics and Astronomy, Michigan State University
Ed Roeber, Assessment Director, Michigan Assessment Consortium
Hillary Rose, Science Teacher, New Tech School High
Ricky Scott, Secondary Science Education Specialist, Utah State Board of Education
Katrina Schneider, Science Teacher, Michigan Great Lakes Virtual Academy
Hakim Shahid, Science Director, New Paradigm for Education
Nathan Spencer, Science Consultant, Wayne Regional Education Service Agency
Chris Standerford, Director, Central Upper Peninsula MiSTEM Region, MiSTEM
Network; The Glenn T. Seaborg Mathematics and Science Center, Northern
Michigan University
Dr. Mary Starr, Executive Director, Michigan Mathematics and Science Learning
Network
Dr. Robert L. Stephenson, Science, Technology, Engineering, and Mathematics
Consultant, Ingham Intermediate School District
Dr. David Stroupe, Associate Professor, Michigan State University
Amanda Sturm, Agriscience Teacher, Montcalm Area Intermediate School District
Susan Tate, Middle School Science Teacher, Whitehall Middle School; Michigan
Science Teachers Association
Board Member
Dr. Marshall Thomsen, Professor, Department of Physics and Astronomy, Eastern
Michigan University
12
Michelle Tindall, Lecturer, Teacher Education, Oakland University
Michelle VandyBogurt, Science Teacher, Northwest Community Schools
Jennifer VanWagnen, Science Teacher, Columbia Schools
Wendi Vogel, Science Consultant, Kent Intermediate School District; Region B
Director, National Science Educational Leadership Association
Cheryl Wilson, Computer Science Consultant, Michigan Department of Education
J. Louise Wilson, Science Teacher, Grand Rapids Public Schools
Richard Wright, Science Teacher, Andrews Academy
Dr. Sandra Yarema, Science Teacher Educator, Wayne State University; Michigan
Science Teachers Association Board Member

Attachment B
13
Standards for the Preparation of Middle
Grades (5-9) and High School (7-12) Science
Teachers
SBE PRESENTATION DRAFT

Attachment B – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) and High School (7-12) Science Teachers
14
MIDDLE GRADES (MG) AND HIGH SCHOOL (HS) SCIENCE TEACHER
PREPARATION STANDARDS
MG/HS.S1. LEARNERS AND LEARNING ENVIRONMENTS
Well-prepared beginning teachers of science:
S1.1 Learn about, consider, and incorporate students’ backgrounds to plan and
adapt instruction that leverages the iterative nature of sense-making and
promotes positive student identities.
S1.2 Monitor and maintain relationships with students while engaging them in
productive struggle and discourse to challenge science ideas and construct science
meaning together, keeping in mind the importance of supporting each student’s
development through scaffolded sense-making.
S1.3 Encourage students to share their thinking about phenomena or problems with
intentional use of talk moves in science to foster an inclusive, equitable and
anti-bias environment that respects students’ cultures.
S1.4 Ensure that group tasks and structures allow all students to build understanding,
identities, and perceptions as science learners, in a variety of environments
(e.g., the laboratory, field, and community) while respecting culturally different
ways of knowing and reinforcing their rightful presence in science.
S1.5 Engage students in learning activities using science and engineering practices and
crosscutting concepts to socially construct explanations of a phenomenon or
solutions to a problem.
MG/HS.S2. CONTENT PEDAGOGY
Well-prepared beginning teachers of science:
S2.1 Elicit and interpret student ideas about anchoring scientific phenomena and
problems in three-dimensional teaching and learning situations to make
instructional decisions that engage students in collaborative, evidence-based sense-
making, while valuing all students’ ways of knowing and doing science.
S2.2 Design three-dimensional learning experiences to connect to and build upon the
lives of learners by leveraging learners’ prior experiences and knowledge, using
varying research-based pedagogies such as talk and group work in ways that are
culturally sustaining and enhance scientific ways of thinking.
S2.3 Uncover and consider students’ verbal and visible thinking to plan and implement
appropriate differentiation and research-based pedagogical strategies to support
and prioritize student needs, perspectives, questions, and problems so that all
students develop conceptual scientific knowledge.
Attachment B – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) and High School (7-12) Science Teachers
15
S2.4 Support students to construct arguments to develop and defend explanations of
scientific phenomena or solutions to engineering problems through the applications
of appropriate scientific practices and crosscutting concepts so that students may
be empowered to contribute to scientific problem solving in their community and in
the world.
S2.5 Create opportunities for students to value diverse ways of thinking that build on
their histories and experiences, while mobilizing social capital to engage all students
in solving problems and using engineering practices.
S2.6 Build mutual trust with students through caring support and alignment of
instruction and assessment strategies which address students’ prior knowledge and
partial understandings while navigating tensions between alternative and canonical
ideas or ways of knowing.
S2.7 Select and integrate science-specific technological tools which engage learners in
three-dimensional learning to explore, describe, and explain phenomena and
support students’ conceptual understanding.
S2.8 Uncover and consider students’ thinking to select appropriate instructional
strategies that illustrate the interdisciplinary nature of science and engineering and
that allow students to demonstrate sense-making and understanding in different,
valid, and informative ways.
S2.9 Support students to persevere in making sense of new observations, information,
or data and to develop arguments supported by credible evidence and valid
reasoning using connections to other core disciplines (mathematics, social studies,
and English language arts).
MG/HS.S3. IMPACT ON LEARNING
Well-prepared beginning teachers of science:
S3.1 Give specific and timely verbal feedback to support student sense-making via
formative assessment used to recognize and assess learners’ ideas, life experiences
and understanding while engaging students in high-level challenges that build
toward citizenship, stewardship, and lifelong community engagement.
S3.2 Prepare constructive written feedback for students from assessments that are
designed to show learning and application of three-dimensional understanding in
order to move them toward productive sense-making situated within their lived
experiences, building on existing ideas, assets, resources, and ways of knowing.
S3.3 Reflect on, interpret, and purposefully disaggregate summative assessment data
to inform future planning and teaching, with particular attention to student
demographics and learning progress, being thoughtful with regard to how
assessment information is used, and the potential impact on students' identities as
scientists.
Attachment B – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) and High School (7-12) Science Teachers
16
MG/HS.S4. SAFETY
Well-prepared beginning teachers of science:
S4.1 Select and modify instructional materials and activities to apprentice students and
build their agency in safety techniques for the purpose of exploring phenomena or
solving problems using knowledge about procurement, preparation, use, storage,
dispensing, supervision, and disposal of chemicals/materials/equipment.
S4.2 Establish shared ownership within the entire classroom community of roles,
routines, and safety protocols to minimize hazardous situations, implement
emergency procedures, maintain safety equipment, and follow policies and
procedures that comply with established state and national guidelines and
standards, and best professional practices while monitoring, coaching, and
providing feedback to collaboratively create an environment that is physically safe.
S4.3 Plan intentionally for discourse around ethical and legal decision-making adhering
strictly to science safety protocols with respect to safe and humane treatment of all
living organisms including their collection, care and use in and out of the classroom,
and creating an environment that is safe for students who have varying
perspectives on the treatment of living organisms.
MG/HS.S5. PROFESSIONAL KNOWLEDGE AND SKILLS
Well-prepared beginning teachers of science:
S5.1 Use and reflect on assessment evidence of the three-dimensional aspects of
student science understanding while honoring students’ multiple ways of knowing,
doing, and communicating their thinking to continually improve instructional
effectiveness and professional growth that ensures a rightful presence in science for
all students.
S5.2 Examine and manage professional growth while engaging in professional learning
to remain current and open to learning from students and colleagues in order to
deepen and grow in teaching knowledge, skills and dispositions, including the ability
to employ science-specific technology and be culturally responsive.
MG/HS.S6. SPECIALIZED CONTENT KNOWLEDGE FOR ALL SCIENCE
TEACHERS
Well-prepared beginning teachers of science:
S6.1 Use tools and strategies to ensure all students have equitable opportunities to
understand the nature of science and the cultural norms and values inherent in the
current and historical development of scientific knowledge, empowering students
toward action that contributes to scientific problem solving in their community and
in the world.
S6.2 As part of planning, unpack big ideas within a learning sequence in order to
identify crosscutting concepts, disciplinary core ideas, science and engineering
practices, and ensure the incorporation of science-specific technologies and
contributions of diverse populations to science.

Attachment B – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) and High School (7-12) Science Teachers
17
S6.3 Reflect and interpret student thinking and understanding while considering
science standards, learning progressions, and sequencing of science content in
order to scaffold and support student sense-making.
S6.4 Select and modify instructional materials that engage students in using grade
appropriate elements of the disciplinary core ideas, science and engineering
practices and crosscutting concepts to explore, describe, and explain phenomena or
design solutions within a classroom environment where all students participate in
the co-construction of knowledge.
S6.5 Engage students in sense-making cycles of activity to develop understanding of
the major scientific concepts, principles, theories, laws, and interrelationships to
explain phenomena and solve problems together as a classroom community of
learners.
S6.5 Details for Middle Grades
S6.5 Details for High School
S6.5A LIFE SCIENCE FOR MIDDLE
GRADES
LS1: From Molecules to Organisms:
Structures and Processes
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
From Molecules to Organisms: Structures
and Processes to explain phenomena and
solve problems together as a classroom
community of learners.
LS2: Ecosystems: Interactions, Energy,
and Dynamics
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Ecosystems: Interactions, Energy, and
Dynamics to explain phenomena and solve
problems together as a classroom
community of learners.
LS3: Heredity: Inheritance and
Variation of Traits
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Heredity: Inheritance and Variation of Traits
to explain phenomena and solve problems
together as a classroom community of
learners.
S6.5A LIFE SCIENCE FOR HIGH
SCHOOL
LS1: From Molecules to Organisms:
Structures and Processes
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
From Molecules to Organisms: Structures
and Processes to explain phenomena and
solve problems together as a classroom
community of learners.
LS2: Ecosystems: Interactions, Energy,
and Dynamics
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Ecosystems: Interactions, Energy, and
Dynamics to explain phenomena and solve
problems together as a classroom
community of learners.
LS3: Heredity: Inheritance and
Variation of Traits
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Heredity: Inheritance and Variation of
Traits to explain phenomena and solve
problems together as a classroom
community of learners.

Attachment B – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) and High School (7-12) Science Teachers
18
LS4: Biological Evolution: Unity and
Diversity
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Biological Evolution: Unity and Diversity
to
explain phenomena and solve problems
together as a classroom community of
learners.
LS4: Biological Evolution: Unity and
Diversity
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Biological Evolution: Unity and Diversity
to
explain phenomena and solve problems
together as a classroom community of
learners.
S6.5B EARTH AND SPACE SCIENCE
FOR MIDDLE GRADES
ESS1: Earth’s Place in the Universe
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Earth’s Place in the Universe
to explain
phenomena and solve problems together as
a classroom community of learners.
ESS2: Earth’s Systems
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Earth’s Systems
to explain phenomena and
solve problems together as a classroom
community of learners.
ESS3: Earth and Human Activity
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Earth and Human Activity
to explain
phenomena and solve problems together as
a classroom community of learners.
S6.5C EARTH AND SPACE SCIENCE
FOR HIGH SCHOOL
ESS1: Earth’s Place in the Universe
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Earth’s Place in the Universe
to explain
phenomena and solve problems together as
a classroom community of learners.
ESS2: Earth’s Systems
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Earth’s Systems
to explain phenomena and
solve problems together as a classroom
community of learners.
ESS3: Earth and Human Activity
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Earth and Human Activity
to explain
phenomena and solve problems together as
a classroom community of learners.
S6.5C PHYSICAL SCIENCE FOR
MIDDLE GRADES
PS1: Matter and Its Interactions
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Matter and Its Interactions
to explain
phenomena and solve problems together as
a classroom community of learners.
S6.5B CHEMISTRY FOR HIGH
SCHOOL
PS1: Matter and Its Interactions
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Matter and Its Interactions
to explain
phenomena and solve problems together as
a classroom community of learners.

Attachment B – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) and High School (7-12) Science Teachers
19
PS2: Motion and Stability: Forces and
Interactions
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Motion and Stability: Forces and
Interactions to explain phenomena and
solve problems together as a classroom
community of learners.
PS3: Energy
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Energy
to explain phenomena and solve
problems together as a classroom
community of learners.
PS4: Waves and Their Applications in
Technologies for Information Transfer
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around the
Waves and Their
Applications in Technologies for Information
Transfer to explain phenomena and solve
problems together as a classroom
community of learners.
PS3:
1
Energy
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Energy
to explain phenomena and solve
problems together as a classroom
community of learners.
PS4: Waves and Their Applications in
Technologies for Information Transfer
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Waves and Their Applications in
Technologies for Information Transfer to
explain phenomena and solve problems
together as a classroom community of
learners.
PSO: Organic Chemistry
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Organic Chemistry
to explain phenomena
and solve problems together as a classroom
community of learners.
PSH: Human Impact on the
Environment
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Human Impact on the Environment
to
explain phenomena and solve problems
together as a classroom community of
learners.
S6.5D PHYSICS FOR HIGH SCHOOL
PS1: Matter and Its Interactions
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Matter and its Interactions
to explain
phenomena and solve problems together as
a classroom community of learners.
1
PS2 concepts are not specifically called out in the high school chemistry but included in
high school physics.

Attachment B – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) and High School (7-12) Science Teachers
20
PS2: Motion and Stability: Forces and
Interactions
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Motion and Stability: Forces and
Interactions to explain phenomena and
solve problems together as a classroom
community of learners.
PS3: Energy
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Energy
to explain phenomena and solve
problems together as a classroom
community of learners.
PS4: Waves and Their Applications in
Technologies for Information Transfer
Well-prepared beginning teachers of science
will engage in sense-making cycles of
activity around key questions related to
Waves and Their Applications in
Technologies for Information Transfer to
explain phenomena and solve problems
together as a classroom community of
learners.
S6.5D ENGINEERING FOR MIDDLE
GRADES
ETS1: Engineering Design
Well-prepared beginning teachers of science
will engage in problem solving through the
design process and facilitate students to
engage in the design process through
engaging in the Engineering Design
questions.
ETS2: Links Among Engineering,
Technology, Science, and Society
Well-prepared beginning teachers of science
will engage in problem solving through the
design process and facilitate students to
engage in the design process through
engaging in the
Links Among Engineering,
Technology, Science, and Society questions.
S6.5E ENGINEERING FOR HIGH
SCHOOL
ETS1: Engineering Design
Well-prepared beginning teachers of science
will engage in problem solving through the
design process and facilitate students to
engage in the design process through
engaging in the Engineering Design
questions.
ETS2: Links Among Engineering,
Technology, Science, and Society
Well-prepared beginning teachers of science
will engage in problem solving through the
design process and facilitate students to
engage in the design process through
engaging in the
Links Among Engineering,
Technology, Science, and Society questions.

Attachment C
21
Standards for the Preparation of Middle
Grades (5-9) Science Teachers in Optional
Disciplinary Specializations
SBE PRESENTATION DRAFT

Attachment C – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) Science Teachers in Optional Disciplinary Specializations
22
OPTIONAL DISCIPLINARY SPECIALIZATION IN BIOLOGY FOR
GRADES 5-9 SCIENCE TEACHERS
MG.B.LS1.A: STRUCTURE AND FUNCTION: How do the structures of organisms
enable life’s functions?
1. What role do specialized cells play in living organisms?
2. How does the difference in structure between unicellular and multicellular
organisms contribute to their function?
3. How does the development of cell theory demonstrate the nature of science?
4. What are the elements of a system that determine an individual’s traits and how
could we model that system?
5. What evidence supports the argument that changes in protein structure alter
functioning of the cell?
6. How can the relationships among the hierarchical system of cells, tissues, organs,
and systems be modeled?
7. How do we use scientific technologies to generate evidence about structures at the
microscopic scale?
8. How does the polarity of the water molecule enable the function of living systems?
9. What evidence supports our understanding of the molecular composition of cells
and their components?
MG.B.LS1.B: GROWTH AND DEVELOPMENT OF ORGANISMS: How do organisms
grow and develop?
1. How can we model the relationships between mitosis, gene expression, and
differentiation to explain the growth and development of multicellular organisms?
2. What argument can be constructed for why meiosis is essential for sexual
reproduction?
3. How can the similarities and differences between mitosis and meiosis be modeled?
MG.B.LS1.C: ORGANIZATION FOR MATTER AND ENERGY FLOW IN
ORGANISMS: How do organisms obtain and use the matter and energy they need to
live and grow?
1. How can the growth of organisms be explained by drawing upon mechanisms for
building complex organic molecules from simple elements?
2. How can we model the interactions between the light and dark reactions to
demonstrate the transformations of matter and energy that occur during
photosynthesis?
3. How do enzymes affect energy use in the breakdown and synthesis of molecules?
MG.B.LS1.D: INFORMATION PROCESSING: How do organisms detect, process, and
use information about the environment?
1. How might functions associated with each of the main regions of the brain impact
behavior?
2. What patterns distinguish reflexes from complex behaviors?
3. How is homeostasis maintained through feedback mechanisms?

Attachment C – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) Science Teachers in Optional Disciplinary Specializations
23
MG.B.LS2.A: INTERDEPENDENT RELATIONSHIPS IN ECOSYSTEMS: How do
organisms interact with the living and nonliving environments to obtain matter and
energy?
1. How do interspecific interactions influence how organisms obtain matter and energy
for survival and reproduction?
2. What evidence would support the claim that complex interactions within an
ecosystem help to maintain relatively stable numbers and types of organisms in
that ecosystem?
MG.B.LS2.B: CYCLES OF MATTER AND ENERGY TRANSFER IN ECOSYSTEMS:
How do matter and energy move through an ecosystem?
1. What models demonstrate how matter and energy move through and within an
ecosystem?
2. What models best illustrate the roles of photosynthesis and respiration in the
movement of carbon through food chains and the cycling of carbon within
ecosystems?
3. How can mathematical modeling be used to depict how the efficiency of energy flow
through ecosystems impacts the number of organisms at increasingly higher trophic
levels?
4. What data could be used to provide evidence that an imbalance in the global carbon
cycle is leading to climate change?
MG.B.LS2.C: ECOSYSTEM DYNAMICS, FUNCTIONING, AND RESILIENCE: What
happens to ecosystems when the environment changes?
1. How can we use models to predict the impact of a disruption to a physical or
biological component of an ecosystem on the populations and communities within
the ecosystem?
2. What data can we use to evaluate the anthropogenic changes that have occurred in
the environment and their impacts on ecosystems?
MG.B.LS2.D: SOCIAL INTERACTIONS AND GROUP BEHAVIOR: How do organisms
interact in groups so as to benefit individuals?
1. What evidence can be used to evaluate the role of group behavior on individual and
species’ chances to survive and reproduce?
MG.B.LS3.A: INHERITANCE OF TRAITS: How are the characteristics of one
generation related to the previous generation?
1. What evidence can be used to support the claim that gene mutations result in
changes in an organism?
2. What evidence was used to develop the structural model of DNA and how does this
demonstrate the nature of science and historical ways that women and minorities
have been challenged in their role in science?
3. How do DNA sequences lend themselves to regulatory functions?

Attachment C – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) Science Teachers in Optional Disciplinary Specializations
24
MG.B.LS3.B: VARIATION OF TRAITS: Why do individuals of the same species vary
in how they look, function, and behave?
1. What are the relationships among alleles and DNA, nucleotide sequences, protein
synthesis, genes, and chromosomes?
2. How can you develop a model that demonstrates both the conservative nature of
DNA replication and how it contributes to variation?
3. What are the mechanisms (including potential for mutation caused by
environmental factors) involved in sexual and asexual reproduction that lead to the
patterns of similarity and difference in how they each generate genetic variability?
4. How do both genetic and environmental factors affect expression/regulation of DNA
to generate particular traits?
5. Why is genetic variability important?
6. How does the calculation of the probability of traits in future generations based on
parental genotypes support making predictions about a population over time?
MG.B.LS4.A: EVIDENCE OF COMMON ANCESTRY AND DIVERSITY: What evidence
shows that different species are related?
1. What forms of evidence are used to infer evolutionary relationships?
2. How do patterns in the fossil record document the existence, diversity, extinction,
and change of life forms throughout the history of life on Earth?
3. How can the patterns inherent in a set of comparative DNA sequences be used to
model evolutionary relationships?
MG.B.LS4.B: NATURAL SELECTION: How does genetic variation among organisms
affect survival and reproduction?
1. How and through what technologies have humans influenced the inheritance of
desired traits in organisms throughout history?
2. How can there be so many similarities among organisms yet so many different
kinds of plants, animals, and microorganisms?
3. How are shifts in the numerical distribution of traits used as evidence to support
that advantageous heritable traits tend to increase in a population?
4. How are natural selection, adaptations, and evolution interrelated?
5. Explain how various scientists contributed to the development of the theories of
evolution by natural selection?
MG.B.LS4.C: ADAPTATION: How does the environment influence populations of
organisms over multiple generations?
1. What is the potential impact of a new pathogen on a population?
2. How has the increase in prescribing antibiotics impacted the evolution of bacteria
(including virulence and resistance)?
MG.B.LS4.D: BIODIVERSITY AND HUMANS: What is biodiversity, how do humans
affect it, and how does it affect humans?
1. In what ways can changes in environmental conditions affect the distribution of
species and/or habitats?
2. How can modeling predict and test the impacts of proposed solutions for protection
of a threatened or endangered species?
Attachment C – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) Science Teachers in Optional Disciplinary Specializations
25
Optional Disciplinary Specialization in Earth and Space
Science Standards for Grades 5-9 Science Teachers
ESS1: EARTH’S PLACE IN THE UNIVERSE: What is the universe, and what is Earth’s
place in it?
MG.E.ESS1.A: THE UNIVERSE AND ITS STARS: What is the Universe and what
goes on in stars?
1. What nuclear reactions take place that result in the Sun radiating energy?
2. How will the nuclear reactions in the Sun change over time?
3. What do the spectral patterns of distant stars reveal about their age and history?
4. How do the spectra of stars and galaxies provide evidence of their chemical
composition?
5. What is the relationship between velocity and relative distance from Earth for these
spectra?
6. How does a star’s mass influence its evolution?
MG.E.ESS1.B: EARTH AND THE SOLAR SYSTEM: What are the predictable patterns
caused by Earth’s motion in the Solar System?
1. How can the mathematical representations of Kepler’s Laws provide predictions of
natural and man-made objects in the solar system?
2. What is the nature and period of oscillations in Earth’s motions?
3. What positive and negative feedback can be seen in these oscillations?
MG.E.ESS1.C: HISTORY OF PLANET EARTH: How do people reconstruct and date
events in Earth’s planetary history?
1. In what ways can the decay of radioactive isotopes be used to establish an absolute
age for Earth materials?
2. How do tectonic processes affect current patterns of continental and ocean floor
features?
3. How do scientists use the mineralogic and chemical compositions of Earth and solar
system materials to understand the conditions of Earth’s earliest history?
4. How does the record of impacts and collisions provide information on the history of
the Solar System?
5. How can a model be used as evidence to illustrate how Earth’s internal and surface
processes operate at different spatial and temporal scales to form continental and
ocean-floor features?
ESS2: EARTH’S SYSTEMS: How and why is Earth constantly changing?
MG.E.ESS2.A: EARTH MATERIALS AND SYSTEMS: How do Earth’s major systems
interact?
1. In what ways can Earth’s dynamic systems be modeled, over both short and long
time spans?
2. How does Earth’s internal energy drive small- and large-scale crustal processes?
3. How is the rate of change in Earth System processes interrelated?
4. How can seismic wave data indicate differences in density in the crust and mantle
of the Earth?

Attachment C – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) Science Teachers in Optional Disciplinary Specializations
26
5. What causes motion in the Earth’s mantle?
6. How can the sequence of rocks in a given area provide evidence of the plate
tectonic environment of their formation?
7. What experimental evidence can be used to identify different types of soil?
8. How can a quantitative model be used to describe the cycling of carbon among the
hydrosphere, atmosphere, geosphere, and biosphere?
MG.E.ESS2.B: PLATE TECTONICS AND LARGE-SCALE SYSTEM INTERACTIONS:
Why do the continents move, and what causes earthquakes and volcanoes?
1. What are the sources of energy that drive Earth’s surface and subsurface
processes?
MG.E.ESS2.C: THE ROLES OF WATER IN EARTH’S SURFACE PROCESSES: How do
the properties and movements of water shape Earth’s surface and affect its systems?
1. How does the storage and movement of water, including but not limited to the
properties of watersheds, mediate and facilitate short- and long-term processes on
the surface and in the subsurface of the Earth?
MG.E.ESS2.D: WEATHER AND CLIMATE: What regulates weather and climate?
1. What energy transformations occur to incoming solar radiation as it is transferred
between Earth systems?
2. What is the evidence in the rock and sediment record for changes in climate?
3. What are drivers for climate change?
4. Based on current rates of change in energy levels, what are some valid
extrapolations for changes in climate and the impact on the biosphere,
hydrosphere, and lithosphere?
5. What causes El Nino and La Nina events and what effect do these events have on
weather, climate, and the environment?
6. How can models be used to describe how variations in the flow of energy into and
out of Earth’s systems result in changes in climate?
MG.E.ESS2.E: BIOGEOLOGY: How do living organisms alter Earth’s processes and
structures?
1. How can organisms impact Earth’s major systems?
2. How would the Earth’s lithosphere, atmosphere, and hydrosphere be different in the
absence of life?
ESS3: EARTH AND HUMAN ACTIVITY: How do Earth’s surface processes and human
activities affect each other?
MG.E.ESS3.A: NATURAL RESOURCES: How do humans depend on Earth’s
resources?
1. How are the Earth’s resources unevenly distributed across the planet and what
caused that distribution?
2. How has technology been employed to develop and promote the use of renewable
energy resources?

Attachment C – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) Science Teachers in Optional Disciplinary Specializations
27
MG.E.ESS3.B: NATURAL HAZARDS: How do natural hazards affect individuals and
societies?
1. How have occurrences of natural hazards in local and regional and global
environments driven human movements and populations in those environments?
MG.E.ESS3.C: HUMAN IMPACTS ON EARTH SYSTEMS: How do humans change the
planet?
1. What changes in human behavior and technology can mitigate the negative impacts
humans have had on Earth systems, including mining, urbanization, and
atmospheric, aquatic, and terrestrial pollution?
2. How do different resource management approaches impact the long-term
availability/sustainability of natural resources?
MG.E.ESS3.D: GLOBAL CLIMATE CHANGE: How do people model and predict the
effects of human activities on Earth’s climate?
1. What technological resources are available to advance Earth’s positive feedback
systems and mitigate negative feedback systems due to the use of resources by
humans?

Attachment C – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) Science Teachers in Optional Disciplinary Specializations
28
Optional Disciplinary Specialization in Physical Science
Standards for Grades 5-9 Science Teachers
Chemistry
MG.P.PS1.A: MATTER AND ITS INTERACTIONS: How can one explain the
structure, properties, and interactions of matter?
1. How does the analysis of data from investigations help determine the nature of
matter?
2. How can the creation of models from data represent the nature of matter and the
properties of its various states of matter?
3. What are chemical and physical properties and how are they used to identify
substances?
4. How can data be used to model the relationships among the number of moles,
volume, temperature, and pressure of gases?
5. What data supports the current model of an atom’s structure?
6. What atomic models are/were commonly used and how were they developed over
time?
7. What observations illustrate the limitations of the Bohr model of the atom?
8. How are patterns in atomic spectra related to our understanding of atomic
structure?
9. How was the Periodic Table developed and what does that process show about the
nature of science?
10. What patterns found in the Periodic Table predict structure, chemical properties,
and physical properties of matter?
11. In what ways do atoms combine to form novel substances?
12. How is the stability of a molecule related to its energy?
13. What patterns exist in the characteristics of different types of bonding and how can
these be predicted using the Periodic Table?
14. How can bonding characteristics be used to determine the shape of a molecule?
15. How can a molecule’s structure be used to determine the polarity of a molecule?
16. What patterns in structure and bonding are used for naming chemical compounds
and writing formulas?
MG.P.PS1.B: CHEMICAL REACTIONS: How do substances combine or change
(react) to make new substances? How does one characterize and explain these
reactions and make predictions about them?
1. What data supports the kinetic molecular theory explanation of chemical
processes?
2. How is energy involved in a chemical reaction?
3. How can data from an investigation explain that a balanced chemical equation
represents conservation of mass in a given chemical reaction?
4. How can the products and yield of a chemical reaction be predicted given the
quantities of reactants?
5. How are observations of chemical reactions used to develop the activity series and
predict the nature of chemical reactions?
6. How can the structure of electrochemical cells be described by chemical half
reactions?

Attachment C – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) Science Teachers in Optional Disciplinary Specializations
29
7. What patterns can be used to predict the resulting reaction given the strength of
an acid and base?
8. How can an investigation be designed to show the change of pH during a titration?
9. In what ways can the rate of a chemical reaction be changed?
10. A change in what factors can shift a system at equilibrium?
11. In what ways are chemical processes used in the mining of metals, minerals, ores,
and elements?
12. How are chemical processes used in biological phenomena?
MG.P.PS1.C: NUCLEAR PROCESSES: What forces hold nuclei together and mediate
nuclear processes?
1. How do the amounts of subatomic particles change during nuclear decay?
2. How does the amount of radioactive materials change over the course of a nuclear
decay reaction?
3. How can half-life be used to mathematically determine the age of rocks and other
natural materials?
MG.P.PS3.A:
2
DEFINITIONS OF ENERGY: What is energy?
1. How can the change of energy during a phase change of a material be represented
in a model?
2. How can the change in enthalpy be calculated during a chemical reaction?
3. How can the spontaneity of a reaction be explained based on the relationship
between enthalpy, entropy, and free energy?
4. How can an investigation show how electrical energy is produced in a voltaic cell?
5. How can electrolysis be explained?
6. What evidence is there for the wave particle duality of electrons?
MG.P.PS3.B: CONSERVATION OF ENERGY AND ENERGY TRANSFER: What is
meant by conservation of energy?
How is energy transferred between objects or systems?
1. How can the relationship between energy of a system and energy of
its surroundings be used to determine the heat of a reaction?
MG.P.PS4.B: ELECTROMAGNETIC RADIATION: What is light? How can one explain
the varied effects that involve light? What other forms of electromagnetic radiation are
there?
1. How do quantum mechanical models and molecular orbital theory improve our
ability to explain chemical behavior?
MG.P.PSO: ORGANIC CHEMISTRY: What is Organic Chemistry? Why is carbon used
in organic molecules?
1. How do models explain how the atoms from glucose molecules combine with other
elements to form more complex organic molecules?
2. What is the effect of the use of enzymes during the synthesis or breaking down of
molecules?
2
PS2 concepts are included in the physics category of physical science

Attachment C – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) Science Teachers in Optional Disciplinary Specializations
30
3. What are the different ways in which carbon atoms combine to make different
classes of organic compounds?
4. How do different functional groups predict the properties and reactivity of organic
compounds?
5. How does the structure of reactants in an organic reaction predict the products?
MG.P.PSH: HUMAN IMPACT ON THE ENVIRONMENT: What impact have humans
caused the environment? What evidence and data can be used to show the impact?
What can humans do to have a positive impact on the environment?
1. What is the greenhouse effect and how do greenhouse gases contribute to climate
change?
2. How can chemistry design solutions to mitigate global climate change?
3. How can chemistry be used to investigate ways to mitigate other air quality
concerns and design solutions?
Physics
MG.P.PS1.C: NUCLEAR PROCESSES: What forces hold nuclei together and mediate
nuclear processes?
1. How is half-life used to determine the age of rocks and other natural materials?
MG.P.PS2.A: FORCES AND MOTION: How can one predict an object’s continued
motion, changes in motion, or stability?
1. How can an object’s continued motion, changes in motion, or stability be predicted?
2. How can models be used to explain relationships among mass, velocity,
acceleration, force, and momentum for macroscopic objects?
3. How can models help explain the nature of different forces?
4. What are the conceptual and mathematical relationships among velocity and mass
for a collection of interacting objects, as in a collision?
5. How can a conceptual model be used to describe the size of collision forces?
MG.P.PS2B: TYPES OF INTERACTIONS: What underlying forces explain the variety
of interactions observed?
1. What is the relationship between electric and magnetic fields, and electric and
magnetic forces?
MG.P.PS2.C: STABILITY AND INSTABILITY IN PHYSICAL SYSTEMS: Why are
some physical systems more stable than others?
1. How do you design an investigation to explore why some systems are more stable
than others?
2. How do feedback mechanisms maintain stability in closed systems?
3. How is the Second Law of Thermodynamics and energy transfer principles applied
to two components in an isolated system?
MG.P.PS3.A: DEFINITIONS OF ENERGY: What is energy?
1. What is energy and how is it measured?
2. What demonstrations can be done to demonstrate the presence of different forms
of energy?
3. How can mathematics be used to describe energy transfer between objects?
Attachment C – SBE PRESENTATION DRAFT: Standards for the Preparation of
Middle Grades (5-9) Science Teachers in Optional Disciplinary Specializations
31
4. How can systems be designed to harness energy to solve practical problems?
MG.P.PS3.B: CONSERVATION OF ENERGY AND ENERGY TRANSFER: What is
meant by conservation of energy? How is energy transferred between objects or
systems?
1. How does the system change when energy (electrical, thermal, and
mechanical) flows in and out of it?
2. How can energy conservation be used to generate mathematical expressions to
predict the behavior of a system?
3. How can electrical circuits be used to demonstrate energy transfer and
transformation?
MG.P.PS3.C: RELATIONSHIP BETWEEN ENERGY AND FORCES: How are forces
related to energy?
1. What are the conceptual and mathematical relationships between two objects
interacting through electrical or gravitational fields?
2. How does calculus help provide insight into the connections between force and
energy?
3. What are the conceptual and mathematical relationships among conservation of
mass, momentum, energy, and charge as applied to systems of objects?
MG.P.PS3.D: ENERGY IN CHEMICAL PROCESSES AND EVERYDAY LIFE: How do
food and fuel provide energy? If energy is conserved, why do people say it is produced
or used?
1. How does Earth receive seemingly unlimited energy?
2. What are some real-world examples of applications of energy conversion?
MG.P.PS4.A: WAVE PROPERTIES: What are the characteristic properties and
behaviors of waves?
1. What happens to light when it interacts with different materials?
2. How can information be digitized and communicated using the electromagnetic
spectrum?
3. What is resonance and how is the concept applied to everyday events?
MG.P.PS4.B: ELECTROMAGNETIC RADIATION: What is light? How can one explain
the varied effects that involve light? What other forms of electromagnetic radiation are
there?
1. What forms of electromagnetic radiation exist?
2. What are the different models for electromagnetic radiation?
3. What are some practical applications of electromagnetic radiation?
4. How does electromagnetic radiation affect matter?
5. How does electromagnetic radiation influence the emission of energy by an atom?

Attachment D
32
Standards for the Preparation of High School
(7-12) Science Teachers in Optional
Disciplinary Specializations
SBE PRESENTATION DRAFT
Attachment D – SBE PRESENTATION DRAFT: Standards for the Preparation of High
School (7-12) Science Teachers in Optional Disciplinary Specializations
33
Optional Disciplinary Specialization in Biology for
Grades 7-12 Science Teachers
HS.B.LS1.A: STRUCTURE AND FUNCTION: How do the structures of organisms
enable life’s functions?
1. What is the role of energy in the making and breaking of polymers?
2. How might a change in the subunits of a polymer lead to changes in structure or
function of the macromolecule?
3. What evidence supports the theory about the origin of eukaryotic cells?
4. How do the mechanisms for transport across membranes support energy
conservation?
5. How are living systems affected by the presence or absence of subcellular
components?
6. How are mathematical models used to help explain the ways in which
osmoregularity mechanisms contribute to the health and survival of organisms?
7. How can we use scientific technologies to explore molecular sequences that provide
insight into the evolutionary relationships between cells and molecules in various
organisms?
HS.B.LS1.B: GROWTH AND DEVELOPMENT OF ORGANISMS: How do organisms
grow and develop?
1. How does the cell cycle aid in the conservation of genetic information?
2. How do disruptions to the cell cycle impact a cell or organism?
3. How does the regulation of gene expression connect with phenotypic differences in
cells and organisms?
HS.B.LS1.C: ORGANIZATION FOR MATTER AND ENERGY FLOW IN
ORGANISMS: How do organisms obtain and use the matter and energy they need to
live and grow?
1. How do organisms use energy or conserve energy to respond to environmental
stimuli?
2. How might changes to the structure of an enzyme affect its function?
3. How does the cellular environment affect enzyme activity?
4. How is variation in the number and types of molecules within cells connected to the
ability of the organism to survive and/or reproduce in different environments?
5. How can rates of transpiration be calculated or investigated?
6. How can rates of enzymatic reactions be investigated?
HS.B.LS1.D: INFORMATION PROCESSING: How do organisms detect, process, and
use information about the environment?
1. What are the molecular bases of signaling mechanisms in cells?
2. How does communication in organisms get explained at varied scales - molecular,
cellular, systems, organisms, ecosystems?
HS.B.LS2.A: INTERDEPENDENT RELATIONSHIPS IN ECOSYSTEMS: How do
organisms interact with the living and nonliving environments to obtain matter and
energy?
1. How can mathematical models help to predict or understand population growth?
Attachment D – SBE PRESENTATION DRAFT: Middle Grades and High School
Science Teacher Preparation Standards Framework
34
2. How do density-dependent and density-independent factors interact to determine
population growth curves?
3. How do we quantify community diversity? How are measures of community
diversity used to evaluate and monitor the quality of ecosystems?
HS.B.LS2.B: CYCLES OF MATTER AND ENERGY TRANSFER IN ECOSYSTEMS:
How do matter and energy move through an ecosystem?
1. How can differences in energy availability lead to different reproductive strategies
related to tradeoffs between number of offspring and resource allocation per
offspring?
2. What is the relationship between metabolic rate per unit body mass and the size of
multicellular organisms?
HS.B.LS2.C: ECOSYSTEM DYNAMICS, FUNCTIONING, AND RESILIENCE: What
happens to ecosystems when the environment changes?
1. How do interspecific interactions result in keystone species having effects on
ecosystems that are disproportionate to their abundance?
2. How do geological and climatological patterns affect habitat change and ecosystem
distribution?
3. How do biogeographical studies illustrate changes in habitat and ecosystem
distribution over time?
HS.B.LS2.D: SOCIAL INTERACTIONS AND GROUP BEHAVIOR: How do organisms
interact in groups so as to benefit individuals?
1. How are behavioral and/or physiological responses of organisms related to changes
in the internal or external environment?
2. How do the behavioral responses of organisms affect their overall fitness and
contribute to the success of the population?
3. How can cooperation or coordination between organisms, populations, and species
result in enhanced movement of, or access to, matter and energy?
4. What behaviors can be investigated and modeled using model organisms such as
Fruit Flies?
HS.B.LS3.A: INHERITANCE OF TRAITS: How are the characteristics of one
generation related to the previous generation?
1. What role does the structure of nucleic acids play in how living systems transmit
information?
2. How does our knowledge of shared, conserved, fundamental processes in genetics
provide evidence for common ancestry?
3. How and what types of interactions regulate gene expression?
4. How do alterations in DNA sequences contribute to variation that can be subject to
natural selection?(evolution?)
5. How are genetic engineering techniques used in analyzing or manipulating DNA?
HS.B.LS3.B: VARIATION OF TRAITS: Why do individuals of the same species vary
in how they look, function, and behave?
1. How does the diversity of a species affect inheritance?
Attachment D – SBE PRESENTATION DRAFT: Middle Grades and High School
Science Teacher Preparation Standards Framework
35
2. How does chromosomal inheritance generate genetic variation in sexual
reproduction?
3. How is a species’ genetic information diversified from generation to generation?
HS.B.LS4.A: EVIDENCE OF COMMON ANCESTRY AND DIVERSITY: What evidence
shows that different species are related?
1. What conditions or changes in conditions cause a population to be more or less
likely to evolve?
2. What and how are data, evidence and models used to provide evidence of the
theory of evolution?
3. What patterns in species interaction encourage or slow changes in species?
4. What and how do mechanisms lead to changes in allele and genotype frequencies in
populations?
HS.B.LS4.B: NATURAL SELECTION: How does genetic variation among organisms
affect survival and reproduction?
1. How can evidence demonstrate that the environment influences populations of
organisms over multiple generations?
2. What model(s) demonstrate that environmental changes impact the distribution of
traits in a population?
3. How can reproductive isolation lead to speciation?
4. What evidence would support that natural selection has occurred in a population?
5. How can mathematical modeling be used to describe how natural selection may
lead to increases and decreases of specific traits in populations over time?
6. What is meant by virulence and resistance?
7. What is the potential impact of a new pathogen on a population?
8. How does reproductive success determine evolutionary fitness?
9. How are changing biotic and abiotic environments predictive of impact on the rate
and direction of evolution?
HS.B.LS4.C: ADAPTATION: How does the environment influence populations of
organisms over multiple generations?
1. How does the process of gradual speciation influence the rate of evolutionary
processes?
2. How does punctuated equilibrium influence evolutionary processes?
3. What type of conditions lead to rapid speciation events?
4. How is evolutionary change impacted by continuous variation across geographic
ranges?
HS.B.LS4.D: BIODIVERSITY AND HUMANS: What is biodiversity, how do humans
affect it, and how does it affect humans?
1. How can an ecosystem model be used to demonstrate the role of biodiversity?
2. How do changes in biodiversity affect humans?
3. How have humans impacted biodiversity?
4. How does human impact on biodiversity affect environmental, economic, and social
considerations of the community?
Attachment D – SBE PRESENTATION DRAFT: Middle Grades and High School
Science Teacher Preparation Standards Framework
36
5. In what ways can changes in environmental conditions (e.g., drought,
deforestation, flood) and the rate of change of the environment affect the
distribution or disappearance of traits in a species?
6. How can modeling predict and test the impacts of proposed solutions for protection
of a threatened or endangered species?
7. How and what type of models can be used to measure human impact on
biodiversity?
8. How can variations in the allele frequencies of a gene be used to provide evidence
of speciation?
Optional Disciplinary Specialization in Chemistry for
Grades 7-12 Science Teachers
HS.C.PS1.A: STRUCTURE AND PROPERTIES OF MATTER: How do particles
combine to form the variety of matter one observes?
1. How does the ionization energy of atoms across periods in the Periodic Table
account for the electron structure of atoms?
2. How does the electron configuration of an atom determine its photoelectric
spectrum?
3. In what ways can the mass spectrum be used to identify and calculate the
abundance of an isotope?
4. How do electron structures which are exceptions to the Octet rule affect the
modeling of those structures?
5. What factors affect the bond length and bond polarity of covalent bonds?
6. How can the use of Lewis diagrams and VSEPR theory predict the structure and
geometry of covalently bonded molecules and polyatomic ions?
7. How do molecular geometry and bonding affect the structural and electronic
properties of molecules?
8. In what ways can Lewis diagrams and formal charges predict resonance
structures?
9. What evidence supports the current model of the changes which occur during the
formation of hybrid orbitals?
10. How does the ideal gas law describe the relationship between the macroscopic
properties of a gas or mixture of gases?
11. How can a particulate model and graphical representations illustrate the
relationship between the motion of particles and the macroscopic properties of
gases?
12. How do interparticle forces and gas volumes influence non-ideal behavior of gases?
13. How can intermolecular interactions between particles be used to predict the
solubility of ionic and molecular compounds in aqueous and nonaqueous solvents?
HS.C.PS1.B: CHEMICAL REACTIONS: How do substances combine or change (react)
to make new substances? How does one characterize and explain these reactions and
make predictions about them?
1. What patterns distinguish polyprotic acids from monoprotic acids?
2. What tests will differentiate acids and bases from other substances?
3. How can a reaction be identified as acid-base, oxidation-reduction, or precipitation
using experimental data and observations?

Attachment D – SBE PRESENTATION DRAFT: Middle Grades and High School
Science Teacher Preparation Standards Framework
37
4. What information is needed to determine a net-ionic equation from a given
chemical reaction?
5. What structural characteristics and chemical properties characterize Lewis,
Arrhenius, and Brønsted-Lowry acids and bases?
6. What patterns exist between Brønsted-Lowry acids, bases, their respective
conjugate acid-base pairs?
7. How does pH change over the course of a titration?
8. What evidence indicates the chemical species present at any point during a
titration?
9. What data is needed to determine the rate equation for a reaction?
10. How can a rate law be determined from a reaction mechanism?
11. Why is half-life a critical parameter for first-order reactions?
12. How can half reactions be used to balance redox reaction equations?
13. What experimental data or observations are necessary to represent and calculate
the equilibrium constants, Kc or Kp?
14. What does the magnitude of K indicate about the relative concentrations of
chemical species at equilibrium?
15. What is the relationship between Q, K, and the direction in which a reversible
reaction will proceed to reach equilibrium?
16. How does Le Châterlier’s principle and equilibrium law explain equilibrium shifts
and changes in concentration of chemical species in a system at equilibrium?
17. What is the effect of a change in pH on the solubility of a salt?
18. How can the solubility of a salt be calculated based on the value of Ksp of the salt?
19. How does the relationship between the Gibbs free energy of a system and the
equilibrium constant for the reaction predict the equilibrium position of the
system?
20. How does the structure of water explain the amphoteric properties of water?
21. How can the values of pH, pOH be calculated for salts, strong acids and bases and
weak acids and bases?
22. What is the relationship between pH, pOH, and Kw?
23. What data is needed to calculate the concentrations of major species in a solution,
pH, and Ka or Kb of a monoprotic weak acid or weak base?
24. How can acid-base equilibrium concentrations be determined for weak acids and
weak bases?
25. How do graphical representations of titrations of strong acids and strong bases
differ from those of weak acids and weak bases?
26. What are the components and characteristics of buffer solutions?
HS.C.PS1.C: NUCLEAR PROCESSES: What forces hold nuclei together and mediate
nuclear processes?
1. How does the structure of an isotope’s nucleus affect the stability of the nucleus
and type of radioactive decay observed by unstable nuclei?
2. What are the properties and uses of the energy and particles emitted from a
radioisotope during nuclear decay?
HS.C.PS3.A:
3
DEFINITIONS OF ENERGY: What is energy?
3
PS2 concepts are included in the physics optional disciplinary specialization
Attachment D – SBE PRESENTATION DRAFT: Middle Grades and High School
Science Teacher Preparation Standards Framework
38
1. How does a graph of enthalpy of a system change during exothermic and
endothermic processes?
2. What data is needed to calculate the change in entropy of a chemical or physical
change?
3. What does the sign and magnitude of change in enthalpy, entropy, or free energy
indicate about a chemical or physical process?
4. How can the spontaneity of a reaction be predicted using enthalpy, entropy, and
free energy?
5. What factors cause a thermodynamically favored reaction to not occur at a
measurable rate?
6. What can be done to drive a thermodynamically unfavorable electrochemical
process?
7. How do changes in temperature, along with K, affect the extent to which a process
is thermodynamically favored?
8. How does the relationship between n, ΔG
o
, and E
o
determine the extent to which a
process is thermodynamically favored?
9. How can the amount of charge flow be calculated based on changes in the
amounts of reactants and products in an electrochemical cell?
10. What is the relationship between cell conditions and changes in cell potential?
11. How do standard cell potential and constituent half-reactions within a cell predict
whether an electrochemical cell is thermodynamically favored?
12. What electrochemical cell characteristics affect the amount of product that is
formed at the electrodes during electrolysis?
13. How is electroplating done and what are its benefits in different industries?
HS.C.PS3.B: CONSERVATION OF ENERGY AND ENERGY TRANSFER: What is
meant by conservation of energy? How is energy transferred between objects or
systems?
1. What are the steps of an investigation that will provide the data needed to calculate
the q of a system undergoing a chemical or physical change?
2. Under what conditions would molar enthalpy of reaction, average bond energies,
and/or standard enthalpies of formation be used to calculate the heat of a system,
q?
3. How do enthalpies of individual steps of a chemical or physical process relate to the
enthalpy of the overall process?
HS.C.PS4.B: ELECTROMAGNETIC RADIATION: What is light? How can one explain
the varied effects that involve light? What other forms of electromagnetic radiation are
there?
1. How does the electronic transition in an atom or molecule give rise to the properties
of an adsorbed or emitted photon?
2. What is the impact of concentration, path length, and/or molar absorptivity on the
amount of light absorbed by a solution of molecules or ions?
HS.C.PSO: ORGANIC CHEMISTRY:
1. What is the importance and purpose of functional groups in organic reactions?
2. What information is needed to identify each type of macromolecule used by living
things?
Attachment D – SBE PRESENTATION DRAFT: Middle Grades and High School
Science Teacher Preparation Standards Framework
39
3. What is an isomer and how can it be modeled?
4. How can the isomeric relationship of molecules be determined?
HS.C.PSH: HUMAN IMPACT ON THE ENVIRONMENT:
1. How do chemical reactions involving greenhouse gases affect the Earth’s
temperature?
2. How does energy consumption relate to global warming?
3. How might forever chemicals (PFAS) be isolated or removed from the environment?
Optional Disciplinary Specialization in Earth and Space
Science Standards for Grades 7-12 Science Teachers
HS.E.VESF 1/ESS2.A: EARTH MATERIALS AND SYSTEMS:
1. How can the dynamo model be used to explain the Earth’s magnetic field?
2. What data can be used to infer the physical and chemical composition of the core?
3. What arguments can be made to support the hypothesis that the Earth’s magnetic
field changes on different time scales?
HS.E.VESF 2/ESS2.B: PLATE TECTONICS AND LARGE-SCALE SYSTEM
INTERACTIONS:
1. What data can be used to support a claim for when and in what patterns of plate
tectonics developed through time on Earth?
2. How can a quantitative model explain the coevolution or coupling of plate tectonics
and mantle convection?
3. What data can be used to support an argument from evidence for the timing of the
onset plate tectonics as it operates today? (Data may include geochronology,
seismic studies, paleomagnetism, topographic features, and characteristic geologic
materials)
HS.E.VESF 3/ESS2.A: EARTH MATERIALS AND SYSTEMS AND ESS3.A NATURAL
RESOURCES:
1. What evidence supports the hypothesis that the uneven distribution of critical
elements is the result of past and current geoscience processes?
2. What are the critical elements needed for a habitable planet, carbon-free energy, or
materials for a modern society?
3. What processes mobilize critical elements through Earth's systems? (Including
magmatism, metamorphism, hydrothermal fluids, weathering, and sedimentation)
4. What roles do critical elements play in Earth history and/or in a modern society?
HS.E.VESF 4/ESS3.B: NATURAL HAZARDS:
1. What geoscience data can be used to support or refute a claim that not all
earthquakes follow the Elastic Rebound model?
2. What are the characteristics of earthquakes and the dynamics that drive them?
(Including aseismic slip and slow, intermediate and fast rupture)
3. What evidence can be used to construct a scientific explanation for how the
occurrence of aseismic slip and slow, intermediate, and fast earthquakes have
influenced human activity?
Attachment D – SBE PRESENTATION DRAFT: Middle Grades and High School
Science Teacher Preparation Standards Framework
40
HS.E.VESF 5/ESS2.B: PLATE TECTONICS AND LARGE-SCALE SYSTEM
INTERACTIONS:
1. What evidence can be used to construct a scientific explanation on how large, rare
volcanic eruptions modify Earth Systems?
2. What are the characteristics and examples of large eruptions? (Including of 10cubic
km or larger, such as Laki, supervolcanoes, and flood basalts)
3. What are the characteristics and potential impacts of large igneous provinces and
their role in mass extinctions? (Including spatial and temporal scales)
HS.E.VESF 8/ESS2.D: WEATHER AND CLIMATE:
1. What data from Earth's past can be used as evidence of the dynamics of the climate
system and be used to predict future change?
2. How can models of paleoclimatic and current climatic change be used to develop an
argument that specific regions within Earth Systems are particularly vulnerable to
rapid and/or sustained changes? (Including coastal and polar regions)
3. What evidence can be used to construct an argument about how feedback loops
that operated in the geologic record are similar to or different from those occurring
today? (Including permafrost melting, polar amplification and other environmental
changes.)
HS.E.VESF 9/ESS2.C: THE ROLES OF WATER IN EARTH’S SURFACE
PROCESSES:
1. What evidence can be used to develop an argument that the Earth’s water cycle is
changing?
2. What evidence can be used to support or refute a claim about how a changing
water cycle will shift the availability of water for human needs? (Including
hydropower, agriculture, water supply)
3. What evidence can be used to describe the impact of climate change on the water
cycle?
HS.E.VESF 10/ESS2.E: BIOGEOLOGY:
1. How do biogeochemical cycles evolve over time?
2. What evidence can be used to construct an explanation of how the biosphere has
evolved and interacted with the chemical makeup of Earth’s surface over geologic
time?
3. What models can be used to illustrate shifting patterns in Earth's biogeochemical
cycles from human activities? (Including carbon, nitrogen, phosphorous)
HS.E.VESF 11/ESS2.E: BIOGEOLOGY:
1. How do geological processes influence biodiversity?
2. What is the relationship between biodiversity and geologic processes? (Including
tectonics, impacts)
3. What arguments can be made to determine a relationship between metabolic
pathways and other evolutionary innovations to major changes in atmospheric and
ocean chemistry and climate? (Examples of metabolic pathways may include:
photorespiration, carbon fixation.)
Attachment D – SBE PRESENTATION DRAFT: Middle Grades and High School
Science Teacher Preparation Standards Framework
41
4. What are the advantages and limitations of using ecosystem response models of
past changes to predict impacts of future climate change? (Including geoclimatic,
geomorphologic)
5. What is the relationship between biodiversity and human impacts? (Including
mining, pollution)
HS.E.NASA/ESS1.B: EARTH AND THE SOLAR SYSTEM:
1. How did the universe and solar system form and change?
2. What drives variations in the Sun, and how do these changes impact the solar
system and drive space weather?
3. How did our solar system originate and change over time?
4. How did the universe begin and evolve, and what will be its destiny?
HS.E.NASA/ESS2.E: BIOGEOLOGY:
1. How did life originate on Earth?
2. What evidence supports the existence of life beyond Earth?
Optional Disciplinary Specialization in Physics for
Grades 7-12 Science Teachers
HS.P.PS1.C: NUCLEAR PROCESSES: What forces hold nuclei together and mediate
nuclear processes?
1. What are the four fundamental forces in nature, and how do they give rise to the
variety of interactions that can be observed experimentally as well as those
observed in everyday life?
2. In what ways is understanding radioactive decay helpful to conceptualize the
broader issues facing society?
3. What types of experiments can be used to demonstrate that mass can be converted
to energy and energy can be converted to mass?
HS.P.PS2.A: FORCES AND MOTION: How can one predict an object’s continued
motion, changes in motion, or stability?
1. How does torque analysis provide insight into the forces acting on an object in
equilibrium?
2. How can we use the mathematical tools of calculus to gain insight into rotational
velocity and rotational acceleration?
3. What types of situations are usefully analyzed with the help of the Principle of
Angular Momentum Conservation?
4. What are the similarities and differences in the way pendulum and simple mass and
spring systems behave as oscillators?
5. When modeling a system, in what situations is pressure a more useful variable to
focus on than force?
6. How can free body diagrams be used to help predict the size of a force that is
needed to cause low density objects to be completely submerged in a fluid?
7. How can Archimedes’s Principle be used to understand why a solid piece of steel will
sink in water but a carefully constructed ship of steel can float?
8. What are some real-world applications of the conservation of mass flow rate in
fluids?
Attachment D – SBE PRESENTATION DRAFT: Middle Grades and High School
Science Teacher Preparation Standards Framework
42
9. How does using a wave function to describe particle motion differ from using a
classical trajectory to describe particle motion?
HS.P.PS2.B: TYPES OF INTERACTIONS: What underlying forces explain the variety
of interactions observed?
1. How can understanding the nature of the electric potential provide insight into the
forces experienced by charges in an electric field?
2. What are the differences between the electric potential associated with a point
charge and that associated with a uniform electric field?
3. For what types of problems is Gauss’s Law helpful in determining the electric field?
4. What are the differences between the electric fields produced by uniformly charged
planes, cylinders, and spheres?
5. What are the differences between the electric potentials produced by uniformly
charged planes, cylinders, and spheres?
6. What information about a system does the electric permittivity provide?
7. How can the principle of conservation of energy be used to make predictions about
the motion of charged particles in an electric field?
8. What does the mathematical expression of the force law for a point charge in a
magnetic field demonstrate about the nature of that magnetic force?
9. What model describes the magnetic field created by electric current in a long
straight wire? What model expresses the force the same long, straight wire
experiences in the presence of another magnetic field?
10. In what situations is Ampere’s Law helpful in calculating the magnetic field, and in
what cases is the Biot-Savart Law more appropriate?
11. What are some practical applications of Faraday's Law?
12. What features of Maxwell’s equations suggest that electromagnetic waves can exist
in vacuum?
HS.P.PS3.B: Conservation of Energy and Energy Transfer: What is meant by
conservation of energy? How is energy transferred between objects or systems?
1. For an object that rolls without slipping, how does the distribution of its mass
affect the proportion of its total kinetic energy that is associated with rotational
kinetic energy?
2. How is the Bernoulli equation used to model fluid flow in simple systems?
3. Under which circumstances is the pressure in a system directly proportional to its
absolute temperature and when is the pressure inversely proportional to its
volume?
4. What role does pressure play in the transfer of energy to and from a gas?
5. What are the similarities and differences in the mathematical models of heat flow
by conduction, convection, and radiation?
6. What types of processes can change the internal energy of a substance?
7. In what ways is understanding thermal conductivity useful when designing
practical devices?
8. How does an understanding of entropy facilitate predictions of the evolution of
thermodynamic systems over time?
9. How is power calculated for a resistor in a circuit, and what energy transformation
does that describe?
Attachment D – SBE PRESENTATION DRAFT: Middle Grades and High School
Science Teacher Preparation Standards Framework
43
10. What is the physical basis for the difference in the behavior of two resistors when
they are connected in series as opposed to in parallel? What is the physical basis
for the difference in the behavior of two batteries when they are connected in
series as opposed to in parallel?
11. What is the connection between Kirchhoff’s Loop rule and the concept of
conservative force?
12. What is the connection between Kirchhoff’s Junction rule and the Conservation of
Electric Charge?
13. What are some examples of how energy stored in a capacitor can be used?
14. How can Faraday’s Law and Lenz’s Law help us understand the behavior of a circuit
consisting of a battery, an inductor, a resistor, and switch, all in series?
HS.P.PHY4.B: ELECTROMAGNETIC RADIATION: What is light? How can one
explain the varied effects that involve light? What other forms of electromagnetic
radiation are there?
1. How are experiments designed differently if one wants to see the wavelike property
of electrons rather than the particle-like properties?
2. In what way does quantum physics provide us with a model for explaining the
results of typical photoelectric effect experiments?
HS.P.PHY4.C: INFORMATION TECHNOLOGIES AND INSTRUMENTATION: How
are instruments that transmit and detect waves used to extend human senses?
1. How can mirrors and lenses be used to manipulate images, changing their location,
size and orientation?
2. How do the properties of slit(s) and light waves affect the resulting patterns
produced in interference and diffraction experiments?

Attachment E
44
Middle Grades and High School Science
Teacher Preparation Standards Framework
SBE PRESENTATION DRAFT
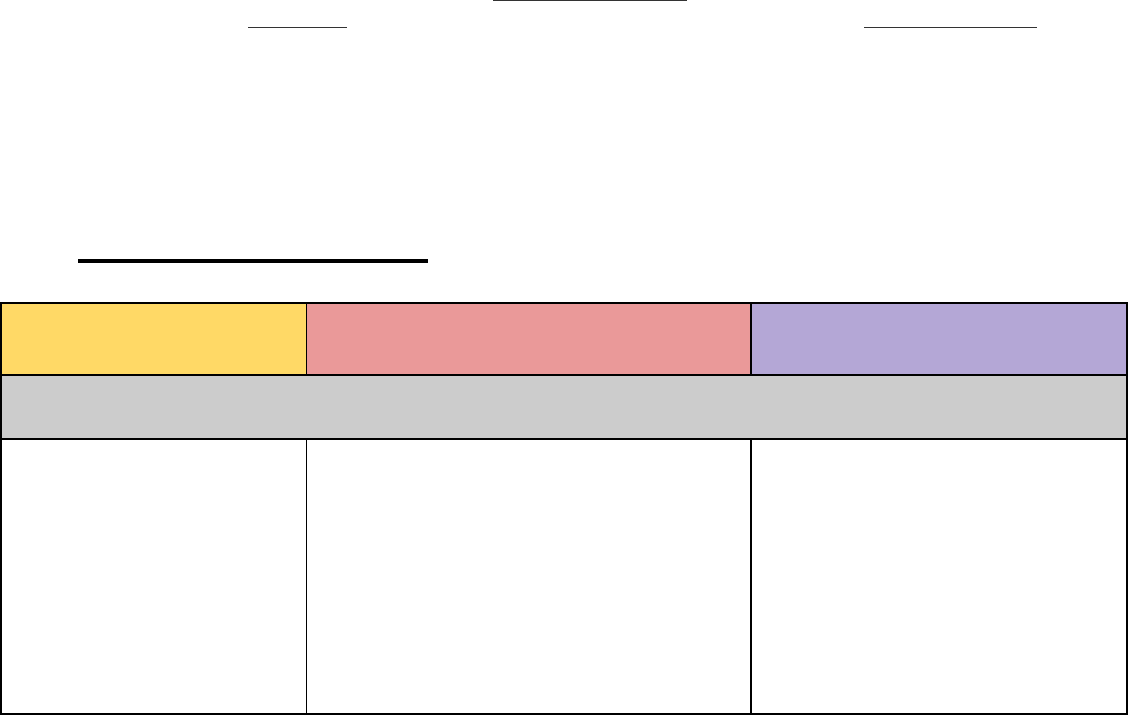
Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
45
Rationale for Faceted Standards
When we define the knowledge needed to teach science (one facet), we are left with
the question of how deeply does one need to understand those core teaching ideas to
engage students productively? We can imagine a teacher candidate who has mastered
the ability to answer multiple choice questions about science concepts and educational
theory that may not be at all prepared to teach students and use that knowledge for
productive teaching and learning.
We can answer the question of how deeply we need to understand core knowledge for
teaching by asking what the precise things are that a typical teacher needs to do with
that knowledge, and the answer becomes, “a teacher needs to understand the core
ideas well enough, deeply enough to engage in the key practices of teaching.” This
second facet helps us to set a depth of knowledge and a performance expectation by
which we can assess if a teacher has the requisite depth of understanding. The
question moves away from whether teachers have memorized core knowledge about
science and the teaching of science and toward whether they can use that knowledge
to effectively enact teaching practices.
Why then layer on the third facet of “guiding principles?” A teacher must be able to
enact teaching practices in a way that not only demonstrates depth of core knowledge
but a flexibility to use that knowledge in varied circumstances according to a set of
shared principles. These principles allow candidates to develop a mindful lens for
decision-making in a variety of situations that span the educational context to promote
science learning for all students. Problem-solving in the context of core knowledge and
core practices requires the demonstration of familiarity, understanding and capacity to
reflect important guiding principles.
Facet Definitions Table
Science Teaching
Practices Facet
Core Knowledge for Teaching Science
Facet
Guiding Principles for Teaching
Science Facet
Definitions
The Science Teaching
Practices are the activities of
teaching which are essential
for:
• engaging students in
learning science
• supporting students’
social emotional
development
Core Knowledge for Teaching Science
reflects specialized knowledge for
teaching:
• fundamental concepts, principles,
and processes in each of the
subdisciplines of science, and of
engineering to address scientific
problems or issues
• how students, depending upon age
and experience, develop
The Guiding Principles for Teaching
Science are intellectual tools and
critical dispositions that:
• serve as a productive lens
through which teachers can
organize their thinking when
making decisions about all
aspects of science teaching
and learning

Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
46
• ensuring responsible,
safe science teaching
practice
understanding of key scientific
concepts, both in terms of breadth
and depth
• approaches to representing these
concepts, principles and processes
in ways more likely to support
student learning in a safe
environment
• provide teachers with an
ethical framework and useful
schema to make
professional judgements and
participate as a member of
the profession

Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
47
The Facets
Science Teaching
Practices
Core Knowledge for Teaching
Science
Guiding Principles for
Teaching Science
1. Building respectful
relationships
2. Eliciting and
interpreting students’
thinking about science
phenomena or solving
problems
3. Engaging students in
sense-making cycles of
activity to explain
phenomenon or solve
problems
4. Leading science
discourse in order to
make sense of
phenomenon or solve
problems
5. Setting up and
managing small group
work to develop
understanding of
problems or phenomena
6. Checking student
understanding and
scientific sense-making
during and at the
conclusion of lessons
7. Providing oral and
written feedback to
students about
phenomena or scientific
problems
8. Learning about
students’ cultural,
religious, family,
intellectual, personal
experiences, and
resources for
mindful/intentional
inclusion in science
instruction
A. Learners and Learning
Environments - teachers
understand how to create and
sustain learning environments
which are safe and inclusive and
which engage students in powerful
learning experiences to support
their developing ability to make
sense of scientific phenomena
B. Content Pedagogy - teachers
understand how to represent and
assess scientific knowledge and
practices in ways which are
responsive to students’
backgrounds, needs, and interests
and support their ability to
effectively solve problems within
and across disciplines
C. Safety- teachers understand how
to produce, maintain, and
evaluate the safety procedures of
the grade band and disciplines
D. Impact on Student Learning -
teachers understand how to
design and make use of formative
and summative assessments to
make informed decisions about
student achievement and about
instruction in the immediate and
longer term
E. Professional Knowledge and
Skills - teachers understand the
role of critical reflection and
ongoing professional learning on
instructional effectiveness
F. Specialized Content
Knowledge - teachers
understand all dimensions of the
key standards and progressions
critical to their grade band and
disciplines
I. Science for All -
teachers are guided by
the value that all
students have a rightful
presence in science
II. Whole Child
Framework- teachers
are guided by the tenets
of the whole child
framework
III.
Scientific Agency -
teachers are guided by
recognition of the
importance of building
scientific agency within
themselves and students
IV. Disciplinary Literacy -
teachers are guided by a
stance that honest
communication is critical
to doing science and
that teachers are
responsible for
apprenticing their
students in that
communication
V. Community
Knowledge Building -
teachers will be guided
by the orientation that
scientific knowledge is
socially constructed

Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
48
Facet Elements
Science Teaching Practices Facet Elements
1. Building respectful relationships
a. Establish rapport with students.
b. Build mutual trust.
c. Implement strategies for creating a classroom culture that values productive
struggle, challenging science ideas, constructing science meaning together, and
enjoying science.
d. Monitor and maintain relationships with students.
e. Develop classroom discussion norms with students or developing student input
on established community norms that include talk that is focused on reasoning,
talk that is respectful, and talk that is equitable.
f. Examine and manage self in relationship with students.
2. Eliciting and interpreting students’ thinking to develop scientific understanding
of phenomenon or solve problems
a. Anticipate student thinking and potential alternative conceptions of science
content based on research.
b. Formulate and pose carefully chosen questions or tasks to allow students to
share their thinking about academic content in order to understand student
thinking.
c. During instruction engage students with additional questions, prompts, and
tasks to probe their thinking about evidence and unpack what they say.
d. Uncover and consider students’ verbal and visible thinking to reveal novel points
of view, new or alternative ideas, partial understandings, and students’ everyday
language and experiences to benefit future instruction.
e. Interpret student ideas to guide instructional decisions and reveal ideas that
may benefit other students.
f. Engage students to make their thinking public.
3. Engaging students in sense-making cycles of activity to explain
phenomenon or solve problems
a. Unpack the big ideas, identify anchoring event, tie the essential question to the
anchoring event, recognize the sense-making that occurs within the unit to
develop the storyline.
b. Engage students in questioning about a phenomenon or problem.
c. Select and modify instructional materials to create learning environments that
engage learners in using the disciplinary core ideas, science and engineering
practices and crosscutting concepts to explore, describe, and explain
phenomena.
d. Engage students in using science and engineering practices and crosscutting
concepts to make sense of a phenomenon or solve a problem.
e. Support students to interpret evidence, construct explanations, and support
explanations with arguments about phenomena or problems.
f. Support students to connect experiences back to the phenomena or problem in
the unit.
Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
49
g. Prepare to allow students to use their everyday language to engage in discourse
with new content/phenomenon and encouraging the use of technical vocabulary
over the course of an instructional unit/cycle.
4. Leading science discourse in order to make sense of phenomenon or solve
problems
a. Plan intentionally for discourse with multiple access points.
b. Determine the goal of the classroom discourse you expect students to engage
in.
c. Anticipate students’ responses to a question or task (e.g. phenomenon, reading,
data) in order to prepare to facilitate conversations.
d. Ensure a safe and collaborative environment by revisiting and reflecting on the
norms as necessary.
e. Activate and elicit students’ ideas about a science phenomenon.
f. Support students to persevere in making sense of new observations, information
or data.
g. Press students for evidence based explanations about a phenomenon or
problem.
h. Use tools and strategies to ensure all students have equitable opportunities to
participate and share their thinking (norms, talk moves, high cognitive demand
tasks).
i. Encourage students to use their everyday language to engage in discourse with
new content/phenomenon.
5. Setting up and managing small group work to develop understanding of problems
or phenomena
a. Ensure that group tasks and structures allow students to see one another as
capable contributors to their learning with understanding that group work,
students’ relationships, identities, and perceptions of one another affect their
learning opportunities.
b. Develop groups intentionally for appropriate size and student representation and
based on patterns of student interaction.
c. Ensure that small group work is intentionally based around high cognitive
demand sense-making tasks.
d. Establish roles and routines, monitoring and coaching and providing feedback on
implementation.
e. Provide the strategies and tools, written and verbal instructions, as well as
scaffolds for students to engage in discourse within a task.
f. Use student and group roles to foster discourse about the phenomenon and
problems posed by the group task.
g. Monitor small group work to ensure that the individual student’s understanding
and group understanding are both critical to the work and valued.
h. Make adjustments based on observations of individual students, groups, and
their sense-making.
Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
50
6. Checking student understanding and scientific sense-making during and at the
conclusion of lessons
a. Elicit student thinking through tools such as summary tables, KLEWS charts,
driving question boards or others that enable students to think critically about
the lesson phenomenon and work to understand how the students’ current
thinking helps explain the anchoring phenomenon or solve a problem.
b. Use or modify both formative and summative assessments that elicit evidence of
the three dimensional aspects of student science understanding.
c. Reflect and interpret student thinking and understanding.
d. Inform and adjust instruction based on student thinking and understanding.
7. Providing oral and written feedback to students about phenomena or scientific
problems
a. Prepare constructive written feedback to students that continues to strengthen
relationships and move students toward productive sense-making.
b. Give specific feedback in a timely fashion to support student thinking and affirm
student knowledge and skills.
c. Use appropriate guiding questions and productive talk to guide students to
reflect on understanding in all three science dimensions and encourage them to
go deeper.
d. Provide equitable and valuable feedback to empower learning and promote
ownership and agency.
e. Provide opportunities for students to self-assess, use feedback and give
feedback to each other.
f. Support and monitor students’ response to the feedback.
8. Learning about students’ cultural, religious, family, intellectual, personal
experiences, and resources for use in science instruction
a. Make intentional connections through use of talk moves to encourage students
to share with the class or small group how they can relate to the phenomenon
or situation personally.
b. Ensure tasks (assessments and activities) are equitable for the diversity of
students in your care.
c. Learn your students’ background, and use this information as you plan for and
adapt instruction.
d. Create opportunities for students to engage in diverse sense-making building on
their community histories, values, and practices.
e. Design learning experiences to grow out of the lives of learners.
f. Support students to use their sense-making repertoires and experiences as
critical tools in engaging with science practices.
g. Notice sense-making repertoires and consider students' diverse sense-making as
connecting to science practices.
Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
51
CORE KNOWLEDGE FACET ELEMENTS
CK A: Learners and Learning Environments
Well-prepared beginning science teachers will understand:
1. how learners make sense of scientific phenomena, ideas, experiences and data,
what scientific sense-making looks like in individuals, and the iterative nature of
sense-making.
2. appropriate and engaging teaching and learning strategies for creating a classroom
culture that values productive struggle, challenging science ideas, engaging in
productive science discourse, constructing science meaning together, and enjoying
science.
3. appropriate and engaging learning activities that foster an inclusive, equitable, and
anti-bias environment and create an inclusive linguistic culture.
4. appropriate and engaging learning activities in a variety of environments (e.g., the
laboratory, field, and community).
5. appropriate and engaging learning activities to include in lesson sequences and/or
assessments to create learning environments that provide opportunities for
sense-making and explanation building through investigation, collaboration,
communication, evaluation, revision, modeling and argumentation related to
scientific phenomena.
CK B: Content Pedagogy
Well-prepared beginning science teachers will understand:
1. the role of scientific phenomena and problems in three-dimensional teaching and
learning and their role in connecting science disciplines.
2. appropriate research-based student-centered, culturally-relevant, disciplinary-based
3D instructional approaches; leveraging learners’ prior experiences and knowledge,
varying activity structures, talk and group work for science. For example, they
should be expected to elicit learners' thinking, cultural and community connections,
and curiosity when making sense of phenomena.
3. appropriate differentiation strategies and research-based pedagogical strategies to
support students with a variety of cognitive, emotional, physical and other needs
and strengths so that all students develop conceptual knowledge.
4. engagement of students in applying science practices and crosscutting concepts,
such as clarifying relationships, and identifying natural patterns from empirical
experiences in lessons, curricula and assessments.
5. engineering practices wherein students design, construct, test and optimize
possible solutions to a problem in support of science learning and how it is similar
or different from science.
6. alignment of instruction and assessment strategies which address students’ prior
knowledge and alternative conceptions to support instructional decision-making and
navigate tensions between alternative ideas and ways of knowing (which may be
derived from various cultures) and canonical science ideas. Example strategies
include: referring to evidence, continuing to consider/debate to work through the
ideas, focusing on the most important disciplinary/explanatory ideas and
understanding when it is appropriate and necessary to create space for learners to
grapple with alternative ideas.
Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
52
7. integration of science-specific technologies to support all students’ conceptual
understanding of science and engineering.
8. appropriate instructional strategies which illustrate the interdisciplinary nature of
fundamental principles, processes and problems in science and engineering.
9. connections to other core disciplines (mathematics, social studies and English
language arts) within the science standards.
CK C: Safety
Well-prepared beginning science teachers will understand:
1. activities appropriate for the abilities of students that demonstrate safe techniques
for the procurement, preparation, use, storage, dispensing, supervision, and
disposal of all chemicals/materials/equipment used within their grade band and
disciplines.
2. how to recognize hazardous situations including overcrowding; implement
emergency procedures; maintain safety equipment; provide adequate student
instruction and supervision; and follow policies and procedures that comply with
established state and national guidelines, appropriate legal state and national
safety standards (e.g., OSHA, NFPA, EPA), and best professional practices (e.g.,
NSTA, NSELA).
3. ethical decision-making with respect to safe and humane treatment of all living
organisms in and out of the classroom, and compliance with the legal restrictions
and best professional practices on the collection, care, and use of living organisms
as relevant to their grade band and disciplines.
CK D: Impact on Student Learning
Well-prepared beginning science teachers will understand how to:
1. use assessments that show students have learned and can apply disciplinary
knowledge, nature of science, science and engineering practices, and crosscutting
concepts in practical, authentic, and real-world situations.
2. use summative purposeful disaggregated assessment data or information to inform
future planning and teaching, with particular attention to student demographics and
learning progress.
3. use formative assessments to recognize and assess learners’ ideas, life experiences
and learning beyond the technical scientific language by evaluating samples of
learners’ work and classroom interactions to determine the nature and depth of
learner sense-making and leverage ongoing changes in student's learning to adjust
instruction.
CK E: Professional Knowledge and Skills
Well-prepared beginning science teachers will understand:
1. critical reflection on science teaching to continually improve instructional
effectiveness.
2. accessing specific opportunities for professional development to deepen their
science-specific content knowledge and pedagogical knowledge as well as practices.
Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
53
CK F: Specialized Content Knowledge
Well-prepared beginning science teachers will understand:
1. the nature of science and the cultural norms and values inherent to the current and
historical development of scientific knowledge.
2. crosscutting concepts, disciplinary core ideas, practices of science and engineering,
the supporting role of science-specific technologies, and contributions of diverse
populations to science.
3. science standards, learning progressions, and sequencing of science content for
teaching the appropriate grade band and discipline area.
4. grade appropriate elements of the practices, disciplinary core ideas, and cross-
cutting concepts within instructional materials.
5. the major concepts, principles, theories, laws, and interrelationships of their grade
band and disciplines and supporting fields (e.g., mathematics).
GUIDING PRINCIPLES FACET ELEMENTS
I. Science for All - Teachers are guided by the value that all students have a
rightful presence in science.
A. Science is a culturally mediated way of thinking and knowing and may
contribute to and/or disrupt social inequities over time.
B. Social and critical justice orientation propels teachers to recognize and disrupt
systemic injustices and inequities manifested in classroom practices.
C. Multicultural representations and respect for culturally different ways of knowing
reinforce students’ rightful presence in science, and expand students’ funds of
knowledge of the multicultural contributions to the field.
D. Pedagogies that are culturally sustaining can be used to leverage and enhance
scientific ways of thinking and to respect students’ cultures.
E. Social capital can be mobilized to ensure opportunities for higher order and
complex thinking for all students in science across school systems.
II. Whole Child Framework- Teachers are guided by the tenets of the whole child
framework.
A. Each student learns in an environment that is physically safe, adhering strictly
to science safety protocols, while also emotionally safe for intellectual risk taking
and building a culture that supports public reasoning (Safety).
B. Each student’s development in using science and engineering practices and
crosscutting concepts is supported by a caring adult through leveraging
community resources and scaffolded sense-making (Supported).
C. Each student is actively engaged in science and engineering practices and using
crosscutting concepts as they solve problems in their school and broader
communities (Engaged).
D. Each student has access to high-level challenges that build citizenship,
stewardship, and lifelong engagement to ensure access to future school and
career opportunities in STEM fields (Challenged).
Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
54
III. Scientific Agency - Teachers are guided by recognition of the importance of
building scientific agency within themselves and students
A. Building and promoting positive science identities is prioritized.
B. Students understand the nature of science and then improve their ability to
navigate and explain their world by actively engaging in science.
C. Learning is best situated within students' lived experiences, building on existing
student ideas, assets, resources, and ways of knowing.
D. Assessments could be tied to negative outcomes on students’ identity as
scientists. Teachers must be thoughtful with regard to modalities, feedback and
how assessment information is used.
E. Students demonstrate understanding in different, valid, and informative ways
and it is necessary to provide different options for how students show their
sense-making.
F. Empowering students toward action that contributes to scientific problem solving
in their community and in the world.
IV. Disciplinary Literacy -Teachers are guided by a stance that communication is
critical to doing science and that teachers are responsible for apprenticing their
students in that communication
A. Evidence-based skepticism is necessary for citizens to be critical consumers of
science who are able to consider the complexity and dynamic nature of science.
B. Agency within a discipline is highly connected to the literacies and
communication practices of that discipline.
C. Honoring multiple ways of knowing, doing, and communicating scientific thinking
ensures a rightful presence in science for all students.
D. Scientific argument must transparently communicate ideas supported by
credible evidence and valid reasoning.
E. Technology is an important means for obtaining, communicating and evaluating
information.
V. Community Knowledge Building - Teachers will be guided by humility and
the stance that scientific knowledge is socially constructed
A. Science understanding is socially constructed in an inquiry-based classroom
environment where all students engage in the building of knowledge or possible
solutions.
B. A scientific community ensures a collaborative evidence-based approach to
addressing beliefs and biases while valuing other ways of knowing and doing
science.
C. Science learning is most meaningful when situated within the community to
ensure authenticity and cultural relevance as problems are solved in real-world
contexts in collaboration with various stakeholders.
D. Science teachers must engage in self-reflection in order to build awareness of
the impact of one’s own culture and biases in the classroom and on the
co-construction of knowledge.
E. Science teachers must be open to learning from students and colleagues in
order to grow in their teaching, scientific understanding, and ability to be
culturally responsive.
Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
55
F. Science teaching must be student-centered; prioritizing student needs,
perspectives, questions and problems within classroom instruction as authentic
scientific work.
G. Classroom culture must promote and support risk-taking inherent in public
reasoning needed for science learning.

Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
56
EXAMPLES
How the facets were used to develop three-faceted performance objectives
EXAMPLE 1:
MG.S1. LEARNERS AND LEARNING ENVIRONMENTS
Well-prepared beginning teachers of science:
S1.1 Learn about, consider and incorporate students’ backgrounds to plan and
adapt instruction that leverages the iterative nature of sense-making and
promotes positive student identities.
Science Teaching
Practices
Core Knowledge Guiding Principles
8c: Learning about
students’ cultural,
religious, family,
intellectual, personal
experiences, and
resources for use in
science instruction
c. Learn students’
background and use
this information as
you plan for and
adapt instruction
CK C: Learners and
Learning
Environments
1. how learners make
sense of scientific
phenomena, ideas,
experiences and data,
what scientific sense-
making looks like in
individuals, and the
iterative nature of
sense-making
IIIA: Scientific
Agency - teachers are
guided by recognition
of the importance of
building scientific
agency within
themselves and
students
A. Building and
promoting positive
science identities is
prioritized

Attachment E – SBE PRESENTATION DRAFT: Middle Grades and High School Science
Teacher Preparation Standards Framework
57
EXAMPLE 2:
MG.S1. LEARNERS AND LEARNING ENVIRONMENTS
Well-prepared beginning teachers of science:
S1.2 Monitor and maintain relationships with students while engaging them in
productive struggle and discourse to challenge science ideas and construct
science meaning together, keeping in mind the importance of supporting
each student’s development through scaffolded sense-making.
Science Teaching
Practices
Core Knowledge Guiding Principles
1d: Building
respectful
relationships
d. Monitor and maintain
relationships with
students.
CK C: Learners and
Learning
Environments
2. appropriate and
engaging teaching
and learning
strategies for
creating a classroom
culture that values
productive struggle,
challenging science
ideas, engaging in
productive science
discourse,
constructing science
meaning together,
and enjoying science
IIB: Whole Child
Framework - teachers
are guided by the tenets
of the whole child
framework.
B. Each student’s
development in using
science and
engineering practices
and crosscutting
concepts is supported
by a caring adult
through leveraging
community resources
and scaffolded sense-
making (Supported)
