Mal J Med Health Sci 16(4): 64-72, Dec 2020
64
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346)
ORIGINAL ARTICLE
Long-Term Effects of Kratom (Mitragyna speciosa) Use
Darshan Singh
1
, Suresh Narayanan
2
, Oliver Grundmann
3
, Nelson Jeng Yeou Chear
1
, Vikneswaran
Murugaiyah
4
, Shahrul Bariyah Sahul Hamid
5
, Nur Sabrina Mohd Yusof
1
, Eshal Bin Dzulkapli
1
, Vicknasingam
Balasingam
1
1
Centre for Drug Research, Universiti Sains Malaysia. 11800 Minden, Penang. Malaysia.
2
School of Social Sciences, Universiti Sains Malaysia. 11800 Minden, Penang. Malaysia.
3
Department of Medicinal Chemistry, College of Pharmacy, University of Florida, 1345 Center Drive, Room P6-20,
Gainesville, FL 32611, USA.
4
School of Pharmacy, Universiti Sains Malaysia. 11800 Minden, Penang. Malaysia.
5
Advanced Medical and Dental Institute, Universiti Sains Malaysia, Bertam, 13200 Kepala Batas, Penang, Malaysia
ABSTRACT
Introduction: Kratom or (Mitragyna speciosa) leaves are consumed as a folk remedy and opioid substitute in the
Southeast Asian region. There is still a lack of information about the long-term or toxic-causing effects of kratom use.
Methods: A total of thirteen regular kratom users, with long-term (>20 twenty years) kratom use history were recruited
for this cross-sectional pilot study. Respondents were required to undergo a blood-test and laboratory anaysis was
conducted to determine the mitragynine content in an acquired street sample of kratom. Results: The regular, long-
term consumption of brewed kratom decoction did not cause any significant alterations in haematological, kidney,
liver, thyroid, inflammatory and gastrointestinal analytes in a cohort of kratom users who had no history of substance
misuse. However, those who had a higher intake (>3 glasses per day) of kratom exhibited higher lipid values (except
for HDL-cholesterol), and a moderate elevation of homocysteine level. Conclusion: Long-term (>20 years with a
daily intake of ≥87.54mg of mitragynine) kratom consumption was not associated with altered biochemical levels,
although prolonged and heavy use (>3 glasses daily) may result in cardiovascular risks. The latter finding, however,
requires further investigation.
Keywords: Mitragynine, Kratom, Toxicity, Haematology, Cardiotoxicity
Corresponding Author:
Darshan Singh , PhD
Email: [email protected]
Tel: +604-653 6029
INTRODUCTION
Kratom (Mitragyna speciosa) leaves are widely used in
rural Southeast Asia for its therapeutic value (1). Rural
folks traditionally use kratom to treat common health
disorders (e.g. cough, hypertension, diabetes, and pain),
while manual labourers rely on kratom to enhance work
productivity (1). Since kratom is believed to have pain
suppressing effects, it is also used as a safe alternative
to opioids (1, 2), since its most abundant alkaloid,
mitragynine has been shown to bind to opioid-receptors
(2, 3). Over the last decade, kratom has gained ground
in the US, chiefly because of its potential to reduce pain,
relieve opioid withdrawal pains, and aid in alleviating
psychological problems like anxiety and depression (2).
Its principal psychoactive alkaloids, both mitragynine
and 7-hydroxymitragynine, were reported to have
an effect on opioid receptors (3). In the West, kratom
consumption is being seen as a public health threat
on account of several kratom toxicity cases triggered
primarily in those who use kratom in combination with
other substances like ethanol, benzodiazepines, narcotics
and pain-relieving medication (acetaminophen) (4, 5). It
is unclear if mitragynine/kratom per se was responsible
for the these adverse health occurrences, or if they were
caused by the concomitant use of kratom with other
illicit drugs (5), or the consumption of unreliable kratom
products with a higher 7-hydroxymitragynine content
than is found naturally (6).
Due to the increase in kratom-related health emergencies
(5), the US Food and Drug Administration (FDA) has
expressed concern about the unapproved sales and
distribution of a variety of kratom products. It is pressing
the government to regulate kratom and its alkaloids in
the US under the Control Substances Act (CSA). Although
kratom remains legal at the Federal level, issues related
to kratom poisoning (e.g. toxicities and deaths) continue
to unfold in the US and are reported to evolve from
the use of kratom products, primarily mitragynine and
7-hydroxymitragynine (7). The latest information from
the US National Poison Data System (NPDS) indicates
that there were about 1807 kratom exposure cases

65
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346)
Mal J Med Health Sci 16(4): 64-72, Dec 2020
reported between 2011 and 2017 (5). In fact, two-thirds
(65%) of the exposure cases occurred between 2016
to 2017, while 51.9% of the exposure incidents were
associated with various adverse medical outcomes such
as seizures, respiratory depression, cardiac arrest, renal
failure, etc. (5).
Based on the available findings, it can be hypothesised
that kratom users in US, specifically those who co-used
kratom with other illicit substances and alcohol, have
higher possibility of experiencing adverse, but not life-
threatening, health problems (4, 5, 7). Findings from
an animal study indicated that higher mitragynine
administration has the potential to alter haematological
and biochemical parameters (8). Several studies have
also highlighted kratom's (mitragynine) association
with biochemical alterations indicative of liver injuries,
neonatal withdrawal symptoms, kratom dependence and
withdrawal, overdose, gastrointestinal and cardiovascular
(e.g. bradycardia, tachycardia, palpitation, etc.) problems
(4, 5, 7, 9, 10, 11). Researchers believe that most of the
mortality incidents in the West were caused by the toxic
effect of combining illicit substances with kratom (7),
or due to the combination of kratom with conventional
drugs that resulted in lethal herb-drug interactions (12).
About 95% of those who have passed away from kratom
use had current drug abuse histories (7).
The present study examines respondents who have a
much longer history of kratom use— more than 20 years,
as compared to a previously studied group consisting of
those who used kratom for between two and eleven years
(13). Furthermore, we also analysed cardiovascular and
gastric cancer markers. Given the unavailability of safety
data on the long-term effects of kratom use, this study
seeks to shed light on whether prolonged kratom use
(>20 years) was associated with adverse health effects.
MATERIALS AND METHODS
Study design, inclusion and exclusion criteria and
measure
Thirteen kratom users consented to participate in this
cross-sectional study. All the respondents were recruited
through purposive sampling from the northern state of
Penang. Rural dwellers in this state still maintain their
traditional kratom using habit. Respondents in this study
only used brewed kratom juice, which is commonly
obtained from illegal kratom suppliers in the community.
None of the respondents have chewed or smoked
kratom before. Respondents were eligible to participate
in the study if they were; 1) below 55 years of age, and 2)
have more than 20 years regular kratom use history. We
recruited those who were less than 55 years of age since
older respondents may be exposed to unrelated health
risks. We excluded those who had current or previous
alcohol and drug use history. All the study data were
collected from January 2019 to March 2019. A trained
research officer conducted the interviews using a semi-
structured questionnaire that collected information on
the respondent's demographic characteristics
(e.g. ethnicity, current age, marital status, etc.), and
kratom use history (e.g. duration of kratom use, first age
of kratom use, etc.). Moreover, respondents were also
asked to share the negative experiences, and any health
problems they encountered in the course of their kratom
use. We also measured the body mass index (BMI) of
each respondent, recorded their daily caloric food intake,
and documented their history of cigarette smoking. This
study was approved by the Human Ethics and Research
Committee of Universiti Sains Malaysia (USM) (USM/
JEPeM/19040224). Respondents were compensated with
RM50 (USD=13.5) for their participation. All gave their
written informed consent.
Haematological and biochemical analyses
Blood samples were analysed at a diagnostic laboratory
(PATHLAB, Malaysia). Details of the haematological and
biochemical analysis have been described in a previous
study (13). Additional tests included prothrombin time
and International Normalised Ratio (INR), cystatin C,
homocysteine, hs-C reactive protein (hsCRP), creatine
phosphokinase, apolipoproteins A1, B and Apo B/Apo
A1 ratio. The cancer antigen 19.9 (CA 19.9) test was also
done to screen for gastric cancer marker.
Study analysis
All the study data were analysed with the Statistical
Package for Social Sciences (SPSS) version 24. Descriptive
statistics were used to describe the sociodemographic
characteristics and kratom use histories of respondents.
The haematological and biochemical parameters of
respondents, and the mean scores and standard deviations
(SD) were calculated to compare with the reference range
scores. Independent t-test were computed to determine
the mean differences in the biochemical parameters
between respondents who consumed <3 glasses (low
quantity) and >3 glasses (high quantity) of kratom juice
per day. We chose to study the relationship between the
low and high dose effects of kratom use because higher
quantities of kratom use were reported to cause adverse
health effects (1). The statistical significance for all tests
was set at p<0.05.
Kratom juice analysis
Reagents and chemicals
Methanol (HPLC grade) was purchased from Merck
(Germany). Mitragynine was extracted from Mitragyna
speciosa leaves following the method described by
Sharma et al. (2019) (14). The chemical structure and
purity of mitragynine was confirmed by 1H & 13C NMR,
MS and HPLC-UV (14).
Sample collection and preparation
In traditional settings, kratom traders typically use fresh
and matured kratom (Mitragyna speciosa) leaves to
produce kratom juice for local consumption. Unlike
in the US where users have a liking for using red and
66
Mal J Med Health Sci 16(4): 64-72, Dec 2020
green-veined kratom powder, local kratom traders
usually combine both the green and red-veined leaves
to prepare kratom juice. On average, respondents in this
study consumed about three packets of kratom decoction
daily (approximately 1,120 mL). It was previously shown
that the major alkaloid detected in local kratom tea/
juice was mitragynine, followed by paynantheine,
speciogynine and speciociliatine (13). Mitragynine is the
major psychoactive alkaloid of kratom. To estimate the
amount of mitragynine consumed by our respondents,
we purchased a sample packet of street kratom. The
acquired sample was measured and freeze-dried to
evaporate off the water content. The alkaloid content of
the lyophilized powder was then pre-concentrated with
methanol prior to GC-MS analyses (13).
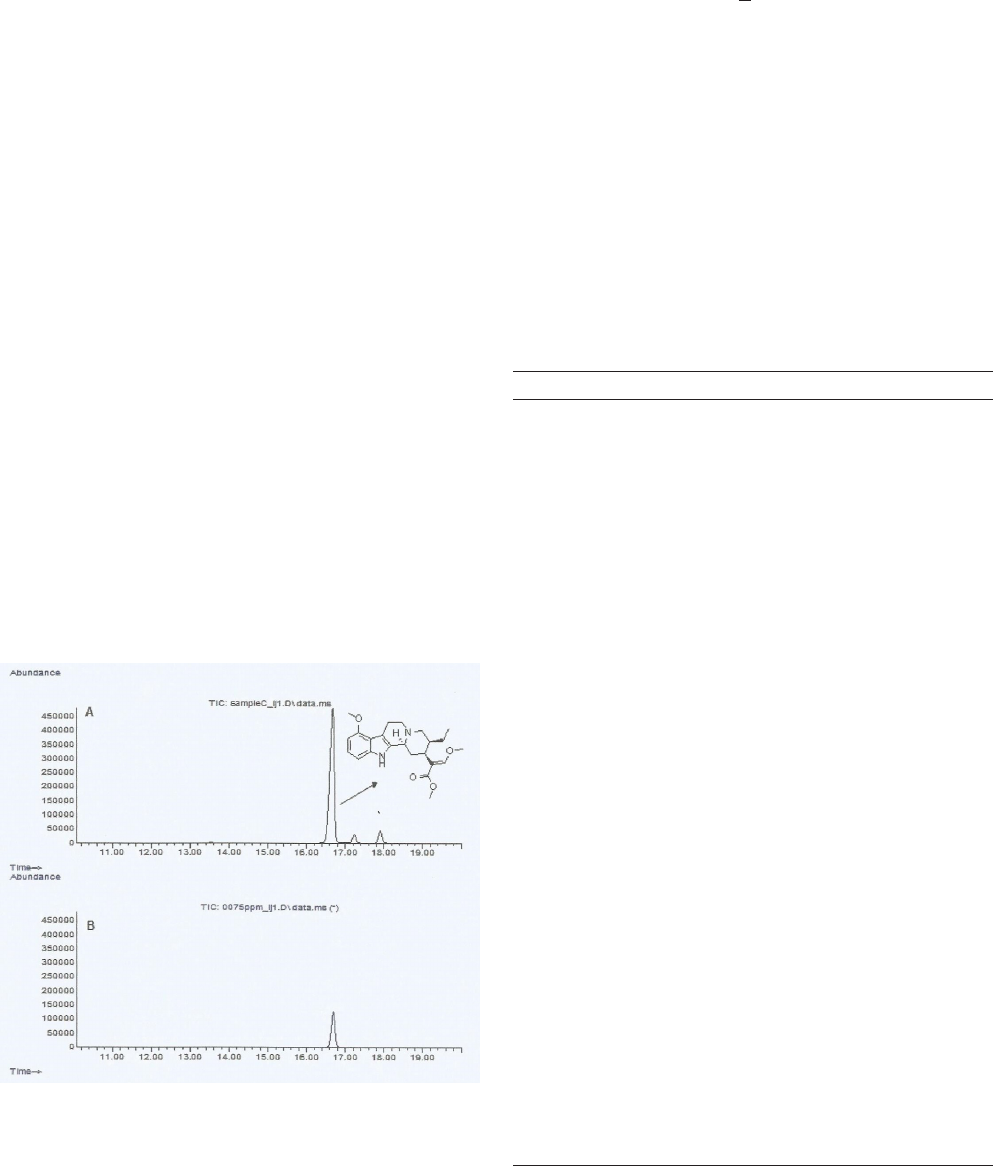
Quantification of mitragynine content using a
validated GC-SIM-MS method
Mitragynine content of the acquired kratom juice/
tea (methanol extract, 20 mg/mL) was estimated using
a validated gas chromatography-mass spectrometry
(GC-MS) method with a selective ion monitoring (SIM)
mode as described in a previous study (13). Detection of
mitragynine (kratom juice) was done by comparing the
retention time (16.8 min) and the major product ion (m/z
214) of the analyte with that of mitragynine standard (>
98% purity) (Figure 1).
RESULTS
The demographic characteristics and kratom use
history of respondents
The demographic characteristics and kratom use
history of respondents are shown in Table I. The sample
consisted only of Malay males (100%, n=13/13). The
mean age of respondents was 45.1 years. The majority
were married (85%, n=11/13), and had completed
upper secondary education (69%, n=9/13). None were
unemployed. The mean age of first kratom use was 27.5
years (SD=4.2), and their mean duration of kratom use
was 20.4 years. Respondents consumed, on average, 4
glasses of kratom juice daily (Table I). Forty-six percent
(n=6/13) consumed >3 glasses of kratom juice daily,
while 54% (n=7/13) used <3 glasses of kratom per day.
The mean BMI of the respondents were 27.2 (SD=4.5),
and their mean intake of daily calories was 2,084.7
(SD=320.6). Almost all (n=12/13) had current history of
cigarette smoking, and their mean duration of smoking
was 26.6 years (SD=2.4).
Haematology blood test findings
There were no significant alterations in the haematology
parameters of the respondents. Only subtle alterations
were observed for RDW value and prothrombin time as
shown in Table I.
Table I: Respondents socio-demographic characteristics and kratom
use history
n (%)
Gender
Male
13 (100)
Ethnicity
Malay
13 (100)
Mean age 45.1 years (SD=6.6)
Marriage
Single
Married
2 (15)
11 (85)
Education
9 years
≥11 years
4 (31)
9 (69)
Employment
Employed 13 (100)
Kratom use history
Mean age of first kratom use
Mean duration of kratom use
Mean frequency of daily kratom use
Mean quantity of daily kratom use
27.5 years (SD=4.2)
20.4 years (SD=0.7)
5.6 times (SD=2.4)
4 glasses (SD=2.5)
Daily quantity of kratom use
≤3 glasses
>3 glasses
7 (54)
6 (46)
Mean BMI 27.2 (SD=4.5)
Mean calories daily 2084.7 calories (SD=320.6)
Mean smoking duration 26.6 years (SD=2.4)
Smoker
Yes
No
12 (92)
1 (8)
Health problems since kratom use
No 13 (100)
Blood group
O RH
AB RH
A RH
B RH
7 (54)
1 (8)
2 (15)
3 (23)
Figure 1: (A) GC-SIM-MS chromatogram of the purchased
kratom juice sample (methanol extract); (B) GC-SIM-MS
chromatogram of mitragynine standard (75 µg/mL).

Mal J Med Health Sci 16(4): 64-72, Dec 2020
67
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346)
Biochemical test findings
Results from the blood chemistry analyses indicated that
there were no significant alterations in the kidney, liver or
thyroid functions, rheumatoid factor, and CA 19.9 level
(Table II). However, the lipid profile and apolipoprotein
parameters were found to be altered. It was apparent that
the level of total cholesterol, LDL, triglycerides, hs-CRP
and homocysteine levels were higher than the reference
range, although the HDL remained at the normal range
(Table II).
Differences in the haematology and bio-chemical
parameters between those who consumed lower (<3
glasses) and higher (>3 glasses) quantity of kratom juice
It has been reported that those who consumed kratom
regularly over prolonged periods may experience weight
loss, decreased appetite and libido, fatigue, etc. (1).
Therefore, we investigated the dose-response effects
of kratom use on haematological and biochemical
parameters. Since chronic kratom use was associated
with gastrointestinal discomfort and elevation in
lipid profile (13), we attempted to determine whether
higher intake of kratom was associated with altered
haematological and biochemical parameters. We found
there was a significant increase beyond the reference
range particularly for RDW and prothrombin time
among those who consumed <3 glasses of kratom per
day, relative to those who consumed >3 glasses of
kratom (Table III). However, there were no significant
alterations in the kidney parameters of the respondents,
except for the cystatin value which appeared higher than
the reference range among those who consumed >3
glasses of kratom juice per day. No significant changes
in the glucose, liver function, thyroid function and CA
19.9 levels were detected between those who reported
consuming more than or less than 3 glasses per day.
However, our results indicated that respondents who
consumed >3 glasses of kratom on a regular basis had
substantial elevations in total cholesterol, LDL, Apo
B, Apo B/A1 ratio when compared to the individual
reference range parameters. There were also elevations
in homocysteine and triglyceride levels, but interestingly
the values turn out to be high among those who reported
consuming lower (<3 glasses) quantity of daily kratom
use. However, hs-CRP was above the reference range
for both groups.
Estimation of mitragynine content in the kratom sample
In this study, approximately 360 mL of kratom tea/juice
was lyophilized to yield 4.39 g of water extract and
subsequently extracted with methanol to yield 3.1 g of
methanolic extract. Based on GC-SIM-MS quantification,
the mitragynine content in the methanol extract (20 mg/
mL) was 0.1883 ± 0.01 mg/mL which is equivalent to
29.18 mg in 3.1 g of methanol extract (0.94% (w/w)).
The mitragynine content in the street sample was found
to be around 29.18 mg per glass (360mL of kratom tea/
juice). It can, therefore, be inferred that the respondents
ingested approximately 87.54 mg of mitragynine per
Table II: Respondents biochemical profile.
Scores
(n=13)
Mean ± (SD)
Unit
Reference
Range
Haematology
ESR
RBC
Haemoglobin
PCV (HCT)
MCV
MCH
MCHC
RDW value
Platelet count
WBC
7.5 (4.2)
5.5 (.74)
15.1 (.94)
41.6 (11.4)
82.5 (10.1)
27.8 (3.4)
33.8 (.90)
15.0 (1.9)*
326.6 (74.4)
9.8 (2.5)
MM/HR
X10^12/L
G/DL
%
FL
PG
G/DL
%
X10^9/L
X10^/L
0-10
4.5-6.5
13.0-18.0
40-54
76-96
27-32
32-36
11.5-14.5
150-400
4.0-11.0
Differential count
Neutrophil
Lymphocyte
Monocyte
Eosinophil
Basophil
50.2 (9.5)
36.1 (7.7)
8.6 (2.4)
4.9 (3.2)
0.31 (.48)
%
%
%
%
%
40-75
20-45
2-10
0-6
0-2
Prothrombin time
Patient time
Control time
INR
14.4 (2.9)*
14.0 (.00)
1.1 (.13)
Seconds
Seconds
9.8-12.1
Diabetes screen
Glucose 5.6 (1.4) mmol/L Fasting 3.9-
5.6
Kidney function test
Urea
Creatinine
Calcium
Inorganic phosphate
Uric acid
Sodium
Potassium
Chloride
Cystatin C
2.9 (.81)
87.5 (10.5)
2.3 (.09)
1.3 (.26)
0.38 (.10)
145.4 (2.3)
4.5 (.36)
101.4 (1.6)
0.97 (.16)*
mmol/L
mmol/L
mmol/L
mmol/L
mmol/L
mmol/L
mmol/L
mmol/L
mg/L
1.7-8.4
62-115
2.12-2.52
0.78-1.65
0.20-0.42
137-150
3.5-5.3
96-108
0.50-0.96
Microalbumin
Urine microalbumin
Urine creatinine
Microalb: creat ratio
6.70 (5.0)
11.5 (7.1)
0.76 (1.2)
mg/L
nmol/L
mg/MMOL <3.4
Lipid profile
Total cholesterol
HDL
LDL
Triglycerides
Total/HDL ratio
Hs-C reactive protein
5.8 (1.1)*
1.3 (.25)*
3.6 (.91)*
2.2 (1.8)*
4.6 (1.4)
5.3 (3.3)*
mmol/L
mmol/L
mmol/L
mmol/L
mg/L
<5.2
>1.04
<2.6
<1.7
<5.0
<4.7
Apolipoproteins
Apolipoprotein A1
Apolipoprotein B
Apo B/APO A1 ratio
Homocysteine
1.3 (.17)
1.3 (.25)
1.0 (.25)
19.2 (13.8)*
g/L
g/L
μmol/L
0.94-1.78
0.63-1.33
<1.00
5.0-15.0
Liver function test
Total protein
Albumin
Globulin
A/G ratio
Total bilirubin
Alkaline phosphatase
SGOT (AST)
SGPT (ALT)
GGT
CPK (Total)
75.2 (3.7)
41.5 (3.3)
33.7 (4.1)
1.3 (.23)
9.5 (2.10)
89.6 (12.7)
30.6 (6.2)
28.1 (10.1)
40.3 (14.8)
162.9 (76.6)
g/L
g/L
g/L
μmol/L
IU/L
IU/L
IU/L
IU/L
U/L
64-83
30-50
20-50
1.2-2.5
<17
39-117
0-40
0-53
<73
39-308
Thyroid screen
Thyroxine (T4)
Rheumatoid factor
111.4 (29.8)
17.3 (25.6)
nmol/L
IU/ML
64.0-167.0
0-35
Tumour marker
CA 19.9 9.4 (5.7) U/ML <37.0
*Denotes values are higher than the reference range.

Mal J Med Health Sci 16(4): 64-72, Dec 2020
68
Table III: Differences in the biochemical parameters of between those who consumed ≤3 glasses and >3 glasses of kratom daily
≤3 glasses
(n=7)
Mean ± (SD)
>3 glasses
(n=6)
Mean ± (SD)
Difference t df
P-value
Reference Range
Haematology
ESR
RBC
Haemoglobin
PCV (HCT)
MCV
MCH
MCHC
RDW value
Platelet count
WBC
7.1 (4.3)
5.5 (1.03)*
14.5 (.72)
37.5 (14.6)
80.9 (13.3)
27.0 (4.5)
33.4 (.98)
15.4 (2.6)*
356.4 (69.8)
9.10 (2.4)
8.0 (4.6)
5.4 (.23)
15.8 (.63)
46.3 (2.2)
84.5 (4.9)*
28.8 (1.2)*
34.3 (.52)
14.6 (.82)
291.8 (68.7)
9.7 (2.7)
0.9
0.1
1.3
8.8
3.6
1.8
0.9
0.8
64.6
0.6
0.349
0.072
3.455
1.455
0.630
0.963
2.032
0.726
1.675
0.215
11
11
11
11
11
11
11
11
11
11
0.782
0.014
0.863
0.105
0.031
0.008
0.153
0.012
0.576
0.911
0-10
4.5-6.5
13.0-18.0
40-54
76-96
27-32
32-36
11.5-14.5
150-400
4.0-11.0
Differential count
Neutrophil
Lymphocyte
Monocyte
Eosinophil
Basophil
49.0 (10.20)
36.9 (9.0)
10.3 (1.5)
3.7 (1.7)
.14 (.38)
51.5 (9.30)
35.2 (6.6)
6.7 (1.8)
6.2 (4.2)*
.50 (.55)*
2.5
1.7
3.6
2.5
0.36
0.459
0.380
4.023
1.432
1.387
11
11
11
11
11
0.894
0.702
0.545
0.012
0.042
40-75
20-45
2-10
0-6
0-2
Prothrombin time
Patient time
Control time
INR
15.5 (3.20)**
14.0 (.00)
1.1 (.2)
13.1 (1.10)**
14.00 (.00)
1.0 (.1)*
2.4
0
0.1
1.554
1.683
11
11
0.166
0.000
9.8-12.1
Diabetes screen
Glucose 5.6 (1.9) 5.6 (.70) 0 0.000 11 0.106 Fasting 3.9-5.6
Kidney function test
Urea
Creatinine
Calcium
Inorganic phosphate
Uric acid
Sodium
Potassium
Chloride
Cystatin C
3.2 (.93)
84.0 (10.1)
2.24 (.10)
1.3 (.30)
.40 (.10)
144.7 (2.6)
4.50 (.27)
101.14 (2.1)
0.88 (.14)
2.6 (.60)
91.5 (10.4)
2.30 (.12)*
1.3 (.30)
.40 (.10)
146.2 (1.6)
4.60 (.47)
101.7 (.8)
1.1 (.13)**
0.6
7.5
0.06
0
0
1.5
0.1
0.56
0.22
1.259
1.316
0.900
0.043
0.759
1.175
0.555
0.568
2.399
11
11
11
11
11
11
11
11
11
0.195
0.886
0.044
0.967
0.916
0.448
0.167
0.315
0.972
1.7-8.4
62-115
2.12-2.52
0.78-1.65
0.20-0.42
137-150
3.5-5.3
96-108
0.50-0.96
Microalbumin
Urine microalbumin
Urine creatinine
Microalb: create ratio
8.3 (5.2)
12.8 (6.4)
1.03 (1.6)
4.9 (4.4)
10.0 (8.1)
0.45 (.24)
3.4
2.8
0.58
1.251
0.704
0.879
11
11
11
0.757
0.502
0.109 <3.4
Lipid profile
Total cholesterol
HDL
LDL
Triglycerides
Total/HDL ratio
Hs-C reactive protein
5.3 (.89)**
1.3 (.31)**
3.1 (.80)**
2.2 (2.2)**
4.2 (1.4)
5.3 (3.4)**
6.5 (1.0)**
1.3 (.18)**
4.1 (.82)**
1.2 (1.5)
5.1 (1.3)
5.2 (3.6)**
1.2
0
1
1
0.9
0.1
2.247
0.005
1.833
0.056
1.110
0.044
11
11
11
11
11
11
0.839
0.353
0.893
0.196
0.860
0.847
<5.2
>1.04
<2.6
<1.7
<5.0
<4.7
Apolipoproteins
Apolipoprotein A1
Apolipoprotein B
Apo B/APO A1 ratio
Homocysteine
1.30 (.20)
1.14 (.20)
.90 (.20)
21.2 (18.2)**
1.30 (.15)
1.41 (.24)**
1.12 (.30)**
16.4 (3.7)**
0
0.27
0.22
4.8
0.217
2.201
1.851
0.572
11
11
11
11
0.661
0.878
0.158
0.119
0.94-1.78
0.63-1.33
<1.00
5.0-15.0
Liver function test
Total protein
Albumin
Globulin
A/G ratio
Total bilirubin
Alkaline phosphatase
SGOT (AST)
SGPT (ALT)
GGT
CPK (Total)
75.0 (3.8)
43.1 (3.4)
31.9 (3.3)
1.4 (.22)
10.4 (2.5)
87.4 (14.1)
33.6 (4.2)
33.0 (11.60)
41.6 (17.3)
173.7 (75.30)
75.3 (3.10)
39.5 (1.6)
35.8 (4.0)
1.1 (.16)
8.5 (3.4)
92.7 (11.40)
27.2 (6.7)
22.3 (3.5)
38.8 (12.7)
150.2 (83.1)
0.3
3.6
3.9
0.3
1.9
5.3
6.4
10.7
2.8
23.5
0.154
2.364
1.950
2.198
1.178
0.729
2.106
2.159
0.320
0.536
11
11
11
11
11
11
11
11
11
11
0.900
0.236
0.603
0.325
0.475
0.608
0.144
0.078
0.465
0.545
64-83
30-50
20-50
1.2-2.5
<17
39-117
0-40
0-53
<73
39-308
Thyroid screen
Thyroxine (T4)
Rheumatoid factor
120.5 (24.4)
22.0 (33.7)
100.7 (34.1)
11.8 (11.7)
19.8
10.2
1.218
0.699
11
11
0.093
0.101
64.0-167.0
0-35
Tumour marker
CA 19.9 7.5 (4.4) 11.3 (6.7) 3.8 1.175 11 0.210 <37.0
*Denotes differences between those who consumed ≤3 or >3 glasses of kratom daily at (p<0.05).
**Denotes values are higher than the reference range.

Mal J Med Health Sci 16(4): 64-72, Dec 2020
69
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346)
day, corresponding to an average of three glasses of
kratom juice.
DISCUSSION
Although kratom leaves have customarily been used
for its broad therapeutic values such as relieving pain,
elevating mood, and as an affordable substitute for opioids
in Southeast Asia, several studies from the West have
emerged linking kratom consumption with poisoning and
death (4, 5, 7). Kratom use for the self-treatment of pain
first was identified in the US in 2007 (1). Subsequently
large scale surveys have shown that kratom was being
used to self-treat chronic pain, opioid withdrawal and
dependence, as well as psychological problems (1, 2).
Despite this, the FDA has kept alleging that kratom's
main alkaloids, mitragynine and 7-hydroxymitragynine,
had a role in the majority of kratom fatalities in the US
(7). In fact, the link between mitragynine and the reported
death rates has been poorly elucidated, since 71% of
the fatalities were reported as accidental (including
misadventure), while 9% were classified as intentional
(suicide) (7). A recent study estimated that the risk of
overdose death is >1000 times greater for opioids than for
kratom (15). In fact, the majority who have encountered
adverse health problems were those who had current
histories of drug use problem, chiefly opioid abuse (7,
15). Meanwhile, in Southeast Asia, traditional kratom
use has, thus far, not been associated with any major
health concerns, although it has been placed under the
Poisons Act 1952 in Malaysia (1). While previous studies
have documented the side-effects of kratom, and its use
as an opioid substitute among heroin users in Malaysia
(1), the safety of long-term consumption remains poorly
investigated. Our preliminary findings show that regular,
long-term kratom use was not associated with altered
biochemical parameters in a small sample of non-drug
using kratom users. This suggests that long-term (>20
years) kratom use may not adversely affect the studied
parameters. Our findings are in line with an earlier study
indicating that kratom use was not harmful (13). However,
more data and clinical investigations of kratom use are
needed to establish its therapeutic value and safety.
In comparison to other haematological parameters, only
the red cell distribution (RDW) value was found to be
raised above the reference range. RDW is commonly
used as a marker for detecting iron deficiency anaemia,
as well as a predictor for inflammatory diseases such as
chronic heart failure (18, 19). A previous study reported
that elevation in RDW value was associated with
increased risk of cardiovascular problems; however,
other factors like poor nutritional intake and age-related
diseases could have played a role in the elevation of
RDW value (16, 19). Similarly, an increase in RDW
value can also arise from defects in red cell production,
or because of increased haemolysis (16). Though there
were significant differences in the RBC, MCV and MCH
values of those who consumed low (<3 glasses) and
higher (>3 glasses) quantities of kratom juice, all values
of the parameters were within the normal reference
range. Only the RDW value was slightly raised beyond
the reference value for those who consumed a lower
quantity of kratom juice (<3 glasses) on a daily basis
(p<0.012). The elevated RDW value can be a sign of
cardiovascular risk or marker for inflammatory cytokines
(16). However, the increase in the prevalence of anaemia
with advancing age can also be partially attributed to
the cytokines that inhibit the proliferation of erythroid
progenitor cells (19).
In the West, users appeared prone to develop liver
and kidney impairments even after using kratom for
short durations (5, 7). This is in contrast to our findings
that suggest that even long-term kratom consumption
appears not to affect liver and kidney functions. Despite
the non-significant differences in the cystatin C level
between those who consumed either more or less
quantity of kratom juice (p<0.972), we found cystatin C
levels were slightly raised beyond the reference range.
A previous study indicated that elevation of cystatin
C level was linked with hypertension, coronary heart
disease, rheumatoid arthritis and older age (20). Based
on this, an inference of a possible link between long-
term kratom use and increased cardiovascular risk might
be made. Confirmation of this link, however, requires
more controlled-clinical studies.
Besides the RDW and cystatin C levels, we found that
long-term kratom users had elevated lipid profiles. The
lipid profile (total cholesterol, LDL and triglycerides) of
respondents were markedly raised beyond the reference
range, regardless of the quantity of kratom consumed.
The hs-CRP, homocysteine and apolipoproteins such as
apolipoprotein B were higher among respondents who
consumed more than 3 glasses of kratom daily. The
elevation in total cholesterol, LDL, triglycerides, hs-CRP,
homocysteine, apolipoprotein B and apolipoprotein B/
APO A1 ratio may be indicative of elevated cardiovascular
risk. Our findings were in line with an earlier study that
also noted the elevation in LDL cholesterol with a higher
intake of kratom juice (13). In another animal model
study, the intake of kratom crude extracts of 100, 500 and
1000mg/kg was associated with a significant increase
in triglycerides and cholesterol parameters in rats (21).
Given its lipid-altering effects, long-term kratom use may
potentially increase the cardiovascular risk among users.
In Asian societies people conceive opium consumption as
having positive effects on cardiometabolic diseases (e.g.
hypertension and dyslipidaemia) (22). It was reported
that opiate use is associated with significant elevation in
lipid profile (23). The mechanism behind this elevation
remains poorly delineated, but the problem was shown
to occur from both the decreased in hepatic clearance of
LDL cholesterol from plasma, as well as the increase in
hepatic synthesis of triglycerides (24). The findings from
a review article indicated that opium (opioids) use is
Mal J Med Health Sci 16(4): 64-72, Dec 2020
70
linked with coronary artery disease (CAD) (22), although
opium was reported to have both positive and negative
cardiovascular effects (25). Opium addiction was found
to have harmful effects on one or more lipid parameters
leading to hypercholesterolemia (26).
Apolipoprotein A 1 and B, and hs-CRP (also known as
a highly sensitive C-reactive protein/CRP) have been
recognised as novel cardiovascular risk factors (28).
Notably, we found kratom users in this study had elevated
hs-CRP. The elevation of hs-CRP is reported to increase
mortality risk (e.g. heart attack and stroke) (28, 29). The
hs-CRP has opsonizing properties, where it can increase
the risk of endothelial dysfunction (28). Perhaps, elevation
in hs-CRP may indicate that kratom users could be at risk
of developing atherosclerosis and myocardial infarction.
This is because hs-CRP is reported to play an important
role in several aspects of atherogenesis (e.g. release of
proinflammatory cytokines, promotion of endothelial
dysfunction, prevent nitric oxide production, etc.) (29).
However, this alteration in hs-CRP parameter needs to
be further investigated through proper clinical studies.
Elevation in LDL and apolipoprotein B are associated
with coronary heart disease (30). We found kratom users
in this cohort had an altered LDL and apolipoprotein B.
Indeed, the elevation in apolipoprotein B was associated
with higher kratom intake (>3 glasses), and may serve
as a risk factor for coronary heart disease. Our results
also indicated that kratom users in this cohort had
hyperhomocysteinemia. It has shown that the elevation
in homocysteine levels can be related to various health
conditions such as cardiovascular risk, neurological
and psychiatric diseases (31). In fact, the prevalence of
hyperhomocysteinemia is shown to be common among
patients with heart diseases or high blood pressure (32).
Despite the elevated cardiovascular risk, we found HDL
cholesterol level was above the reference range among
kratom users in this study, while apolipoprotein A was
within the normal reference range.
We also found there were no alterations in the thyroid
function and rheumatoid factor parameters of the
respondents. It appears that regular kratom consumption
in the form of a decoction did not impair their thyroid
function. Since kratom use was associated with
gastrointestinal discomfort like constipation, we also
determined whether long-term kratom use can cause a
severe gastrointestinal problem. CA 19.9 is usually used
as a prognostic indicator in diagnosing gastric cancer
(33). Respondents' CA 19.9 markers for gastric cancer
were either negative or within the normal range. Thus,
we found no link between regular kratom consumption
and elevated risk of gastric cancer.
This study has a few limitations. First, our sample size was
relatively small and consisted only of male respondents
who were recruited through purposive sampling from
one particular state without a control group. We decided
not to recruit a control-group because the aim of the
study was to determine the long-term (>20 years) effects
of kratom use. We tried to recruit more long-term kratom
users; however, most were excluded from the study since
they had existing medical problems such as diabetes and
hypertension, as well as previous illicit drug use history.
Due to the small sample size, our findings cannot be
generalised. Second, although all the respondents were
long-term users (>20 years), some of the alterations in the
haematological and biochemical parameters, specifically
in the lipid profile (total cholesterol, LDL, triglycerides,
hs-CRP and apolipoproteins B) could have been caused
by other factors such as respondent's history of cigarette
smoking, diet and lifestyle, and not kratom use per se. As
such, it is vital that future studies attempt to determine
the long-term effects of kratom use in regular users
through a longitudinal clinical study. Finally, although
the respondents self-reported the absence of pre-existing
medical problems, we could not conclusively rule this
out. This may have affected our findings.
CONCLUSION
Notwithstanding the vast evidence highlighting kratom's
utility as a safe substitute to opioids, the popular notion
that kratom is an opioid has compelled regulatory
agencies to consider banning kratom use. In fact, though
kratom consumption has been implicated in some deaths
in the US, it has not been conclusively demonstrated
that kratom was primarily or solely responsible for
them (34). A recent study clearly pinpointed that 80%
of kratom-related deaths occurred among those with
history of substance misuse, and 90% had no evidence
of a history of supervised pain care, suggesting strongly
that the majority of the deaths were caused by the used
of multiple drugs and not just kratom (35). Our findings
are among the first to show in a cohort of non-drug using
kratom users, that prolonged kratom use (>20 years with
an average daily intake of ≥87.54mg of mitragynine), in
the form of a brewed solution, was not associated with
significant alterations in haematological and biochemical
profile. However, there were indications that kratom use
may increase cardiovascular risk, especially when used
in large quantity for an extended period of time; this
possible link necessitates further investigation.
ACKNOWLEDGEMENTS
This study was financially supported by the Ministry of
Higher Education Malaysia under the Higher Institution
Centres of Excellence (HICoE) grant.
REFERENCES
1. Singh D, Narayanan S, Vicknasingam B. Traditional
and non-traditional uses of Mitragynine (Kratom):
A survey of the literature. Brain Res. Bull. 2016;
126: 41-46.
2. Grundmann O. Patterns of Kratom use and health
impact in the US-Results from an online survey.
Mal J Med Health Sci 16(4): 64-72, Dec 2020
71
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346)
Drug Alcohol Depend. 2017; 176: 63-70.
3. Kruegel AC, Grundmann O. The medicinal
chemistry and neuropharmacology of kratom: A
preliminary discussion of a promising medicinal
plant and analysis of its potential for abuse.
Neuropharmacology. 2018; Vol. 134: 108-120.
4. Anwar M, Law R, Schier J. Notes from the Field:
Kratom (Mitragyna speciosa) exposures reported
to poison centers - United States, 2010-2015.
MMWR Morbidity and Mortality Weekly Report.
2016: 65(29); 748-749.
5. Post S, Spiller HA, Chounthirath T, Smith GA.
Kratom exposure reported to United States poison
control centers: 2011-2017. Clin. Toxicol. 2019:
Vol. 57(10); 847-854.
6. Lydecker AG, Sharma A, McCurdy CR,
Avery BA, Babu KM, Boyer EW. Suspected
Adulteration of Commercial Kratom Products with
7-Hydroxymitragynine. J. Med. Toxicol. 2016:
12(4); 341-349.
7. Corkery JM, Streete P, Claridge H. et al.
Characteristics of deaths associated with kratom
use. J Psychopharmacol. 2019: Vol. 33(9); 1102-
1123.
8. Sabetghadam A, Ramanathan S, Sasidharan
S, Mansor SM, 2013. Subchronic exposure to
mitragynine, the principal alkaloid of Mitragynine
speciosa, in rats. J Ethnopharmacol. 2013: 146;
815-823.
9. Matsunaga T, Morikawa Y, Kamase K, et al.
Enhancement of Endothelial Barrier Permeability
by Mitragynine. Biol. Pharm. Bull. 2017: 40; 1779-
1783.
10. Lu J, Wei H, Wu, J. et al. Evaluation of the
Cardiotoxicity of Mitragynine and Its Analogues
Using Human Induced Pluripotent Stem Cell-
Derived Cardiomyocytes. PloS ONE. 2014: 9(12);
e115648.
11. Tay YL, Teah YF, Chong YM. Mitragynine and
its potential blocking effects on specific cardiac
potassium channels. Toxicol. Appl. Pharmacol.
2016: 305; 22-39.
12. Manda VK, Avula B, Dale OR. et al. PXR mediated
induction of CYP3A4, CYP1A2, and P-gp by
Mitragyna speciosa and its alkaloids. Phytother
Res. 2017: 31; 1935-1945.
13. Singh D, Muller CP, Murugaiyah V. et al. Evaluating
the hematological and clinical-chemistry
parameters of kratom (Mitragyna speciosa) users
in Malaysia. J Ethnopharmacol. 2018a: 214; 197-
206.
14. Sharma A, Kamble SH, Leon F. et al. Simultaneous
quantification of ten key kratom alkaloids
in Mitragyna speciosa leaf extracts and
commercial products by ultra-performance liquid
chromatography-tandem mass spectrometry. Drug
Test Anal. 2019: Vol. 11, Issue 8.
15. Henningfield, JE., Grundmann, O., Babin, JK.,
Fant, RV., Wang, DW., Cone, EJ. Risk of death
associated with kratom use compared to opioids.
Prev Med. 2019: Vol. 128; 105851.
16. Felker GM, Allen LA, Pocock SJ. Red Cell
Distribution Width as a Novel Prognostic Marker
in Heart Failure. J Am Coll Cardiol. 2007: Vol. 50;
40-47.
17. Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z,
Prohaszka Z, Janoskuti L. Red cell distribution
width in heart failure: Prediction of clinical events
and relationship with markers of ineffective
erythropoiesis, inflammation, renal function, and
nutritional state. Am Heart J, Vol. 2009: 158(4);
659-666.
18. Cheng S, Han F, Wang Y. et al. The red distribution
width and the platelet distribution width as
prognostic predictors in gastric cancer. BMC
Gastroenterology. 2017: 17: 163; 1-11.
19. Patel KV, Semba RD, Ferrucci L. Red Cell
Distribution Width and Mortality in Older Adults:
A Meta-analysis. J Gerontol A Biol Sci Med Sci.
2010: Vol. 65A (3); 258-265.
20. Wasen E, Isoaho R, Mattila K, Vahlberg T, Kivela SL,
Irjala K. Serum Cystatin in the Aged: Relationship
With Health Status. Am J Kidney Dis. 2003: Vol.
42; 36-43.
21. Harizal SN, Mansor SM, Hasnan J, Tharakan JKJ,
Abdullah, J. Acute toxicity study of the standardized
methanolic extract of Mitragyna speciosa Korth in
Rodent. J Ethnopharmacol. 2010: 131; 404-409.
22. Masoudkabir F, Sarrafzadegan N, Eisenberg MJ.
Effects of opium consumption on cardiometabolic
diseases. Nat. Rev. Cardiol. 2013: 10; 733-740.
23. Bryant HU, Story JA, Yim GKW. Morphine-induced
alterations in plasma and tissue cholesterol levels.
Life Sci. 1987: Vol. 41(5); 545-554.
24. Mohammadi A, Darabi M, Nasry M. et al. Effect
of opium addiction on lipid and atherosclerosis
formation in hypercholesterolemic rabbits. Exp.
Toxicol. Pathol. 2009: Vol. 61; 145-149.
25. Najafipour H, Beik A. The Impact of Opium
Consumption on Blood Glucose, Serum Lipids and
Blood Pressure, and Related Mechanisms. Front.
Physiol. 2016: Vol. 7; 436, 1-12.
26. Aghadavoudi O, Eizadi-Mood N, Najarzadegan,
MR. Comparing cardiovascular factors in opium
abusers and non-users candidate for coronary
artery bypass graft surgery. Adv Biomed Res. 2015:
4; 12.
27. Asgary S, Sarrafzadegan N, Naderi GA, Rozbehani
R. Effects of opium addiction on new and
traditional cardiovascular risk factors: do duration
of addiction and route of administration matter?
Lipids Health Dis. 2008: 7:42; 1-5.
28. Kamath DY, Xavier D, Sigamani A, Pais P.
High sensitivity C-reactive protein (hsCRP) &
cardiovascular disease: An Indian perspective.
Indian J Med Res. 2015: 142; pp261-268.
29. Shrivastava AK, Singh HV, Raizada A, Singh SK.
C-reactive protein, inflammation and coronary
Mal J Med Health Sci 16(4): 64-72, Dec 2020
72
heart disease. Egypt Heart J. 2015: 67; 89-97.
30. Contois JH, McConnell JP, Sethi AA. Et al.
Apolipoprotein B and Cardiovascular Disease Risk:
Position Statement from the AACC Lipoproteins
and Vascular Diseases Division Working Group on
Best Practices. Clin Chem. 2009: 55:3; 407-419.
31. Chung, K. H., Chiou, H. Y., & Chen, Y. H.
Association between serum homocysteine levels
and anxiety and depression among children and
adolescents in Taiwan. Scientific Reports. 2017:
Vol. 7; 8330.
32. Asfar S, Safar HA. Homocysteine levels and
peripheral arterial occlusive disease: a prospective
cohort study and review of the literature. J
Cardiovasc Surg. 2007: Vol. 48; 601-605.
33. Jo JC, Ryu MH, Koo DH. Et al. Serum CA 19.9 as a
prognostic factor in patients with metastatic gastric
cancer. Asia Pac J Clin Oncol. 2013: Vol. 9; 324-
330.
34. Henningfield, JE., Fant RV., Wang, DW. The
abuse potential of kratom according the 8 factors
of the controlled substances act: implications for
regulation and research. Psychopharmacology
(Berl.). 2018: Vol. 235(2): 573-589.
35. Kuehn B. Kratom-Related Deaths. JAMA. 2019:
Vol. 321; No. 20.
