
Barriers to Birth Control: An Analysis of
Contraceptive Coverage and Costs for Patients with
Private Insurance
Staff Report
Committee on Oversight and Reform
U.S. House of Representatives
October 25, 2022
oversight.house.gov

1
EXECUTIVE SUMMARY
The Affordable Care Act (ACA), signed into law by President Obama in 2010, protects
Americans’ access to affordable birth control. The ACA requires private health plans and issuers
of health insurance to cover the full range of birth control methods approved by the Food and
Drug Administration (FDA), without patient cost-sharing.
In May 2022, following reports that patients were facing barriers to accessing birth-
control without cost-sharing, Oversight Committee Chairwoman Carolyn B. Maloney opened an
investigation into contraceptive coverage for individuals enrolled in private health plans. The
Committee sought information from five of the nation’s largest health insurers and four of the
largest pharmacy benefit managers (PBMs) to assess how companies are providing patients with
access to FDA-approved birth control—without cost-sharing—as required by the ACA.
1
As part of this investigation, the Committee reviewed cost-sharing requirements and
coverage exclusions for 120 contraceptive products across approximately 68 health plans and
formularies and analyzed each company’s process for approving or denying exceptions to cost-
sharing requirements and coverage exclusions.
The Committee’s investigation identified more than 30 birth control products for which
most health insurers and PBMs reviewed impose cost-sharing requirements or coverage
exclusions. The investigation found that the processes established for patients seeking
exceptions to cost-sharing and coverage restrictions can be burdensome for patients and
providers, and that companies deny exception requests on average four or more times out of ten.
These practices raise barriers to accessing zero-cost birth control, contrary to Congress’s goal in
the ACA.
In the wake of the Supreme Court’s decision in Dobbs v. Jackson Women’s Health
Organization overturning the constitutional right to abortion, access to contraception has become
even more important for the 64 million women of reproductive age in the United States.
2
The
Biden Administration has recently taken steps to further clarify health insurers’ obligations to
provide access to contraception without cost-sharing. It is critical that the federal government
build on this progress to ensure Americans have meaningful, equitable access to the full range of
FDA-approved contraceptive methods—without facing financial or procedural barriers.
This staff analysis, the first congressional report of contraceptive coverage in the private
health insurance market, presents the findings of the Committee’s investigation:
1
These nine companies are Aetna, Cigna, CVS Caremark, Elevance Health (formerly Anthem, Inc.)
Express Scripts, Humana, UnitedHealthcare, Optum Rx, and Prime Therapeutics.
2
The Court’s decision to eliminate the constitutional right to abortion also threatens the right to
contraception, established in Griswold v. Connecticut. In a concurring opinion to Dobbs, Justice Clarence Thomas
urged the Supreme Court to “reconsider all of this Court’s substantive due process precedents, including Griswold,
Lawrence, and Obergefell,” asserting, “Because any substantive due process decision is ‘demonstrably erroneous,’
we have a duty to ‘correct the error’ established in those precedents.” 597 U. S. ____ (2022) (citations omitted).

2
• Health Plans and PBMs Have Coverage Exclusions or Cost-Sharing
Requirements for at Least 34 Different Contraceptive Products. The
Committee identified 34 contraceptive products for which the majority of
companies reported coverage exclusions or cost-sharing on at least one plan or
formulary.
3
Of these products, 12 have no equivalent product on the market.
Half of the 34 products—six of which have no equivalent product on the
market—are subject to exclusions or cost-sharing requirements by each of the
companies surveyed. The companies reported cost-sharing obligations of up to
$178 per month for certain non-pill contraceptives like the Twirla patch, and
approximately $218 per month for certain birth control pills.
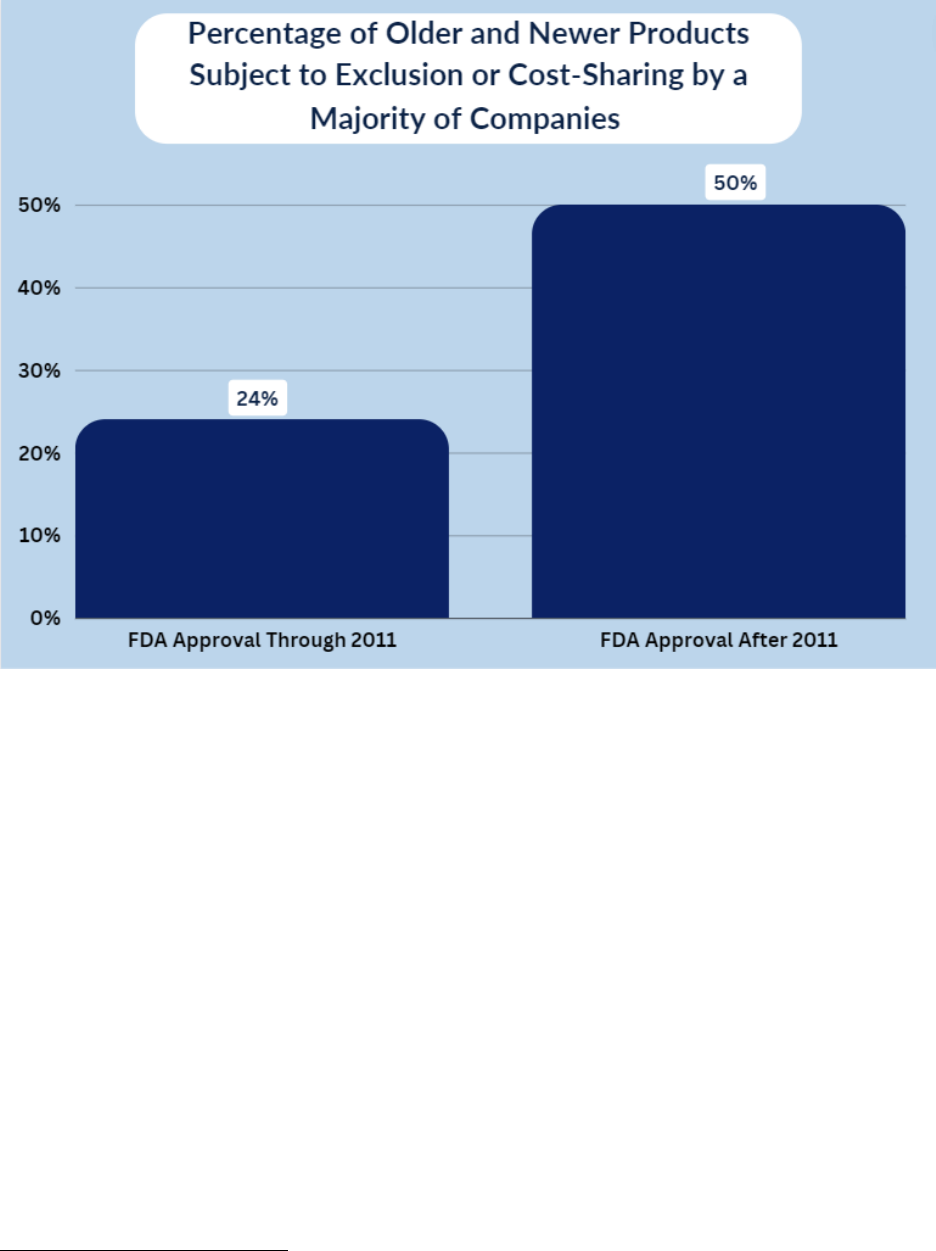
• Insurers and PBMs Disproportionately Impose Cost-Sharing or Coverage
Exclusions for Newer Contraceptive Products. New products may reflect
recent innovations and provide alternatives to traditional contraception. However,
for approximately 50% of the contraceptive products examined by the Committee
that were approved by the FDA after 2011, a majority of the companies imposed
coverage exclusions or required cost-sharing.
• Many Contraceptive Products Used by Patients with Distinct Health Care
Needs or Disproportionately Used by People with Lower Incomes Are
Subject to Cost-Sharing or Exclusions. At least five of the 17 products for
which all companies require cost-sharing or have coverage exclusions provide
particular clinical benefit to patients with distinct health care needs. Four of the
17 products are non-pill products, which are disproportionately used by patients
with less income and non-white patients.
• Health Insurers and PBMs Deny an Average of at Least 40% of Exception
Requests. The majority of companies surveyed reported denying an average of at
least 40% of exception requests from individuals seeking coverage for
contraceptive products from 2015 to 2021. One company denied more than 80%
of requests each year.
• Exceptions Processes Are Inadequate. The Committee obtained documents
suggesting the companies’ processes for considering exceptions to cost-sharing or
coverage exclusions are not sufficiently accessible, expedient, or transparent to be
considered “reasonable” medical management under ACA guidance. One
company reported a delay of up to 15 days to process exception requests, and two
companies request providers document prior medications a patient has
unsuccessfully tried in order for that patient to receive an exception.
3
A formulary is a list of drugs or medical products that are covered by an insurer or a Pharmacy Benefit
Manager.

3
I. BACKGROUND ON CONTRACEPTIVE COVERAGE
On May 26, 2022, following reports that people were being denied access to
contraceptives without cost-sharing, as required by the ACA, Chairwoman Maloney sent letters
to five of the largest health insurers and four of the largest PBMs in the United States, seeking
information on coverage of contraceptive products and related services for individuals enrolled
in private health plans.
4
Collectively, these five health insurance companies cover more than
150 million people in the United States, and the four PBMs manage prescription drug benefits
for more than 260 million members.
5
This staff report presents key findings from the Committee’s review of information
obtained from these companies across approximately 68 formularies and plans.
6
The staff report
also proposes policy recommendations to ensure that patients can access contraceptives without
cost-sharing, consistent with the spirit of the ACA’s contraceptive coverage requirement, and to
address other barriers that restrict access to contraceptives.
A. Contraceptive Coverage in the Private Health Insurance Market
People typically obtain private health insurance coverage through their employer or by
purchasing it directly from an insurer through a federal or state health insurance marketplace.
7
In 2020, approximately 201 million people in the United States had private health insurance
4
Committee on Oversight and Reform, Press Release: Chairwoman Maloney Seeks Answers from Insurers
and Pharmacy Benefit Managers on Birth Control Coverage (May 27, 2022) (online at
oversight.house.gov/news/press-releases/chairwoman-maloney-seeks-answers-from-insurers-and-pharmacy-benefit-
managers-on).
5
CVS Caremark, About Us (online at www.caremark.com/about-us.html) (accessed Aug. 15, 2022);
Express Scripts, About Us (online at www.express-scripts.com/corporate/about) (accessed Aug. 15, 2022); Optum
Rx, Serving 28 Million Americans Nationwide (online at
www.optum.com/content/dam/optum/resources/whitePapers/Rx-about-rx.pdf) (accessed Aug. 15, 2022); Prime
Therapeutics, Company Information (Sept. 24, 2021) (online at www.primetherapeutics.com/news/company-
information/); 20 Things to Know About UnitedHealth Group, Becker’s Payer Issues (Dec. 22, 2021) (online at
www.beckerspayer.com/payer/20-things-to-know-about-unitedhealth-group.html); Anthem Doubles Profit to $1.1B,
Issues Conservative 2022 Guidance, Healthcare Dive (Jan. 26, 2022) (online at
www.healthcaredive.com/news/anthem-profit-doubles-2022-q4-guidance-payer/617743/); CVS Health, Press
Release: Aetna 2022 Medicare Plans Feature More Benefits, Lower Member Costs and Greater Affordability (Oct.
1, 2021) (online at www.cvshealth.com/news-and-insights/press-releases/aetna-2022-medicare-plans-feature-more-
benefits-lower-member-costs); Humana’s Q2 Profit Jumps to $696M on Lower Medical Costs, Healthcare Dive
(July 27, 2022) (online at www.healthcaredive.com/news/humanas-q2-profit-jumps-to-696m/628246/).
6
The companies surveyed provided information about coverage of contraceptive products across a total of
approximately 68 plans and formularies. Companies provided information about a wide range of plans and
recommended formularies. Of the nine companies, seven provided information about one to seven plans or
formularies, one provided information about 13 formularies, and one provided information about 35 plans. The
Committee understands that the companies surveyed may offer additional plans or formularies. While Pharmacy
Benefit Managers may make recommendations regarding formularies to their customers, which include insurers,
employers, and government entities, Pharmacy Benefit Managers’ customers make final coverage determinations for
the formularies they select.
7
United States Census Bureau, Health Insurance Coverage in the United States: 2018 (Nov. 2019) (online
at www.census.gov/content/dam/Census/library/publications/2019/demo/p60-267.pdf).

4
coverage.
8
Insurers and the covered individual each pay a certain percentage of medical
expenses accrued, as set out by a plan developed by the insurer. The amount contributed by the
covered individual is referred to as cost-sharing.
9
Common forms of cost-sharing include
copayments, coinsurance, and deductibles.
10
PBMs serve as intermediaries between health insurers and pharmaceutical companies.
11
As part of their role, PBMs manage prescription drug benefits for insurers by developing and
maintaining lists of covered medications, called formularies.
12
These formularies sort
medications by tier, with “preferred” tiers corresponding with lower out-of-pocket costs to the
patient.
13
The price that an individual is required to pay for a particular medication at the
pharmacy counter is determined by their health plan’s cost-sharing requirements for prescription
medications and how that medication is listed on their PBM formulary. When a product is
excluded from a formulary, it is not covered by the patient’s plan and the patient must pay for the
medication out of pocket.
14
B. The Affordable Care Act’s Contraceptive Coverage Requirements
Under the ACA and related guidance issued by the Department of Health and Human
Services, Department of Labor, and Department of the Treasury (collectively, the Tri-
Departments), private health plans and issuers of health insurance coverage, including PBMs,
must cover the full range of FDA-approved contraceptive methods and services without cost-
sharing.
15
The Tri-Departments are responsible for implementing and enforcing this
requirement.
16
8
Centers for Disease Control and Prevention, National Health Statistics Reports: Demographic Variation
in Health Insurance Coverage: United States, 2020 (Feb. 11, 2022) (online at
www.cdc.gov/nchs/data/nhsr/nhsr169.pdf).
9
Stanford University, How U.S. Health Insurance Works (online at https://vaden.stanford.edu/insurance-
referral-office/health-insurance-overview/how-us-health-insurance-works) (accessed Aug. 15, 2022).
10
Id.
11
The Commonwealth Fund, Pharmacy Benefit Managers and Their Role in Drug Spending (Apr. 22,
2019) (online at www.commonwealthfund.org/publications/explainer/2019/apr/pharmacy-benefit-managers-and-
their-role-drug-spending).
12
Id.
13
Academy of Managed Care Pharmacy, Formulary Management (July 18, 2019) (online at
www.amcp.org/about/managed-care-pharmacy-101/concepts-managed-care-pharmacy/formulary-management).
14
Verywell Health, Why Isn’t This Prescription Drug on My Health Plan’s Drug Formulary? (May 27,
2022) (online at www.verywellhealth.com/why-isnt-my-rx-drug-on-my-health-plan-drug-formulary-1738477).
15
Grandfathered health plans and issuers are excluded from these requirements. Department of Health and
Human Services, Department of Labor, and Department of the Treasury, FAQs About Affordable Care Act
Implementation Part 51, Families First Coronavirus Response Act and Coronavirus Aid, Relief, and Economic
Security Act Implementation (Jan. 10, 2022) (online at www.dol.gov/sites/dolgov/files/EBSA/about-ebsa/our-
activities/resource-center/faqs/aca-part-51.pdf).
16
See Department of Health and Human Services, Department of Labor, and Department of the Treasury,
FAQs About Affordable Care Act Implementation (Part XII) (Feb. 20, 2013) (online at
www.dol.gov/sites/dolgov/files/EBSA/about-ebsa/our-activities/resource-center/faqs/aca-part-xii.pdf); Department
of Health and Human Services, Department of Labor, and Department of the Treasury, FAQs About Affordable Care

5
In 2015, the Tri-Departments issued guidance clarifying that although plans or issuers
may use “reasonable medical management techniques” in administering contraceptive benefits,
each plan or issuer must cover without cost-sharing at least one form of contraception in each of
the 18 categories of FDA-approved contraceptive methods.
17
For patients who need to use a
different contraceptive product, the 2015 guidance stated that plans and issuers must have “an
easily accessible, transparent, and sufficiently expedient exceptions process that is not unduly
burdensome on the individual or a provider.”
18
In July 2022, the Tri-Departments issued guidance further clarifying that plans or issuers
must cover all contraceptive products and related services without cost-sharing “if the
individual’s attending provider recommends a particular service or FDA-approved, cleared, or
granted product,” based on a determination that the contraceptive product is medically
appropriate for the individual. The July 2022 guidance also provided additional clarity on how
plans and issuers should determine whether medical management techniques are “reasonable,”
and whether the exceptions processes they have in place are “easily accessible” and
“transparent.”
19
Act Implementation (Part XXVI) (May 11, 2015) (online at www.cms.gov/cciio/resources/fact-sheets-and-
faqs/downloads/aca_implementation_faqs26.pdf); Department of Health and Human Services, Department of Labor,
and Department of the Treasury, FAQs About Affordable Care Act Implementation Part 31, Mental Health Parity
Implementation, and Women’s Health and Cancer Rights Act Implementation (Apr. 20, 2016) (online at
www.dol.gov/sites/dolgov/files/EBSA/about-ebsa/our-activities/resource-center/faqs/aca-part-31.pdf); Department
of Health and Human Services, Department of Labor, and Department of the Treasury, FAQs About Affordable Care
Act Implementation Part 51, Families First Coronavirus Response Act and Coronavirus Aid, Relief, and Economic
Security Act Implementation (Jan. 10, 2022) (online at www.dol.gov/sites/dolgov/files/EBSA/about-ebsa/our-
activities/resource-center/faqs/aca-part-51.pdf); Department of Health and Human Services, Department of Labor,
and Department of the Treasury, FAQs About Affordable Care Act Implementation Part 54 (July 28, 2022) (online at
www.cms.gov/files/document/faqs-part-54.pdf).
17
Department of Health and Human Services, Department of Labor, and Department of the Treasury, FAQs
About Affordable Care Act Implementation (Part XXVI) (May 11, 2015) (online at
www.cms.gov/cciio/resources/fact-sheets-and-faqs/downloads/aca_implementation_faqs26.pdf). To be FDA-
approved, the registering company has to complete a rigorous pre-market approval process. Typically, higher risk
medical devices have to undergo this approval process. In contrast, lower risk medical devices can be “cleared” to
enter the market if they are substantially equivalent to an existing device, or “granted” approval by the FDA if they
are utilized to treat a rare or unique condition or are low or moderate risk devices without a predicate. Rimsys, FDA
Listed, Cleared, Approved, Granted - What Do These Mean, and What’s the Difference? (Mar. 3, 2022) (online at
www.rimsys.io/blog/fda-listed-cleared-approved-granted).
18
Department of Health and Human Services, Department of Labor, and Department of the Treasury, FAQs
About Affordable Care Act Implementation (Part XXVI) (May 11, 2015) (online at
www.cms.gov/cciio/resources/fact-sheets-and-faqs/downloads/aca_implementation_faqs26.pdf); Department of
Health and Human Services, Department of Labor, and Department of the Treasury, FAQs About Affordable Care
Act Implementation Part 54 (July 28, 2022) (online at www.cms.gov/files/document/faqs-part-54.pdf).
19
Department of Health and Human Services, Department of Labor, and Department of the Treasury, FAQs
About Affordable Care Act Implementation Part 54 (July 28, 2022) (online at www.cms.gov/files/document/faqs-
part-54.pdf). According to the July 2022 guidance, a medical management technique is “unreasonable” if the plan
or issuer requires patients to “fail first” on other contraceptives before approving coverage for an “FDA-approved,
cleared, or granted contraceptive product.” A plan or issuer’s exceptions process is considered “easily accessible” if
plan documentation includes information on how to access and pursue an exceptions process, instructions to request
an exception, and “contact information for a representative of the plan or issuer who can answer questions related to
the exceptions process.” A plan or issuer’s exceptions process is considered “transparent” if information relevant to

6
The contraceptive coverage requirements in the ACA have successfully expanded access
to contraceptive products and services for millions of Americans. Yet public reporting and
information obtained by the Committee indicate that people still experience barriers to accessing
their preferred method of contraception without cost-sharing, and that the exceptions processes
some health insurance companies and PBMs have in place do not appear to meet regulatory
guidelines for complying with the ACA’s coverage requirements.
For example, a recent analysis by the advocacy organization Power to Decide determined
that the majority of plan documents did not mention an exceptions process for contraceptive
products, meaning that patients and providers may not be aware that they can request an
exception after coverage of a contraceptive product is denied.
20
According to Power to Decide,
calls to customer service representatives to inquire about the exceptions process “were met with
ignorance, inadequate information, or misinformation.”
21
The Power to Decide analysis also
found that patients may be required to utilize a plan’s regular exceptions process or prior
authorization process, which the organization found “do not appear to meet the ACA standards
for contraceptive coverage, as they include medical review by the plan, typically set narrow
criteria for an exception, and require information beyond what the ACA requires to make a
determination of medical necessity.”
22
II. INVESTIGATIVE FINDINGS
Information obtained by the Committee shows five of the largest insurers and four of the
largest PBMs routinely require cost-sharing for certain birth control products or exclude them
from coverage altogether. The Committee’s investigation identified 34 different contraceptive
products which the majority of companies exclude from coverage or require cost-sharing on at
least one plan or formulary. The investigation also showed that insurers and PBMs are more
likely to exclude or impose cost-sharing requirements for newer contraceptive products, products
used by people with distinct health care needs, and products disproportionately used by people
with less income.
Although cost-sharing burdens can place financial strain on any person seeking to access
contraceptive products, these cost barriers pose the greatest harm to patients with less income
who may have difficulty affording cost-sharing. One report found that nearly one-quarter of
low-income contraceptive users would prefer to use another method if cost were not a
consideration.
23
the exceptions process “is included and prominently displayed in plan documents” as well as “any other plan
materials that describe the terms of the plan’s or issuer’s coverage of contraceptive items and services.” Id.
20
Power to Decide, When Your Birth Control Isn’t Covered: Health Plan Non-Compliance with the
Federal Contraceptive Coverage Requirement (online at https://powertodecide.org/sites/default/files/2022-
04/ACA%20Contraception%20Exception%20Report.pdf) (accessed Aug. 15, 2022).
21
Id.
22
Id.
23
Megan L. Kavanaugh, Emma Pliskin, and Rubina Hussain, Associations Between Unfulfilled
Contraceptive Preferences Due to Cost and Low-Income Patients’ Access to and Experiences of Contraceptive Care

7
A. Insurers and PBMs Impose Exclusions or Cost-Sharing Requirements for at
Least 34 Different Contraceptive Products
The Committee’s review identified 34 contraceptive products for which at least five of
the nine companies reported imposing patient cost-sharing or coverage exclusions as of June
2022.
24
For half of these products, all of the companies exclude the product or require cost-
sharing on at least one plan or formulary.
25
For 12 of these products, there are no equivalent
products on the market.
26
Although cost-sharing obligations vary by plan and by product, the
companies surveyed reported monthly cost-sharing obligations of up to $178 per month for
certain non-pill contraceptives like the Twirla patch, and approximately $218 per month for
certain birth control pills.
in the United States, 2015-2019, Contraception: X (2022) (online at
www.sciencedirect.com/science/article/pii/S2590151622000053).
24
This includes products for which a company reported cost-sharing requirements or coverage exclusions
on at least one plan or formulary. These 34 products are: Annovera, Balcoltra, Beyaz, Caya, Depo-Provera, Depo-
SubQ Provera 104, Estrostep Fe, Generess Fe, Loestrin 1.5/30-21, Loestrin 1/20-21, Loestrin Fe 1.5/30, Loestrin Fe
1/20, Lo Loestrin Fe, LoSeasonique, Minastrin 24 Fe, Mircette, Natazia, Nextstellis, NuvaRing, Ortho-Novum
7/7/7, Ortho Micronor, Ortho Tri-Cyclen Lo, ParaGard, Phexxi, Plan B One Step, Quartette, Safyral, Seasonique,
Slynd, Taytulla, Twirla, Tyblume, Yasmin 28, Yaz.
25
These 17 products are: Balcoltra, Beyaz, Depo-Provera, Depo-SubQ Provera 104, Generess Fe,
LoSeasonique, Minastrin 24 Fe, Mircette, Nextstellis, Phexxi, Quartette, Safyral, Seasonique, Slynd, Twirla, Yasmin
28, and Yaz. For six of these products, there are no equivalent products on the market: Balcoltra, Depo-SubQ
Provera 104, Nextstellis, Phexxi, Slynd, and Twirla.
Because plans and formularies vary by company, there are certain contraceptive products for which the
Committee did not receive information on formulary coverage and cost-sharing obligations from all nine companies.
In instances where a company did not provide information on formulary coverage and cost-sharing obligations for a
certain product, the Committee did not consider that product to be excluded or to require cost-sharing. However, it
is likely that products the companies excluded from their document productions to the Committee are also not
included without cost-sharing on all plans or formularies.
26
These 12 products are Annovera, Balcoltra, Caya, Depo-SubQ Provera 104, Lo Loestrin Fe, Natazia,
Nextstellis, ParaGard, Phexxi, Plan B One-Step, Slynd, and Twirla. Another one of these 34 products, Tyblume, is a
generic product.

8
B. Insurers and PBMs Often Require Cost-Sharing for Newer Contraceptive
Products
The Committee received information regarding coverage of approximately 20
contraceptive products approved by the FDA after 2011. Ten of these 20 products are excluded
from coverage or subject to cost-sharing requirements by the majority of companies reviewed.
27
These products represent nearly a third of the 34 contraception products the Committee
identified as being subject to cost-sharing or coverage exclusions by a majority of companies.
27
In particular, six of the nine companies have at least one plan or formulary that excludes or requires cost-
sharing for at least ten of the 20 products approved by the FDA since 2011. For seven of these ten products, all nine
companies have at least one plan or formulary that excludes or requires cost-sharing.
9
Many contraceptive products approved after 2011 reflect recent innovations and provide
alternatives to traditional contraceptives. Products approved after 2011 that are subject to cost-
sharing requirements by many insurers and PBMs include:
• The only vaginal ring that lasts for a year instead of a month;
• The only progestin-only birth control pill that does not have to be taken at the
same time every day;
• Two birth control pills that utilize different estrogen formulations than traditional
birth control pills; and
• A patch with lower levels of hormones than other patches.
28
Studies indicate that when patients are faced with cost-sharing requirements and coverage
exclusions for a particular contraceptive product, they are less likely to access that product. A
September 2016 study on the ACA’s zero cost-sharing coverage requirements for birth control
found that patients were more likely to choose long-term contraceptive methods when their cost-
28
The brand names for these products are Annovera, Slynd, Nextstellis, Lo Loestrin Fe, and Twirla.

10
sharing burdens were eliminated.
29
In addition, administrative burdens like lengthy exceptions
processes have been shown to negatively impact patients and reduce patients’ ability to use their
preferred contraceptive product.
30
C. Contraceptive Products for Patients with Distinct Health Care Needs and
Those Used by People with Less Income Are Disproportionately Likely to
Require Cost-Sharing or Be Excluded from Coverage
There are many reasons that a patient may need to use a specific birth control product—
including duration of protection, effectiveness, contraindications, potential side effects or
allergies, and lifestyle.
31
The Tri-Departments’ 2015 guidance states that determining the “medical necessity” of a
specific product “may include considerations such as severity of side effects, differences in
permanence and reversibility of contraceptives, and ability to adhere to the appropriate use of the
item or service.”
32
The Committee’s investigation revealed that at least five of the 17 products for which
health plans and PBMs reported having cost-sharing requirements or formulary exclusions—
none of which have therapeutic equivalents—provide particular clinical benefit to patients with
distinct health care needs. Four of these products are non-pill methods, which are
disproportionately used by non-white patients and patients with less income. For example:
• The majority of companies investigated by the Committee have at least one
formulary or health insurance plan that excludes or requires cost-sharing for
Annovera, a vaginal ring contraceptive product that prevents pregnancy for up to
29
Caroline S. Carlin, Angela R. Fertig, and Bryan E. Dowd, Affordable Care Act’s Mandate Eliminating
Contraceptive Cost Sharing Influenced Choices of Women with Employer Coverage, Health Affairs (Sept. 2016)
(online at https://doi.org/10.1377/hlthaff.2015.1457); see also Vanessa K. Dalton et al., The Impact of Cost Sharing
on Women’s Use of Annual Examinations and Effective Contraception, Original Research Gynecology (July 1,
2018) (online at https://doi.org/10.1016/j.ajog.2018.04.051); Hope C. Norris et al., Utilization Impact of Cost-
Sharing Elimination for Preventative Care Services: A Rapid Review, Medical Care Research and Review (June 22,
2021) (online at https://doi.org/10.1177/10775587211027372).
30
See Mitchell A. Psotka et al., Challenges and Potential Improvements to Patient Access
to Pharmaceuticals, Circulation (Aug. 24, 2020) (online at
www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.119.044976); Lori Frohwirth et al., Access to Preferred
Contraceptive Strategies in Iowa: A Longitudinal Qualitative Study of Effects of Shifts in Policy and Healthcare
Contexts, Journal of Health Care for the Poor and Underserved (Aug. 2022) (online at
https://muse.jhu.edu/article/862431).
31
Department of Health and Human Services, Choose the Right Birth Control (Oct. 11, 2022) (online at
https://health.gov/myhealthfinder/healthy-living/sexual-health/choose-right-birth-control); Rebecca Voelker, Nearly
All Medications Contain Potentially Allergenic Inactive Ingredients, Analysis Shows, JAMA (May 8, 2019) (online
at jamanetwork.com/journals/jama/article-abstract/2733397).
32
Department of Health and Human Services, Department of Labor, and Department of the Treasury, FAQs
About Affordable Care Act Implementation (Part XXVI) (May 11, 2015) (online at
www.cms.gov/cciio/resources/fact-sheets-and-faqs/downloads/aca_implementation_faqs26.pdf).

11
a year, as compared to other vaginal ring products that only last for a month.
33
Annovera may benefit patients who are unable to regularly get to the pharmacy
each month due to work schedules, disability, caregiving responsibilities, a lack of
reliable transportation, or other barriers. Annovera may also benefit patients
residing in medically underserved areas where primary care and other health
services are more difficult to access.
34
• All the companies in the Committee’s investigation have at least one
formulary or health insurance plan that excludes or requires cost-sharing for
Slynd, a progestin-only pill used by patients for whom estrogen is
contraindicated—such as people with certain health conditions, including high
blood pressure, migraines, a family history of breast cancer, or being a current or
former smoker.
35
Many of the contraindications for estrogen are more common
in Black women.
36
Slynd is the only progestin-only pill that does not need to be
taken at the same time every day, which may be particularly beneficial for people
with irregular work schedules.
37
• All nine companies have at least one formulary or health insurance plan that
excludes or requires cost-sharing for Nextstellis, a combination pill that uses
both estrogen and progestin. Nextstellis may be preferable for those who have
contraindications to traditional estrogen or experience side effects from other
forms of estrogen, because the estrogen contained in Nextstellis is different from
other pills.
38
33
Food and Drug Administration, Press Release: FDA Approves New Vaginal Ring for One Year of Birth
Control (Aug. 10, 2018) (online at www.fda.gov/news-events/press-announcements/fda-approves-new-vaginal-ring-
one-year-birth-control).
34
Department of Health and Human Services, What Is Shortage Designation? (Aug. 2022) (online at
https://bhw.hrsa.gov/workforce-shortage-areas/shortage-designation#mups). Research has found that “pharmacy
deserts”—or communities with little or no access to pharmacies—are often disproportionately concentrated in
communities of color and communities with less income. See University of Southern California Schaeffer Center,
“Pharmacy Deserts” Disproportionately Affect Black and Latino Residents in Largest U.S. Cities (May 3, 2021)
(online at https://healthpolicy.usc.edu/article/pharmacy-deserts-disproportionately-affect-black-and-latino-residents-
in-largest-u-s-cities/); National Academies of Sciences, Engineering, Medicine, Exploring the Importance of
Pharmacies to Public Health (Apr. 22, 2020) (online at www.nationalacademies.org/news/2020/04/exploring-the-
importance-of-pharmacies-to-public-health).
35
Slynd, Slynd: Home (online at https://slynd.com/) (accessed Aug. 15, 2022); National Center for
Biotechnology Information, Estrogen (June 28, 2022) (online at www.ncbi.nlm.nih.gov/books/NBK538260/).
Contraceptive products without estrogen are typically also preferred for people who are breastfeeding.
36
More than half of Black women have hypertension, and Black women are more likely than white women
to have breast cancer during their reproductive years due to longstanding health inequities. Clement G. Yediou et
al., Health and Racial Disparity in Breast Cancer, Advances in Experimental Medicine and Biology (Jan. 3, 2020)
(online at www.ncbi.nlm.nih.gov/pmc/articles/PMC6941147/).
37
Slynd, Slynd: Home (online at https://slynd.com/) (accessed Aug. 15, 2022).
38
U.S. FDA Approves NEXTSTELLIS, New Oral Contraceptive, PR Newswire (Apr. 16, 2021) (online at
www.prnewswire.com/news-releases/us-fda-approves-nextstellis-new-oral-contraceptive-301270669.html); Birth
Control Pharmacist, Nextstellis: A New Drug Update (Sept. 20, 2021) (online at
https://birthcontrolpharmacist.com/2021/09/20/nextstellis/).

12
• The majority of companies in the Committee’s investigation have at least one
formulary or health insurance plan that excludes or requires cost-sharing for
Lo Loestrin Fe, a combination pill that contains the lowest dose of estrogen
currently available on the market.
39
Higher doses of estrogen can cause side
effects like breast tenderness, nausea, and headaches.
40
• All the companies surveyed by the Committee have at least one formulary
that excludes or requires cost-sharing for Twirla, a combination hormonal
contraceptive patch with a lower dose of estrogen than other contraceptive
patches.
41
Twirla’s lower dose of estrogen may be beneficial for people who need
a non-pill hormonal contraceptive but experience side effects with a higher
estrogen dose. Twirla also lasts for a week before needing to be replaced, which
could be beneficial for people who cannot take a pill at the same time every day
due to their work schedule or other lifestyle factors.
42
In addition to raising barriers to access specific products, exclusions and cost-sharing
requirements undermine efforts to ensure equitable access to convenient contraception. Research
indicates that Black and Hispanic individuals are less likely to use birth control pills for
contraception than white individuals, and people with incomes below the federal poverty level
use implants at higher rates.
43
There are generally fewer products available in non-pill
contraceptive categories, and the information obtained by the Committee indicates that many
non-pill methods are excluded from coverage or require cost-sharing.
• The majority of companies exclude or require cost-sharing on at least one plan for
half of the ring products about which companies provided information—including
Annovera—and for half of available implantable progestin rod products.
• Twirla, discussed above, is one of only three patch products about which
companies provided information. All companies surveyed exclude or require
cost-sharing for Twirla on at least one formulary.
39
Lo Loestrin, Lo Loestrin: Home (online at www.loloestrin.com/) (accessed Aug. 15, 2022).
40
National Center for Biotechnology Information, Estrogen (June 28, 2022) (online at
www.ncbi.nlm.nih.gov/books/NBK538260).
41
Twirla, A Contraceptive Patch Purposefully Designed for Her (online at www.twirla.com/hcp/)
(accessed Aug. 15, 2022).
42
Twirla, Application Schedule (online at www.twirla.com/hcp/application-schedule) (accessed Aug. 15,
2022); Planned Parenthood, What Are the Benefits of the Birth Control Patch? (online at
www.plannedparenthood.org/learn/birth-control/birth-control-patch/what-are-benefits-birth-control-patch) (accessed
Aug. 15, 2022).
43
Guttmacher Institute, Fact Sheet: Contraceptive Use in the United States (May 2021) (online at
www.guttmacher.org/fact-sheet/contraceptive-method-use-united-states).

13
• All companies surveyed exclude or require cost-sharing on at least one formulary
for Phexxi, the only vaginal pH regulator gel on the market.
44
Phexxi is a non-
hormonal birth-control method that provides an alternative for those for whom the
common spermicide nonoxynol-9 is contraindicated.
45
• For the remaining types of non-pill prescription contraceptive products—IUDs,
diaphragms, and sponges with spermicide—companies reported only one type,
the sponge with spermicide, as generally being covered without exclusions or
cost-sharing.
o One-third of companies indicated that at least one formulary excludes or
requires cost-sharing for three of the four hormonal IUD products about
which companies provided information.
o One-third of companies indicated that at least one formulary excludes or
requires cost-sharing for the diaphragms about which companies provided
information. Because some companies did not provide data on a particular
diaphragm product, this finding may underestimate the extent to which
diaphragm products are subject to exclusion or cost-sharing requirements.
D. Companies Have Inadequate Processes for Patients Requesting Exceptions to
Cost-Sharing or Coverage Exclusions
Although the ACA does not require that health insurers cover all contraceptive products
without cost-sharing, the Tri-Department guidance makes clear that patients should have access
without cost-sharing to products deemed medically appropriate by a health care provider.
Patients seeking to access contraceptive products that are either excluded from coverage or
subject to cost-sharing requirements must submit claims through an exceptions process so that
the company can evaluate whether to waive patient cost-sharing or the coverage exclusion.
46
Although all nine companies described having an exceptions process in place, some companies’
processes have deficiencies that may substantially impede patients’ ability to access the
contraceptive of their choosing.
i. Most Health Insurers and PBMs Deny an Average of at Least 40% of
Contraceptive Exception Requests Each Year
44
One PBM, Prime Therapeutics, indicated that it will begin covering Phexxi without cost-sharing
beginning in January 2023.
45
Phexxi, Phexxi: About (online at www.phexxi.com/aboutphexxi) (accessed Aug. 15, 2022); What
Spermicide Users Should Know, But Often Don’t, National Public Radio (Feb. 6, 2012) (online at
www.npr.org/sections/health-shots/2012/02/06/146343080/what-spermicide-users-should-know-but-often-dont)
(describing that nonoxynol-9 is best suited for people in monogamous relationships and is contraindicated for high-
frequency users or women at high risk for HIV).
46
As described above, the Committee’s investigation identified at least 34 products for which patients
would need to seek an exception on at least one plan or formulary offered by most companies, because of a cost-
sharing requirement or coverage exclusion.

14
The majority of companies reported denying an annual average of at least 40% of
exception requests for contraceptive products from 2015 through 2021.
• One PBM, Prime Therapeutics, reported denying at least 77.5% of exception
requests each year from 2015 to 2021, denying more than 80% of requests in
several years.
• Another PBM, Optum Rx, denied more than 60% of exception requests each year
from 2015 to 2020.
• Cigna reported denying significantly fewer exception requests than the other eight
companies, denying less than 5% of exception requests on average from 2015 to
2021.
• Although some companies reported a decrease in denials of exception requests
during the period reviewed, five out of nine companies reported denying more
than 40% of requests in 2021, the most recent year examined.
47
ii. UnitedHealthcare’s 15-Day Review Period for Requested Exceptions
Tri-Department guidance states that the contraceptive exceptions process that plans and
issuers have in place must be “expedient.” Although most companies reported that they take
approximately 24 to 72 hours to process a contraception exception request, UnitedHealthcare
told the Committee that it “generally provides determinations on exception requests within 15
days or less (depending on state regulations), with an average turnaround time in 2021 of
approximately 13 days.”
48
Although the Tri-Departments have not released specific guidance as to what constitutes
an “expedient” exceptions process, this waiting period of up to 15 days is notably longer than the
eight other companies from which the Committee received information. The other eight
companies reported an average turnaround time of just over 40 hours.
49
No other company
reported taking more than 72 hours on average to process an exception request.
50
47
The companies that denied more than 40% of exception requests in 2021 are Aetna, CVS Caremark,
Humana, Optum Rx, and Prime Therapeutics. The companies with a downward trend in exception request denials
are Express Scripts, UnitedHealthcare, and Optum Rx.
48
Letter from UnitedHealth Group to Chairwoman Carolyn B. Maloney, Committee on Oversight and
Reform (June 16, 2022). UnitedHealthcare is the health insurance company owned by UnitedHealth Group. United
Healthcare, About Us (2022) (online at www.uhc.com/about-us/).
49
The average exception request turnaround time was determined by considering information provided by
the companies for a standard exception request. Where a company reported that requests were processed
immediately, the Committee considered this to be zero hours. Where a single number was provided, the Committee
understood that number to already reflect the average time for exception requests to be processed.
50
Two companies (Cigna and Express Scripts) stated that exceptions are granted upon submission using
Electronic Prior Authorization (ePA) vendors. Four companies (Aetna, CVS Caremark, Humana, and Elevance
Health) reported a determination within 72 hours for a standard exception request, and 24 hours for an expedited

15
A long waiting period for processing contraception exception requests delays a patient’s
ability to begin taking a contraceptive product. This can increase the likelihood a patient
experiences health impacts and unintended pregnancy. It is recommended that patients
switching contraceptive methods proceed immediately to a new method; in some cases, medical
professionals even recommend overlapping both forms of contraception by starting the new
product a few days before stopping the old method.
51
iii. Health Insurers and PBMs Require Documentation that Patients
Have Tried Other Forms of Contraception Before Waiving Out-of-
Pocket Costs
While plans and issuers are permitted to use “reasonable” medical management
techniques for contraceptive coverage, the Tri-Departments have explained that plans and issuers
must “defer to the determination of the attending provider” as to whether a patient needs a
product that is not covered without cost-sharing by their plan or formulary. In July 2022, the
Tri-Departments clarified that “requiring individuals to fail first using numerous other services or
FDA-approved, cleared, or granted contraceptive products” before approving coverage for a
product determined by the patient’s health care provider to be medically appropriate is
considered unreasonable medical management.
52
Information provided to the Committee indicates that as of June 2022, two companies,
Elevance Health (formerly Anthem, Inc.) and Prime Therapeutics, require providers to provide
documentation of prior medications or products the patient has previously failed or any
contraindications for at least some exception requests. Elevance Health specifically requires the
provider to attest the patient has previously tried to utilize a covered drug or has a documented
drug interaction as part of an exception request. A third company, Humana, requires that for
some exception requests providers include information on why the medications included on the
patient’s formulary are inappropriate for the patient.
It is unclear whether any of these companies have changed their medical management
techniques following the July 2022 guidance.
The other six companies in the Committee’s investigation described a process where, at
the point of prescribing a contraceptive product that would typically be excluded or subject to
cost-sharing, the provider is simply required to attest that the selected product is medically
necessary in order to bypass the cost-sharing.
exception request. The remaining two companies (Optum Rx and Prime Therapeutics) reported an average response
time of 36 and 41.3 hours.
51
American Academy of Family Physicians, How to Switch Birth Control Methods (Mar. 1, 2011) (online
at www.aafp.org/pubs/afp/issues/2011/0301/p575.html); American Academy of Family Physicians, Preventing
Gaps When Switching Contraceptives (Mar. 1, 2011) (online at
www.aafp.org/pubs/afp/issues/2011/0301/p567.html).
52
Department of Health and Human Services, Department of Labor, and Department of the Treasury, FAQs
About Affordable Care Act Implementation Part 54 (July 28, 2022) (online at www.cms.gov/files/document/faqs-
part-54.pdf).

16
iv. Patients May Have Difficulty Learning About Contraceptive
Exceptions Processes
Federal guidance calls for plans and issuers to have “an easily accessible, transparent, and
sufficiently expedient exceptions process” for contraceptive coverage.
53
According to the Tri-
Departments’ July 2022 guidance, a plan or issuer’s contraception exceptions process is
considered to be easily accessible if the “plan documentation includes relevant information
regarding the exceptions process under the plan or coverage.” An exceptions process is
transparent if the exceptions process is “included and prominently displayed in plan documents,”
as well as “any other plan materials that describe the terms of the plan’s or issuer’s coverage of
contraceptive items and services (such as a prescription drug formulary).”
54
In response to the Committee’s inquiry, most companies described ways in which they
inform patients about their contraception exceptions process. Several companies stated that
information on the exceptions process is included within membership documents. However, the
Committee’s investigation reveals that some companies put the onus on patients to seek out
information about the exceptions process:
• Elevance Health explained that it makes this information available to patients if
they call a toll-free number on their membership card or ask a pharmacist.
• UnitedHealthcare reported that “members and providers can find information
about the cost-share waiver process online” or by calling the phone number on the
member’s health plan ID card.
55
Requiring patients to seek out information about the contraceptive exceptions process by
calling a hotline, asking a pharmacist, or seeking out information online, appears to be
inconsistent with the July 2022 guidance. It is unclear whether these companies have updated
the manner in which they inform their members of about the contraceptive exceptions process
following the July 2022 guidance.
III. RECOMMENDATIONS
The information obtained by the Committee’s investigation indicates that some health
insurers and PBMs may not be in compliance with the Tri-Department guidance to cover without
cost-sharing all contraceptive products deemed medically necessary by a patient’s attending
53
Department of Health and Human Services, Department of Labor, and Department of the Treasury, FAQs
About Affordable Care Act Implementation (Part XXVI) (May 11, 2015) (online at
www.cms.gov/cciio/resources/fact-sheets-and-faqs/downloads/aca_implementation_faqs26.pdf); Department of
Health and Human Services, Department of Labor, and Department of the Treasury, FAQs About Affordable Care
Act Implementation Part 54 (July 28, 2022) (online at www.cms.gov/files/document/faqs-part-54.pdf).
54
Department of Health and Human Services, Department of Labor, and Department of the Treasury, FAQs
About Affordable Care Act Implementation Part 54 (July 28, 2022) (online at www.cms.gov/files/document/faqs-
part-54.pdf).
55
Letter from UnitedHealth Group to Chairwoman Carolyn B. Maloney, Committee on Oversight and
Reform (June 16, 2022).

17
provider, including by having in place an “easily accessible, transparent, or sufficiently
expedient” exceptions process. The Committee’s investigation also shows that products
approved by the FDA in the past decade are less likely to be covered without cost-sharing or
other out-of-pocket costs, even though these products may provide important alternatives for
people with contraindications to other products. The findings in this staff report are consistent
with public accounts of patients being denied access to contraception at no cost.
To address coverage gaps and better ensure that all people have access to the most
appropriate contraceptive products without out-of-pocket payment obligations, the Tri-
Departments should consider further updating guidance to:
• Clarify requirements regarding appropriate medical management for
coverage of contraceptives. The Tri-Departments could issue guidance
clarifying that all FDA-approved contraceptive products that do not have a
therapeutic equivalent should be covered without cost-sharing as part of every
plan or formulary—allowing health plans and PBMs to use medical management
techniques to prioritize the use of generic pharmaceuticals where possible, while
ensuring that patients have access without cost-sharing to products that do not yet
have a generic version.
56
• Encourage exceptions processes that are automatic at the point of
prescribing. To ensure the exceptions process is “easily accessible, transparent,
and sufficiently expedient,” this process could be automatic at the point of
prescribing, so that a patient’s provider would not have to take any additional
steps to ensure the patient has access to medically appropriate contraceptive
products without cost-sharing. The exceptions process would remain important
even if all contraceptive products without a therapeutic equivalent are covered
without cost-sharing, because some patients will be unable to use the therapeutic
equivalent to a branded product—for example, some patients may be allergic to
the color, flavoring, or preservatives used.
56
The FDA maintains an “Orange Book” that lists therapeutic equivalents for FDA-approved drug
products. This document is available online at www.fda.gov/drugs/development-approval-process-drugs/orange-
book-preface/.
