
University of Kentucky University of Kentucky
UKnowledge UKnowledge
Epidemiology and Environmental Health Faculty
Publications
Epidemiology and Environmental Health
1-11-2019
Patterns and Predictors of Chronic Opioid Use in Older Adults: A Patterns and Predictors of Chronic Opioid Use in Older Adults: A
Retrospective Cohort Study Retrospective Cohort Study
GYeon Oh
University of Kentucky
Erin L. Abner
University of Kentucky
, erin.abner@uky.edu
David W. Fardo
University of Kentucky
, david.fardo@uky.edu
Patricia R. Freeman
University of Kentucky
, [email protected].edu
Daniela C. Moga
University of Kentucky
, daniela.moga@uky.edu
Follow this and additional works at: https://uknowledge.uky.edu/epidemiology_facpub
Part of the Epidemiology Commons, and the Substance Abuse and Addiction Commons
Right click to open a feedback form in a new tab to let us know how this document beneBts you. Right click to open a feedback form in a new tab to let us know how this document beneBts you.
Repository Citation Repository Citation
Oh, GYeon; Abner, Erin L.; Fardo, David W.; Freeman, Patricia R.; and Moga, Daniela C., "Patterns and
Predictors of Chronic Opioid Use in Older Adults: A Retrospective Cohort Study" (2019).
Epidemiology and
Environmental Health Faculty Publications
. 63.
https://uknowledge.uky.edu/epidemiology_facpub/63
This Article is brought to you for free and open access by the Epidemiology and Environmental Health at
UKnowledge. It has been accepted for inclusion in Epidemiology and Environmental Health Faculty Publications by
an authorized administrator of UKnowledge. For more information, please contact UKnowledge@lsv.uky.edu.

Patterns and Predictors of Chronic Opioid Use in Older Adults: A Retrospective Patterns and Predictors of Chronic Opioid Use in Older Adults: A Retrospective
Cohort Study Cohort Study
Digital Object IdentiBer (DOI)
https://doi.org/10.1371/journal.pone.0210341
Notes/Citation Information Notes/Citation Information
Published in
PLOS ONE
, v. 14, no. 1, e0320341, p. 1-14.
© 2019 Oh et al.
This is an open access article distributed under the terms of the Creative Commons Attribution License,
which permits unrestricted use, distribution, and reproduction in any medium, provided the original author
and source are credited.
This article is available at UKnowledge: https://uknowledge.uky.edu/epidemiology_facpub/63

RESEARCH ARTICLE
Patterns and predictors of chronic opioid use
in older adults: A retrospective cohort study
GYeon Oh
ID
1
, Erin L. Abner
1,2,3
, David W. Fardo
2,3
, Patricia R. Freeman
4
, Daniela
C. Moga
1,2,4
*
1 Department of Epidemiology, University of Kentucky, Lexington, Kentucky, United States of America,
2 Sanders-Brown Center on Aging, University of Kentucky, Lexington, Kentucky, United States of America,
3 Department of Biostatistics, University of Kentucky, Lexington, Kentucky, United States of America,
4 Department of Pharmacy Practice and Science, University of Kentucky, Lexington, Kentucky, United States
of America
Abstract
Background
Given the controversy around the effectiveness of opioid treatment for chronic pain and the
lack of detailed guidance for prescribing opioids in older adults, the objectives of this study
were to estimate the trajectories and predictors of opioid use in older adults.
Methods
Data were extracted from the National Alzheimer’s Coordinating Center (2005–2017). Group-
based trajectory modeling was used to identify the patterns of opioid use (any or strong)
among participants age 65+. We used multivariable logistic regression with backward selection
to evaluate demographics and comorbidities as potential predictors of trajectory membership.
Results
Among 13,059 participants, four trajectories were identified for the use of both any opioids
and strong opioids (minimal-users, incident chronic-users, discontinuing-users, and preva-
lent chronic-users). For any opioids, female sex (adjusted odds ratio = 1.23; 95% confidence
interval = 1.03–1.46), black vs. white (1.47; 1.18–1.82), year of education (0.96; 0.94–0.99),
type of residence (independent group vs. private: 1.77; 1.38–2.26, care facility vs. private:
1.89; 1.20–2.97), hypertension (1.44; 1.20–1.72), cardiovascular disease (1.30; 1.09–1.55),
urinary incontinence (1.45; 1.19–1.78), dementia (0.73; 0.57–0.92), number of medications
(1 to 4 vs. none: 0.48; 0.36–0.64, 5 or more vs. none: 0.67; 0.50–0.88), and antidepressant
agent (1.38; 1.14–1.67) were associated with incident chronic-use vs. non-use. For strong
opioids, female sex (1.27; 1.04–1.56), type of residence (independent group vs. private:
1.90; 1.43–2.53, care facility vs. private: 2.37; 1.44–3.90), current smoking (1.68; 1.09–
2.60), hypertension (1.49; 1.21–1.83), urinary incontinence (1.45; 1.14–1.84), dementia
(0.73; 0.55–0.97), number of medications (1 to 4 vs. none: 0.46; 0.32–0.65, 5 or more vs.
none: 0.59; 0.42–0.83), and antidepressant agent (1.55; 1.24–1.93) were associated with
incident chronic-use vs. non-use.
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 1 / 14
a1111111111
a1111111111
a1111111111
a1111111111
a1111111111
OPEN ACCESS
Citation: Oh G, Abner EL, Fardo DW, Freeman PR,
Moga DC (2019) Patterns and predictors of chronic
opioid use in older adults: A retrospective cohort
study. PLoS ONE 14(1): e0210341. https://doi.org/
10.1371/journal.pone.0210341
Editor: Danijela Gnjidic, University of Sydney,
AUSTRALIA
Received: September 21, 2018
Accepted: December 20, 2018
Published: January 11, 2019
Copyright: © 2019 Oh et al. This is an open access
article distributed under the terms of the Creative
Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in
any medium, provided the original author and
source are credited.
Data Availability Statement: "NACC data are
owned by the National Alzheimer’s Coordinating
Center and are available by request from https://
www.alz.washington.edu/. Two types of data files
are now available, each based on the most recent
data freeze at the time of request. • The QUICK-
ACCESS FULL DATA FILE contains the complete
UDS and Neuropathology data sets from the latest
quarterly data archive. It can be provided more
quickly — shortly after submitting this request and
signing NACC’s Data Use Agreement — but may
require more effort on the part of the investigator
afterward in understanding the data elements. •

Conclusion
Given that chronic opioid use was more prevalent in participants who were more vulnerable
(i.e., older age, with multiple comorbidities, and polypharmacy), further studies should eval-
uate the safety and efficacy of using opioids in this population.
Introduction
Over 50% of the elderly population reported pain in the United States (US) in 2011, and about
75% of those reported pain in multiple sites [1]. Although chronic pain is prevalent in older
adults, appropriate treatment is challenging for this population due to the high rate of poly-
pharmacy and potential of adverse events [2]. Older adults with dementia may be especially
vulnerable due to inherent difficulties in assessing and treating pain [3–5]. Long-term (90
days) opioid prescriptions have dramatically increased over the past decade, though the effec-
tiveness of this therapy for chronic pain is yet to be established [6, 7]. The prevalence of long-
term opioid use in US adults increased from 1.8% in 1999–2000 to 5.4% in 2013–2014 [8].
Among these long-term opioid users, 25% were adults age 65 years or older [8]. Opioid-related
negative outcomes, such as addiction, misuse, and overdose deaths, have also risen [9–12].
Long-term opioid use has also been associated with opioid overdose-related hospitalization in
older adults [13].
A recent study in Australia showed that opioid initiation with a transdermal formulation,
higher oral morphine equivalents, older age, history of mental health comorbidities, use of
non-opioid analgesics, and use of benzodiazepines were the predictors of persistent prescrip-
tion opioid in adults 18 years and older [14]. A prospective study with participants in a large
nonprofit health care system in Washington State reported that patients’ expectations of long-
term opioid use was the main predictor of using opioids 30 or more days [15]. Although sev-
eral studies reported the predictors of chronic opioid use in different populations, is the evi-
dence is still limited regarding predictors of long-term opioid use in older adults in the US
population. Older adults are more sensitive to negative outcomes (e.g., cognitive impairment,
falls) from opioids, in part due to age-related decreases in liver and kidney function and poly-
pharmacy [2, 9, 10, 12]. The Centers for Disease Control and Prevention (CDC) recently
issued guidelines aimed at improving the safety and effectiveness of chronic pain treatment
[16, 17]. These guidelines recommend increasing monitoring to minimize the risks of opioids
in older adults, yet lack detailed guidance on opioid prescribing [16, 17]. Identifying the char-
acteristics associated with opioid use in older adults can help identify factors that could
improve risk-benefit assessment and prevent inappropriate use. Therefore, the purpose of this
study was to investigate patterns of longitudinal opioid utilization in older adults using group-
based trajectory models and to identify predictors associated with the trajectories indicating
chronic use.
Methods
Study participants
Study data were drawn from the National Alzheimer’s Coordinating Center’s (NACC) Uni-
form Data Set (UDS), which comprises participants enrolled in longitudinal studies at
National Institute on Aging-funded Alzheimer’s Disease Centers (ADC) throughout the US.
Participants included subjects with cognitive status ranging from normal to dementia that are
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 2 / 14
The CUSTOM FILE is created for the investigator
after he or she has carefully specified the file
criteria, with or without the guidance of NACC’s
research scientists. The custom file is generally
provided less than a week after the criteria are fully
specified. The authors of this study did not enjoy
any special access privileges which would preclude
other researchers from requesting access to these
data. Data are retained beyond each quarter. Other
researchers would be able to request the full data
file used in our study by requesting the September
2017 Uniform Data Set (UDS) data freeze with the
variables listed in our cover letter and also attached
with this submission. Researchers will have to
apply our eligibility criteria; inclusion criteria: (1) 65
years or older at their initial UDS visit, and (2)
medication data recorded at every visit.
Participants with fewer than three visits were
excluded from our study. Additional information
regarding access to these data, including the data
dictionary, can be found at: https://www.alz.
washington.edu/"
Funding: This study was supported in part by grant
R01AG054130 to DCM from the National Institute
on Aging. There was no additional external funding
received for this study. The NACC database is
funded by NIA/NIH Grant U01 AG016976. NACC
data are contributed by the NIA-funded. ADCs: P30
AG019610 (PI Eric Reiman, MD), P30 AG013846
(PI Neil Kowall, MD), P50 AG008702 (PI Scott
Small, MD), P50 AG025688 (PI Allan Levey, MD,
PhD), P50 AG047266 (PI Todd Golde, MD, PhD),
P30 AG010133 (PI Andrew Saykin, PsyD), P50
AG005146 (PI Marilyn Albert, PhD), P50
AG005134 (PI Bradley Hyman, MD, PhD), P50
AG016574 (PI Ronald Petersen, MD, PhD), P50
AG005138 (PI Mary Sano, PhD), P30 AG008051
(PI Thomas Wisniewski, MD), P30 AG013854 (PI
M. Marsel Mesulam, MD), P30 AG008017 (PI
Jeffrey Kaye, MD), P30 AG010161 (PI David
Bennett, MD), P50 AG047366 (PI Victor
Henderson, MD, MS), P30 AG010129 (PI Charles
DeCarli, MD), P50 AG016573 (PI Frank LaFerla,
PhD), P50 AG005131 (PI James Brewer, MD,
PhD), P50 AG023501 (PI Bruce Miller, MD), P30
AG035982 (PI Russell Swerdlow, MD), P30
AG028383 (PI Linda Van Eldik, PhD), P30
AG053760 (PI Henry Paulson, MD, PhD), P30
AG010124 (PI John Trojanowski, MD, PhD), P50
AG005133 (PI Oscar Lopez, MD), P50 AG005142
(PI Helena Chui, MD), P30 AG012300 (PI Roger
Rosenberg, MD), P30 AG049638 (PI Suzanne
Craft, PhD), P50 AG005136 (PI Thomas
Grabowski, MD), P50 AG033514 (PI Sanjay
Asthana, MD, FRCP), P50 AG005681 (PI John
Morris, MD), P50 AG047270 (PI Stephen
Strittmatter, MD, PhD).

recruited through clinician referral, self-referral by patients or family members, active recruit-
ment, and volunteers. Data from subjects and their study partners (co-participants) are col-
lected annually by trained clinicians and other ADC research personnel until they are
deceased or decline to participate. Data collected at initial and annual follow-up visits include
sociodemographic characteristics, family history, medical history, neurological evaluations,
and medication use information [18–20]. For this study, we included participants from 38
ADCs with data available in the September 2017 UDS data freeze, meeting the following inclu-
sion criteria: (1) 65 years or older at their initial UDS visit, and (2) medication data recorded at
every visit. Participants with fewer than three visits were excluded to facilitate assessing trajec-
tory trends with quadratic components; in addition participants with cancer history [21], were
also excluded given that opioid medications are highly prevalent in this population (Fig 1).
ADC study procedures are approved by local Institutional Review Boards (IRBs), and all par-
ticipants provided written informed consent. Research using the NACC database is approved
by the University of Washington IRB. Because the NACC data are de-identified, no additional
IRB approval was necessary for this secondary data analysis.
Opioid use assessment
Medication information was provided by the participant and/or the caregiver/legally autho-
rized representative and was based on each participant’s reported medication use within two
weeks of each study visit. In assessing opioid use, opioid medications used as antitussives were
not considered. Participants were considered to be “any opioid” users if they reported use of
any opioid analgesic medications, and “strong opioid” users were defined among any opioid
users if they reported use of opioid analgesics stronger than or equal to morphine’s potency
[22, 23] (e.g., buprenorphine, fentanyl, hydrocodone, hydromorphone, methadone, morphine,
opium, oxycodone, oxymorphone) (S1 Table).
Participant characteristics
Baseline characteristics of interest were recorded at the participant’s initial UDS visit. Demo-
graphic information included age at enrollment (reference category [ref]: 65–74 years), sex
(ref: male), race (ref: white), years of education, and type of residence (ref: single- or multiple-
family private dwelling). Self-reported medical history information included current smoking,
as well as ever-history of alcohol abuse, and other abused substances; hypertension, diabetes,
urinary incontinence, and cardiovascular conditions. Medication information included num-
ber of medications reported (excluding opioid analgesics); use of nonsteroidal anti-inflamma-
tory medication (NSAID), antidepressant agent, antipsychotic agent (including miscellaneous
antipsychotics, psychotherapeutic combinations, phenothiazine psychotics, thioxanthenes,
and atypical antipsychotics), and anxiolytic, sedative, or hypnotic agent (including barbiturates
and benzodiazepines, and miscellaneous anxiolytics, sedatives, and hypnotics). Reference cate-
gory for all medical history and medication variables was the absence of condition or medica-
tion use. Clinician-determined agitation (ref: no agitation) and cognitive status (ref: no
dementia) were also included in the analysis [see S2 Table for detailed descriptions].
Statistical analysis
Group-based trajectory models (GBTM) [24, 25] were used to identify participants with similar
longitudinal patterns of opioid analgesic use. With this approach, latent trajectories are estimated
by the model, and every individual is assigned a probability of belonging to each trajectory, with
total probability of membership summing up to 1.0; we used maximum probability assignment to
determine group membership. The shapes of each trajectory are defined by polynomial terms
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 3 / 14
Competing interests: The authors have declared
that no competing interests exist.

(cubic, quadratic, or linear). Since the time scale was study time, and participants could have up to
12 visits, follow-up was truncated when more than 95% of participants did not have data available
for a particular visit. As a result, data from visits 11 and 12 were not included in the analysis.
Models considering between 2 and 6 trajectories were fit to the data, and the optimal final
model was determined by the Bayesian Information Criterion (BIC) with the least negative
value [26, 27]. In addition, for judging model adequacy, we used the approach proposed by
Nagin that the average posterior probability of membership in the assigned group is greater
than 0.7 [26, 27].
Once optimal GBTM models were selected, we assessed face validity by tabulating the pro-
portion of total study visits in each trajectory group where opioid analgesic use was reported
(e.g., participants assigned to the higher use groups should have higher proportion of visits
where opioid analgesic use was reported). We then used multivariable logistic regression with
backward selection to identify participant characteristics significantly associated with trajec-
tory group membership. Our preliminary analyses indicated that there were participants who
could be described as chronic users, such that they reported using the drugs at most visits.
Since our primary interest was in identifying risk factors for this group of participants, the out-
come for the logistic models was membership in a chronic user group vs. membership in a
group that did not use opioid analgesics chronically.
Participants with missing values, including “unknown”, were excluded from this analysis
with the exception of type of residence, which had a large number of participants in the
“unknown or other” category (>600). Here, we treated unknown/other as a category. Adjusted
odds ratios (OR
adj
) with 95% confidence intervals (CI) were obtained from the full and
reduced models. To identify the best fitting logistic models, Akaike’s Information Criterion
(AIC) values were compared between full and reduced models. All data analyses were
Fig 1. Sample selection flowchart.
https://doi.org/10.1371/journal.pone.0210341.g001
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 4 / 14

conducted using SAS 9.4, and 0.05 was set as the significance level. PROC TRAJ [24] was used
to estimate GBTM, and PROC LOGISTIC was used to fit the logistic regression models.
Results
A total of 13,059 participants were included in our analyses after applying inclusion and exclu-
sion criteria (Fig 1). The mean (SD) number of follow-up visits was 5.4 (2.2), ranging between
3 and 10 visits. The mean (SD) baseline age was 75.8 (6.9) years. The majority of participants
were female (56.7%), white (83.1%), and resided in private dwellings (89.9%). The most com-
mon comorbidity was hypertension (55.0%), and 55.8% of participants reported taking 5 or
more medications. At the initial visit, there were 498 (3.8%) users of any opioid and 284 (2.2%)
users of strong opioids (Tables 1 and 2).
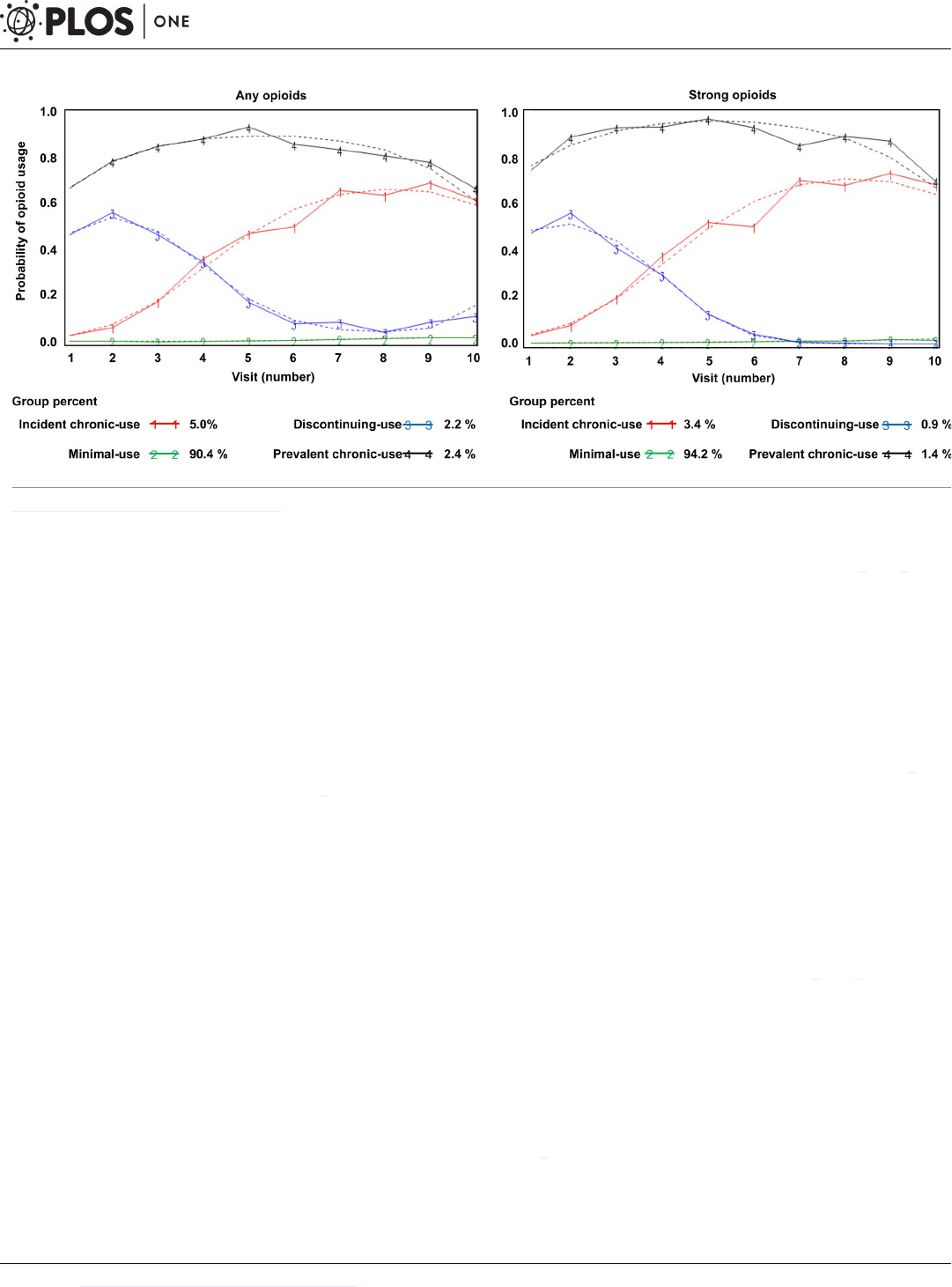
Using GBTM, four trajectories were identified for both any opioid use and strong opioid
use (Fig 2). The optimal number of trajectories was determined based on the BIC in combina-
tion with the requirement that the average posterior probability in all assigned trajectory
groups was at least 0.70 [26, 27]. The shapes of the trajectories for any opioid use were qua-
dratic or cubic, and the parameter estimates of the quadratic or cubic function for each trajec-
tory were all statistically significant (S3 Table). For strong opioid use, the shapes of the
trajectories were all quadratic, and the parameter estimates of the quadratic function for each
trajectory were significant in 3 of the 4 groups. The final optimal models were adequate based
on the criterion of the average posterior probability [26, 27] (S3 Table).
GBTM for any opioids
A majority of participants (90.4%) were assigned to the “minimal-users” group, meaning they
reported no use or low use over time. Participants (5.0%) who did not report opioid use at
their first visit, but initiated use during the study period and continued their use during fol-
low-up were assigned to the “incident chronic-users” group. “Discontinuing-users” were par-
ticipants who used opioids at the first visit but discontinued during follow-up (2.2%).
“Prevalent chronic-users” (2.4%) were participants who reported opioid use at baseline and
consistently during follow-up (Fig 2). The median percentage (IQR) of UDS visits with any
opioid use were 0% (0–0), 33.3% (25.0–40.0), 40.0% (33.3–60.0), and 85.7% (75.0–100) for
minimal-users, incident chronic-users, discontinuing-users, and prevalent chronic-users,
respectively. Participant characteristics for each group are presented in Table 1.
GBTM for strong opioids
Similar trajectories—minimal-users (94.3%), incident chronic-users (3.4%), discontinuing-
users (0.9%), and prevalent chronic-users (1.4%)—were identified for use of strong opioids.
The median percentage (IQR) of UDS visits with strong opioid use were 0% (0–0), 33.3%
(25.0–40.0), 50.0% (33.3–60.0), and 83.3% (70.0–100.0) for minimal-users, incident chronic-
users, discontinuing-users, and prevalent chronic-users, respectively. Participant characteris-
tics for each group are presented in Table 2.
Predictors of prevalent chronic-use trajectory membership
Multivariable logistic regression models were used to identify t predictors of prevalent
chronic-use trajectory membership compared to discontinuing-users or minimal-users for
both, any and strong opioids. All variables listed in Table 1 were considered for inclusion (full
model) (S4 and S5 Tables), and the reduced models are reported in Tables 3 and 4. After back-
ward selection, urinary incontinence was negatively associated with prevalent chronic-use vs.
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 5 / 14
discontinuing-use for both any opioids (OR
adj
= 0.66 [95% CI = 0.45, 0.98]) and strong opioids
(0.45 [0.25, 0.80]).
Several factors emerged as significant predictors of prevalent chronic-use vs. minimal-use
in both models (any opioid and strong opioids): age (any opioid: 1.83 [1.28, 2.61]; strong
Table 1. Baseline characteristics across trajectory groups of any opioid use.
Baseline Characteristics Pattern of Any Opioid Use
Minimal
(N = 11,806)
Discontinuing
(N = 287)
Incident chronic
(N = 657)
Prevalent chronic
(N = 309)
Baseline age
65–74 5,479 (46.4) 131 (45.6) 266 (40.5) 132 (42.7)
75–84 4,976 (42.2) 108 (37.6) 294 (44.8) 121 (39.2)
85+ 1,351 (11.4) 48 (16.7) 97 (14.8) 56 (18.1)
Female 6553 (55.5) 203 (70.7) 423 (64.4) 226 (73.1)
Race
White 9,869 (83.7) 216 (75.5) 508 (77.6) 236 (76.6)
Black 1,453 (12.3) 59 (20.6) 131 (20.0) 67 (21.8)
Other
a
464 (3.9) 11 (3.9) 16 (2.4) 5 (1.6)
Education, mean (SD) 15.3 (3.4) 14.5 (3.6) 14.6 (3.4) 14.5 (3.4)
Type of Residence
Private
b
10,371 (87.9) 247 (86.1) 531 (80.8) 243 (78.6)
Independent group
c
854 (7.2) 28 (9.8) 88 (13.4) 44 (14.2)
Care facility
d
228 (1.9) 5 (1.7) 25 (3.8) 14 (4.5)
Unknown 353 (3.0) 7 (2.4) 13 (2.0) 8 (2.6)
Current smoking 377 (3.2) 18 (6.3) 26 (4.0) 17 (5.5)
Ever alcohol abuse 513 (4.4) 19 (6.6) 31 (4.7) 19 (6.2)
Ever other abused substances 63 (0.5) 4 (1.4) 4 (0.6) 5 (1.6)
Agitation 652 (5.5) 10 (3.5) 31 (4.7) 16 (5.2)
Ever hypertension 6,326 (53.7) 200 (69.7) 432 (65.9) 205 (66.6)
Ever diabetes 1,401 (11.9) 54 (18.8) 104 (15.8) 59 (19.2)
Ever cardiovascular disease 2,953 (25.3) 82 (28.9) 214 (32.8) 103 (33.6)
Ever urinary incontinence 1,742 (14.8) 79 (27.5) 147 (22.4) 62 (20.1)
Dementia diagnosis 2,047 (17.3) 41 (14.3) 103 (15.7) 38 (12.3)
Number of medications
e
0 898 (7.6) 6 (2.1) 76 (11.6) 10 (3.2)
1 to 4 4,489 (38.0) 58 (20.2) 170 (25.9) 61 (19.7)
5 or more 6,419 (54.4) 223 (77.7) 411 (62.6) 238 (77.0)
Antidepressant agent 2,645 (22.4) 109 (38.0) 184 (28.0) 121 (39.2)
Antipsychotic agent 292 (2.5) 4 (1.4) 24 (3.7) 11 (3.6)
Anxiolytic, sedative, or hypnotic agent 1,134 (9.6) 64 (22.3) 83 (12.6) 73 (23.6)
NSAID use 4,022 (34.1) 116 (40.4) 219 (33.3) 143 (46.3)
Any opioid use 121 (1.0) 162 (56.5) 9 (1.4) 206 (66.7)
Strong opioid use 67 (0.6) 90 (31.4) 6 (0.9) 121 (39.2)
(All results presented are N (%) unless otherwise noted). Abbreviations: SD, standard deviation; NSAID, nonsteroidal anti-inflammatory medication. Note
a = American Indian, Alaska Native, Native Hawaiian, Other Pacific Islander, Asian, or Other
b = single-or multiple family private living
c = retirement community, or independent group living
d = assisted living, nursing home, or hospital
e = number of opioids was excluded from the total number of medications; the minimal-use group includes participants who reported no use or low use over time.
https://doi.org/10.1371/journal.pone.0210341.t001
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 6 / 14

opioids:2.10 [1.34, 3.28]), female sex (1.76 [1.35, 2.30]; 1.71[1.22, 2.40]), black vs. white (1.92
[1.41, 2.61]; 1.97 [1.34, 2.91]), independent group living vs. private living (1.74 [1.21, 2.49];
1.68 [1.06, 2.65]), care facility living vs. private living (2.02 [1.07, 3.83]; 3.46 [1.73, 6.94]), 5 or
more medications vs. none (2.52 [1.25, 5.08]; 4.89 [1.53, 15.65]), use of antidepressant agent
Table 2. Baseline characteristics across trajectory groups of strong opioid use.
Baseline Characteristics Pattern of strong opioid use
Minimal
(N = 12,317)
Discontinuing
(N = 116)
Incident chronic
(N = 444)
Prevalent chronic
(N = 182)
Baseline age
65–74 5,699 (46.3) 57 (49.1) 176 (39.6) 76 (41.8)
75–84 5,192 (42.2) 40 (34.5) 197 (44.4) 70 (38.5)
85+ 1,426 (11.6) 19 (16.4) 71 (16.0) 36 (19.8)
Female 6,904 (56.1) 79 (68.1) 289 (65.1) 133 (73.1)
Race
White 10,232 (83.2) 92 (80.0) 362 (81.7) 143 (78.6)
Black 1,584 (12.9) 20 (17.4) 70 (15.8) 36 (19.8)
Other
a
479 (3.9) 3 (2.6) 11 (2.5) 3 (1.7)
Education, mean (SD) 15.2 (3.4) 14.6 (3.7) 14.9 (3.4) 14.6 (3.1)
Type of Residence
Private
b
10,806 (87.7) 99 (85.3) 351 (79.1) 136 (74.7)
Independent group
c
911 (7.4) 12 (10.3) 64 (14.4) 27 (14.8)
Care facility
d
236 (1.9) 4 (3.5) 20 (4.5) 12 (6.6)
Unknown 364 (3.0) 1 (0.9) 9 (2.0) 7 (3.9)
Current smoking 391(3.2) 10 (8.7) 23 (5.2) 14 (7.7)
Ever alcohol abuse 537 (4.4) 13 (11.2) 20 (4.5) 12 (6.6)
Ever other abused substances 65 (0.5) 2 (1.7) 6 (1.4) 3 (1.7)
Agitation 672 (5.5) 4 (3.5) 26 (5.9) 7 (3.9)
Ever hypertension 6,673 (54.3) 84 (72.4) 287 (64.8) 119 (65.4)
Ever diabetes 1,505 (12.3) 23 (19.8) 62 (14.0) 28 (15.4)
Ever cardiovascular disease 3,112 (25.5) 40 (34.8) 139 (31.5) 61 (33.9)
Ever urinary incontinence 1,857 (15.1) 37 (31.9) 101 (22.8) 35 (19.2)
Dementia diagnosis 2,124 (17.2) 16 (13.8) 69 (15.5) 20 (11.0)
Number of medications
e
0 936 (7.6) 1 (0.9) 50 (11.3) 3 (1.7)
1 to 4 4,599 (37.3) 21 (18.1) 119 (26.8) 39 (21.4)
5 or more 6,782 (55.1) 94 (81.0) 275 (61.9) 140 (76.9)
Antidepressant agent 2,795 (22.7) 51 (44.0) 138 (31.1) 75 (41.2)
Antipsychotic agent 307 (2.5) 3 (2.6) 16 (3.6) 5 (2.8)
Anxiolytic, sedative, or hypnotic agent 1,219 (9.9) 29 (25.0) 56 (12.6) 50 (27.5)
NSAID 4,235 (34.4) 42 (36.2) 141 (31.8) 82 (45.1)
Any opioid use 259 (2.1) 81 (69.8) 28 (6.3) 130 (71.4)
Strong opioid use 82 (0.7) 78 (67.2) 3 (0.7) 121 (66.5)
(All results presented are N (%) unless otherwise noted). Abbreviations: SD, standard deviation; NSAID, nonsteroidal anti-inflammatory medication. Note
a = American Indian, Alaska Native, Native Hawaiian, Other Pacific Islander, Asian, or Other
b = single-or multiple family private living
c = retirement community, or independent group living
d = assisted living, nursing home, or hospital
e = number of opioids was excluded from the total number of medications.
https://doi.org/10.1371/journal.pone.0210341.t002
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 7 / 14
(1.89 [1.46, 2.44]; 1.89 [1.36, 2.63]), use of anxiolytic, sedative, or hypnotic agent (2.26 [1.69,
3.02]; 2.51 [1.76, 3.57]), and dementia (0.46 [0.32, 0.68]; 0.39 [0.23, 0.65]) (Tables 3 and 4).
Predictors of incident chronic-use trajectory memberships
Four multivariable logistic regression models were used to identify predictors associated with
incident chronic-users compared to discontinuing-users or minimal-users for both any and
strong opioid groups. Use of anxiolytic, sedative, or hypnotic agent (any opioids: 0.57 [0.39,
0.83]; strong opioids: 0.53 [0.31, 0.90]), 1 to 4 medications vs. none (0.26 [0.11, 0.62]; 0.12
[0.02, 0.95]), and 5 or more medications vs. none (0.17 [0.07, 0.39]; 0.07 [0.01, 0.52]) were sig-
nificant predictors of incident chronic-use vs. discontinuing-use in both models (Tables 3
and 4).
Several factors emerged as significant predictors of incident chronic-use vs. minimal-use in
both models (any opioid and strong opioids): female sex (any opioid: 1.23 [1.03, 1.46]; strong
opioids: 1.27 [1.04, 1.56]), independent group living vs. private living (1.77 [1.38, 2.26]; 1.90
[1.43, 2.53]), care facility living vs. private living (1.89 [1.20, 2.97]; 2.37 [1.44, 3.90])hyperten-
sion (1.44 [1.20, 1.72]; 1.49 [1.21, 1.83]), urinary incontinence (1.45 [1.19, 1.78]; 1.45 [1.14,
1.84]), use of antidepressant agent (1.38 [1.14, 1.67]; 1.55 [1.24, 1.93]), 1 to 4 medications vs.
none (0.48 [0.36, 0.64]; 0.46 [0.32, 0.65]), 5 or more medications vs. none (0.67 [0.50, 0.88];
0.59 [0.42, 0.83]) and dementia (0.73 [0.57, 0.92]; 0.73 [0.55, 0.97]) (Tables 3 and 4).
Discussion
This study investigated the patterns of opioid analgesics (any opioid or strong opioids) use and
identified predictors of inclusion in different use trajectories over 10 years of follow-up in
older adults. The prevalence of any opioid use (3.8%) at enrollment was lower than that
reported in a previous study (6.5%) using National Health and Nutritional Examination Sur-
vey (NHANES) from 1999 to 2014 [8]. In addition, the prevalence of any opioid use in our
study was lower than that reported in other countries. A previous study from Canada has
reported that the prevalence of prescription opioid use was 16.7% in the population aged 65
Fig 2. Estimated group-based trajectories for any opioid and strong opioid use in National Alzheimer’s Coordinating Center (NACC) participants (2005–2017).
https://doi.org/10.1371/journal.pone.0210341.g002
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 8 / 14

+ in 2009 [28]. A recent study of Australians conducted by Lalic et al. has reported that the
prevalence of prescription opioid analgesic use in people without cancer (ages 18–99 years)
was 15.37% in 2016–2017 [29]. Another study has examined that 14.1% of residents (aged 65
+) in the State of Victoria, Australia, filled the prescription of oxycodone in 2013 [30]. This
could be due to the different definition of identifying opioid use (reported medications used
within two weeks of visit vs. prescription opioid use in the past 30 days) and using different
study population. Our study identified four longitudinal trends—minimal-users, incident
chronic-users, discontinuing-users, and prevalent chronic-users—for use of both any and
strong opioids. We found that participants who were older, female, black, residing in indepen-
dent group living or care facilities, or taking antidepressant agents were more likely to be
chronic-users compared to minimal-users in both the “any opioid” and “strong opioid” user
Table 3. Predictors associated with chronic-use (prevalent or incident) vs. discontinuing-use or minimal-use of any opioid in multivariable logistic regression
model (reduced models adjusted for the covariates retained by backward selection).
Prevalent chronic-use Incident chronic-use
vs. discontinuing-use
a
vs. minimal use
b
vs. discontinuing-use
c
vs. minimal-use
d
Baseline age
65–74 - Ref. - -
75–84 - 1.11 (0.85, 1.44) - -
85+ - 1.83 (1.28, 2.61) - -
Female vs. male - 1.76 (1.35, 2.30) 0.72 (0.52, 0.98) 1.23 (1.03, 1.46)
Race
White - Ref. - Ref.
Black - 1.92 (1.41, 2.61) - 1.47 (1.18, 1.82)
Other - 0.44 (0.18, 1.08) - 0.62 (0.37, 1.04)
Education (1-year difference) - 0.95 (0.91, 0.98) - 0.96 (0.94, 0.99)
Type of Residence
Private - Ref. - Ref.
Independent group - 1.74 (1.21, 2.49) - 1.77 (1.38, 2.26)
Care facility - 2.02 (1.07, 3.83) - 1.89 (1.20, 2.97)
Unknown - 0.88 (0.42, 1.81) - 0.66 (0.37, 1.19)
Hypertension - - - 1.44 (1.20, 1.72)
Diabetes - 1.44 (1.05, 1.97) - -
Cardiovascular disease - - - 1.30 (1.09, 1.55)
Urinary incontinence 0.66 (0.45, 0.98) - - 1.45 (1.19, 1.78)
Number of medications
None - Ref. Ref. Ref.
1 to 4 - 1.33 (0.65, 2.71) 0.26 (0.11, 0.62) 0.48 (0.36, 0.64)
5 or more - 2.52 (1.25, 5.08) 0.17 (0.07, 0.39) 0.67 (0.50, 0.88)
Antidepressant agent - 1.89 (1.46, 2.44) - 1.38 (1.14, 1.67)
Anxiolytic, sedative, or hypnotic agent - 2.26 (1.69, 3.02) 0.57 (0.39, 0.83) -
NSAID - 1.36 (1.06, 1.75) - -
Dementia - 0.46 (0.32, 0.68) - 0.73 (0.57, 0.92)
Note: Where the reference category is not specified, the comparison is either yes vs. no or ever vs. never.
a = Number of observations used in the model is 578 (prevalent chronic-user: 298 and discontinuing-user: 280)
b = Number of observations used in the model is 11,458 (prevalent chronic-user: 298 and minimal-users: 11,458)
c = Number of observations used in the model is 920 (incident chronic-user: 640 and discontinuing-user: 280)
d = Number of observations used in the model is 12,098 (incident chronic-user: 640 and minimal-users: 11,458
https://doi.org/10.1371/journal.pone.0210341.t003
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 9 / 14

groups. These results are consistent with previous studies that reported that older adults and
women experience pain more frequently than younger adults and men [13, 31–33], and that
older women have a higher prevalence of long-term opioid use [34]. Also, previous studies
have shown that long-term opioid use is highly prevalent in nursing home residents compared
to people in a community setting [35], and having depression was associated with long-term
opioid use in older adults [13].
We found that taking anxiolytic, sedative, or hypnotic agents (including barbiturates and
benzodiazepines) was significantly associated with prevalent chronic-use in both the any opi-
oid and strong opioid user groups compared to minimal-use. We also observed that the preva-
lence of taking benzodiazepines was higher in prevalent chronic-users (2.9%) than in
minimal-users (0.7%). In a recent study including adult participants of the NHANES, long-
term use of opioids was associated with concurrent benzodiazepine use [8]. Similarly, a study
Table 4. Predictors associated with chronic-use (prevalent or incident) vs. discontinuing-use or non-use of strong opioids in multivariable logistic regression model
(reduced models adjusted for the covariates retained by backward selection).
Prevalent chronic-use Incident chronic-use
vs. discontinuing-use
a
vs. non-users
b
vs. discontinuing-use
c
vs. non-users
d
Baseline age
65–74 - Ref. - -
75–84 - 1.13 (0.80, 1.59) - -
85+ - 2.10 (1.34, 3.28) - -
Female vs. male - 1.71 (1.22, 2.40) - 1.27 (1.04, 1.56)
Race
White - Ref. - -
Black - 1.97 (1.34, 2.91) - -
Other - 0.54 (0.17, 1.73) - -
Type of Residence
Private Ref. Ref. - Ref.
Independent group 2.20 (1.00, 4.85) 1.68 (1.06, 2.65) - 1.90 (1.43, 2.53)
Care facility 3.14 (0.83, 11.91) 3.46 (1.73, 6.94) - 2.37 (1.44, 3.90)
Unknown 6.53 (0.77, 55.73) 1.32 (0.60, 2.87) - 0.67 (0.33, 1.36)
Current smoking - 2.34 (1.31, 4.16) - 1.68 (1.09, 2.60)
Alcohol abuse - - 0.44 (0.20, 1.00) -
Hypertension - - - 1.49 (1.21, 1.83)
Urinary incontinence 0.45 (0.25, 0.80) - - 1.45 (1.14, 1.84)
Dementia - 0.39 (0.23, 0.65) - 0.73 (0.55, 0.97)
Number of medications
None - Ref. Ref. Ref.
1 to 4 - 2.59 (0.79, 8.45) 0.12 (0.02, 0.95) 0.46 (0.32, 0.65)
5 or more - 4.89 (1.53, 15.65) 0.07 (0.01, 0.52) 0.59 (0.42, 0.83)
Antidepressant agent - 1.89 (1.36, 2.63) - 1.55 (1.24, 1.93)
Anxiolytic, sedative, or hypnotic agent - 2.51 (1.76, 3.57) 0.53 (0.31, 0.90) -
NSAID 1.68 (1.01, 2.78) - - -
Note: Where the reference category is not specified, the comparison is either yes vs. no or ever vs. never.
a = Number of observations used in the model is 290 (prevalent chronic-user: 178 and discontinuing-user: 112)
b = Number of observations used in the model is 12,131 (prevalent chronic-user: 178 and minimal-users: 11,953)
c = Number of observations used in the model is 545 (incident chronic-user: 433 and discontinuing-user: 112)
d = Number of observations used in the model is 12,386 (incident chronic-user: 433 and minimal-users: 11,953)
https://doi.org/10.1371/journal.pone.0210341.t004
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 10 / 14

from Australia reported that previous use of benzodiazepines was one of the predictors of per-
sistent opioid use [14]. Considering the overdose risk of co-prescribing benzodiazepine and
opioids [36], the CDC guidelines suggest avoiding the use of opioids and benzodiazepines
together [16, 17]. Therefore, further studies are needed to investigate the effect of using opioids
and benzodiazepines together on opioid-related adverse outcomes in older adults.
We found that patients with dementia were less likely to become chronic users of either any
or strong opioids compared to non-users. This trend might be due to inherent difficulties in
assessing and treating pain in these patients [4, 5], as well as potential concerns about the
added burden of cognitive impairment and risk of other adverse events from opioids. Given
the concern about serious problems (e.g., depression, anxiety, and agitation) that could result
from under-treating pain in older adults [33, 37–40], future studies are required to thoroughly
address the patterns of opioid use in patients with dementia.
Reporting a higher number of medications was positively associated with prevalent
chronic-use of both any opioid and strong opioids; however, with respect to incident chronic-
use, the results showed that participants with higher number of medications were less likely to
be incident chronic-users compared to discontinuing-users or minimal-users. Since ADC par-
ticipants may be more likely to receive medical care than the general population through their
contacts with ADC clinicians, there is a possibility that the participants with polypharmacy
were monitored more closely with regard to newly prescribed opioids. Thus, this result may
not be generalizable to all older adults in the US.
Neither comorbidities nor number of medications significantly predicted prevalent
chronic-use vs. discontinuing use. A recent prospective study concluded that neither baseline
chronic pain risk score nor depression were predictors of long-term opioid use; rather, a
patient’s expectation of long-term opioid use was the strongest predictor [15]. In a recent
study, long-term opioid use was significantly associated with physicians who have high-inten-
sity of prescribing opioids [41]. We also examined group percentages of discontinuing- and
chronic-users among the different ADCs (S6 Table) and observed that some centers had a
higher proportion of discontinuing users than others. Thus, it is possible that clinicians at dif-
ferent ADCs implement varying approaches in the management of pain and the de-escalation
and discontinuation of opioids in participants who use these medications chronically. Future
studies that include other factors (e.g., clinician characteristics or patient’s expectation) are
needed to fully understand how the prevalent chronic opioid-user group is different from the
discontinuing group.
This study has several limitations. First, because opioids were identified by reported medi-
cations used within two weeks of UDS visit, we could not classify participants by continuous
long-term use of opioids. Given the short exposure window, participants could be misclassified
if they used opioids only between visits. However, with up to 10 years of annual assessments,
we believe that we have meaningful information regarding longitudinal use patterns. Addi-
tionally, ADC participants tend to be highly educated, which may limit generalizability [18].
Also, participants who were excluded from the study had a higher rate of comorbidities and a
higher rate of using any opioid/strong opioids at baseline (S7 Table). The selection bias from
this exclusion criterion may result in an underestimate of opioid usage in this cohort. How-
ever, since the mean number of visits for each trajectory group in any opioid users was similar
(minimal user: 5.38; incident chronic user: 5.75; discontinuing-user: 5.13; prevalent chronic
user: 5.06), participants with less of follow up didn’t affected the participants being in their tra-
jectory group. Finally, we did not consider time-varying covariates, which may have resulted
in different associations.
In conclusion, the present study showed that potentially inappropriate opioid use was dis-
proportionately prevalent among vulnerable NACC participants (i.e., older age, with multiple
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 11 / 14

comorbidities and polypharmacy). Further studies are required to thoroughly address the risk
and benefit of using opioids in older adults, and it is essential to provide evidence-based guide-
lines for opioid use in this population.
Supporting information
S1 Table. List of drugs included in “any opioids” and “strong opioids”.
(PDF)
S2 Table. Description of variables used in the study.
(PDF)
S3 Table. Description of estimated trajectories and number of participants in each trajec-
tory.
(PDF)
S4 Table. Factors associated with chronic-use (prevalent or incident) vs. discontinuing-use
and chronic-use (prevalent or incident) vs. non-use of any opioids in multivariable logistic
regression model (full model).
(PDF)
S5 Table. Factors associated with chronic-use (prevalent or incident) vs. discontinuing-use
and chronic-use (prevalent or incident) vs. non-use of strong opioids in multivariable
logistic regression model (full model).
(PDF)
S6 Table. The frequency distribution across trajectory groups of any opioid use among
Alzheimer’s Disease Centers (ADC).
(PDF)
S7 Table. Participant characteristics: Included participants vs. participants excluded for
having fewer than 3 visits.
(PDF)
Acknowledgments
We thank Dr. I-Chen Chen and Dr. Bobby Jones for assistance with GBTM modeling.
Author Contributions
Conceptualization: GYeon Oh, Erin L. Abner, Daniela C. Moga.
Formal analysis: GYeon Oh.
Funding acquisition: Daniela C. Moga.
Methodology: GYeon Oh, Erin L. Abner, David W. Fardo, Patricia R. Freeman, Daniela C.
Moga.
Supervision: Erin L. Abner, David W. Fardo, Patricia R. Freeman, Daniela C. Moga.
Writing – original draft: GYeon Oh.
Writing – review & editing: Erin L. Abner, David W. Fardo, Patricia R. Freeman, Daniela C.
Moga.
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 12 / 14

References
1. Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the
United States: findings from the 2011 National Health and Aging Trends Study. Pain. 2013; 154
(12):2649–57. https://doi.org/10.1016/j.pain.2013.07.029 PMID: 24287107
2. Wilder-Smith OH. Opioid use in the elderly. Eur J Pain. 2005; 9(2):137–40. https://doi.org/10.1016/j.
ejpain.2004.07.011 PMID: 15737802
3. Mantyselka P, Hartikainen S, Louhivuori-Laako K, Sulkava R. Effects of dementia on perceived daily
pain in home-dwelling elderly people: a population-based study. Age Ageing. 2004; 33(5):496–9.
https://doi.org/10.1093/ageing/afh165 PMID: 15271639
4. Scherder E, Herr K, Pickering G, Gibson S, Benedetti F, Lautenbacher S. Pain in dementia. Pain. 2009;
145(3):276–8. https://doi.org/10.1016/j.pain.2009.04.007 PMID: 19409705
5. Bell JS, Laitinen ML, Lavikainen P, Lonnroos E, Uosukainen H, Hartikainen S. Use of strong opioids
among community-dwelling persons with and without Alzheimer’s disease in Finland. Pain. 2011; 152
(3):543–7. https://doi.org/10.1016/j.pain.2010.11.003 PMID: 21247697
6. Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of
long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Path-
ways to Prevention Workshop. Ann Intern Med. 2015; 162(4):276–86. https://doi.org/10.7326/M14-
2559 PMID: 25581257
7. Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, et al. Effect of Opioid vs Nono-
pioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoar-
thritis Pain: The SPACE Randomized Clinical Trial. JAMA. 2018; 319(9):872–82. https://doi.org/10.
1001/jama.2018.0899 PMID: 29509867
8. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf.
2017.
9. Miller M, Sturmer T, Azrael D, Levin R, Solomon DH. Opioid analgesics and the risk of fractures in older
adults with arthritis. J Am Geriatr Soc. 2011; 59(3):430–8. https://doi.org/10.1111/j.1532-5415.2011.
03318.x PMID: 21391934
10. Rolita L, Spegman A, Tang X, Cronstein BN. Greater number of narcotic analgesic prescriptions for
osteoarthritis is associated with falls and fractures in elderly adults. J Am Geriatr Soc. 2013; 61(3):335–
40. https://doi.org/10.1111/jgs.12148 PMID: 23452054
11. Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths—United
States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016; 65(5051):1445–52.
12. Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates.
J Intern Med. 2006; 260(1):76–87. https://doi.org/10.1111/j.1365-2796.2006.01667.x PMID: 16789982
13. Kuo YF, Raji MA, Chen NW, Hasan H, Goodwin JS. Trends in Opioid Prescriptions Among Part D Medi-
care Recipients From 2007 to 2012. Am J Med. 2016; 129(2):221 e21–30.
14. Lalic S, Gisev N, Bell JS, Korhonen MJ, Ilomaki J. Predictors of persistent prescription opioid analgesic
use among people without cancer in Australia. Br J Clin Pharmacol. 2018; 84(6):1267–78. https://doi.
org/10.1111/bcp.13556 PMID: 29451672
15. Thielke SM, Shortreed SM, Saunders K, Turner JA, LeResche L, Von Korff M. A Prospective Study of
Predictors of Long-term Opioid Use Among Patients With Chronic Noncancer Pain. Clin J Pain. 2017;
33(3):198–204. https://doi.org/10.1097/AJP.0000000000000409 PMID: 27428547
16. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United
States, 2016. JAMA. 2016; 315(15):1624–45. https://doi.org/10.1001/jama.2016.1464 PMID:
26977696
17. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United
States, 2016. MMWR Recomm Rep. 2016; 65(1):1–49. https://doi.org/10.15585/mmwr.rr6501e1
PMID: 26987082
18. Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, et al. The Uniform Data Set (UDS):
clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis
Assoc Disord. 2006; 20(4):210–6. https://doi.org/10.1097/01.wad.0000213865.09806.92 PMID:
17132964
19. Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, et al. The National Alzheimer’s Coordi-
nating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007; 21(3):249–
58. https://doi.org/10.1097/WAD.0b013e318142774e PMID: 17804958
20. Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, et al. The National Alzheimer’s
Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord.
2004; 18(4):270–7. PMID: 15592144
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 13 / 14

21. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: A population-based
matched cohort study among individuals with and without a history of cancer. Cancer. 2017; 123
(21):4286–93. https://doi.org/10.1002/cncr.30839 PMID: 28782114
22. Pasero C, McCaffery M. Pain assessment and pharmacologic management. St. Louis, MO: Mosby;
2010.
23. Horgas AL, Snigurska U, Farland MZ, Marsiske M. Analyzing Analgesic Medications in Community-
Dwelling Older Adults. Pain Med. 2018.
24. Jones BL, Nagin DS, Roeder K. A SAS Procedure Based on Mixture Models for Estimating Develop-
mental Trajectories. Sociological Methods & Research. 2001; 29(3):374–93.
25. Jones B, Nagin D. Advances in group-based trajectory modeling and an SAS procedure for estimating
them. Sociological methods and research. 2007; 35(4):542–71.
26. Nagin D. Group-based modeling of development. Cambridge, Mass.: Harvard University Press; 2005.
x, 201 p. p.
27. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol.
2010; 6:109–38. https://doi.org/10.1146/annurev.clinpsy.121208.131413 PMID: 20192788
28. Shield KD, Jones W, Rehm J, Fischer B. Use and nonmedical use of prescription opioid analgesics in
the general population of Canada and correlations with dispensing levels in 2009. Pain Res Manag.
2013; 18(2):69–74. PMID: 23662288
29. Lalic S, Ilomaki J, Bell JS, Korhonen MJ, Gisev N. Prevalence and incidence of prescription opioid anal-
gesic use in Australia. Br J Clin Pharmacol. 2018.
30. Berecki-Gisolf J, Hassani-Mahmooei B, Clapperton A, McClure R. Prescription opioid dispensing and
prescription opioid poisoning: Population data from Victoria, Australia 2006 to 2013. Aust N Z J Public
Health. 2017; 41(1):85–91. https://doi.org/10.1111/1753-6405.12568 PMID: 27624336
31. Hurley RW, Adams MC. Sex, gender, and pain: an overview of a complex field. Anesth Analg. 2008;
107(1):309–17. https://doi.org/10.1213/01.ane.0b013e31816ba437 PMID: 18635502
32. Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001; 17(3):417–
31, v. PMID: 11459713
33. Molton IR, Terrill AL. Overview of persistent pain in older adults. Am Psychol. 2014; 69(2):197–207.
https://doi.org/10.1037/a0035794 PMID: 24547805
34. Campbell CI, Weisner C, Leresche L, Ray GT, Saunders K, Sullivan MD, et al. Age and gender trends
in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010; 100(12):2541–7.
https://doi.org/10.2105/AJPH.2009.180646 PMID: 20724688
35. Hunnicutt JN, Chrysanthopoulou SA, Ulbricht CM, Hume AL, Tjia J, Lapane KL. Prevalence of Long-
Term Opioid Use in Long-Stay Nursing Home Residents. J Am Geriatr Soc. 2017.
36. Jones CM, McAninch JK. Emergency Department Visits and Overdose Deaths From Combined Use of
Opioids and Benzodiazepines. Am J Prev Med. 2015; 49(4):493–501. https://doi.org/10.1016/j.amepre.
2015.03.040 PMID: 26143953
37. Morrison RS, Siu AL. A comparison of pain and its treatment in advanced dementia and cognitively
intact patients with hip fracture. J Pain Symptom Manage. 2000; 19(4):240–8. PMID: 10799790
38. McAuliffe L, Brown D, Fetherstonhaugh D. Pain and dementia: an overview of the literature. Int J Older
People Nurs. 2012; 7(3):219–26. https://doi.org/10.1111/j.1748-3743.2012.00331.x PMID: 22830419
39. Kunik ME, Snow AL, Davila JA, Steele AB, Balasubramanyam V, Doody RS, et al. Causes of aggres-
sive behavior in patients with dementia. J Clin Psychiatry. 2010; 71(9):1145–52. https://doi.org/10.
4088/JCP.08m04703oli PMID: 20361896
40. Shega J, Emanuel L, Vargish L, Levine SK, Bursch H, Herr K, et al. Pain in persons with dementia: com-
plex, common, and challenging. J Pain. 2007; 8(5):373–8. https://doi.org/10.1016/j.jpain.2007.03.003
PMID: 17485039
41. Barnett ML, Olenski AR, Jena AB. Opioid-Prescribing Patterns of Emergency Physicians and Risk of
Long-Term Use. N Engl J Med. 2017; 376(7):663–73. https://doi.org/10.1056/NEJMsa1610524 PMID:
28199807
Long-term opioid use in older adults
PLOS ONE | https://doi.org/10.1371/journal.pone.0210341 January 11, 2019 14 / 14
