
National Research
Action Plan on Long COVID
August 2022

2
National Research Action Plan on Long COVID
Preparation Notice
This National Research Action Plan on Long COVID was prepared by the Department of Health
and Human Services.
Electronic Access
This publication may be downloaded from COVID.gov - Find COVID-19 guidance for your
community
Disclaimer Notice
This publication lists non-Federal resources to provide additional information. The views and
content in those resources have not been formally approved by the Department of Health and
Human Services (HHS). Listing of the resources is not an endorsement by HHS or its
components.
Public Domain Notice
All material appearing in this publication is in the public domain and may be reproduced or
copied without permission from Department of Health and Human Services. Citation of the
source is appreciated.
Suggested Citation
Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022.
National Research Action Plan on Long COVID, 200 Independence Ave SW, Washington, DC
20201.

3
National Research Action Plan on Long COVID
Contents
Executive Summary................................................................................................ 5
Letter from the Secretary of Health and Human Services .................................... 10
Chapter 1: Introduction ....................................................................................... 12
Recognizing Long COVID: A Brief Timeline ................................................................................ 12
The Challenges of Long COVID: Public Health, Economic, and Societal Impacts ...................... 12
A Foundational Challenge: Understanding Long COVID and Associated Conditions ............... 14
The Need for a National Research Action Plan on Long COVID ................................................ 15
The Purpose of the National Research Action Plan on Long COVID ......................................... 16
Guiding Principles of the National Research Action Plan on Long COVID ................................ 17
Development of the National Research Action Plan................................................................. 18
References ................................................................................................................................. 19
Chapter 2: Summary of Partner Input .................................................................. 23
Chapter 3: The Current United States Government Long COVID Research Portfolio
............................................................................................................................. 26
1. Characterizing the Full Clinical Spectrum of Long COVID and Diagnostic Strategies ........... 27
2. Pathophysiology .................................................................................................................... 32
3. Surveillance and Epidemiology ............................................................................................. 34
4. Long COVID and Overall Well-Being ...................................................................................... 41
5. Therapeutics and Other Health Interventions ...................................................................... 46
6. Human Services, Supports, and Interventions ...................................................................... 48
7. Health Services and Health Economics Research ................................................................. 50
References ................................................................................................................................. 54
Chapter 4: Accelerating Research and Innovation in Long COVID ........................ 57
Research Priorities for Long COVID ........................................................................................... 57
Strategic U.S. Government Actions for Long COVID Research ................................................. 60
Implementing the National Research Action Plan on Long COVID ........................................... 65
References ................................................................................................................................. 65
National Research Action Plan on Long COVID......................................................... 1

4
National Research Action Plan on Long COVID
Appendix A: Report Abbreviations ....................................................................... 66
Appendix B: Contributing U.S. Government Departments ................................... 68
Appendix C: Terminology and Definitions ............................................................ 69
Appendix D: Highlights of Current U.S. Government Research Portfolio on Long
COVID
.................................................................................................................. 74
Appendix E: U.S. Government Long COVID Research, Interim Implementation Plan
............................................................................................................................. 83

5
National Research Action Plan on Long COVID
Executive Summary
On April 5, 2022, President Biden issued the Memorandum on Addressing the Long-Term Effects
of COVID-
19 outlining actions needed to support the American people in addressing the longer-
term effects of COVID-19. The President charged the Secretary of Health and Human Services
with coordinating a government-wide response to Long COVID. An element of that response
was for the Department of Health and Human Services, in collaboration with federal partners,
to develop two reports
• Services and Supports for Longer-Term Impacts of COVID-19 Report (Services Report)
• National Research Action Plan on Long COVID (the Plan).
The Services Report outlines federal services and mechanisms of support available to the
American public in addressing the longer-term effects of COVID-19. The Plan provides the first
U.S. government-wide national research agenda focused on advancing prevention, diagnosis,
treatment, and provision of services and supports for individuals and families experiencing Long
COVID.
The end of the COVID-19 public health emergency will not signal the end of the effects of the
pandemic. These lingering effects may impact the health of the nation for years to come.
Recovery from infection with SARS-CoV-2, the virus that causes COVID-19, can vary from person
to person. Most individuals seem to recover quickly and completely. However, some report
symptoms that persist or emerge weeks or even months after the initial phase of the infection
has passed, even when the infection was asymptomatic. These sets of conditions are often
referred to as “Long COVID.” This term was created and has been promulgated by patients and
is used in this Plan. The Plan also recognizes the importance of two technical terms, Post-
COVID-19 conditions, or PCC, broadly equivalent to Long COVID, and Post-acute Sequalae of
SARS-CoV-2 infection, focused on the direct effects of the virus.
Research into the causes and consequences of Long COVID and associated conditions, the
underlying biological mechanisms, the therapies that work, and services and supports needed
for persons experiencing Long COVID are necessary to develop strategies to prevent, treat, and
support those with Long COVID. The Plan was developed with four guiding principles: orienting
research towards improving patient care and outcomes, health equity, accelerating and
expanding existing research, and partner engagement. The Plan ensures an effective,
comprehensive, and equitable research strategy to inform the national response to Long
COVID’s impacts on individuals, families, communities, and all of society, inclusive of age,
gender, race, ethnicity, geographic location, socioeconomic status, insurance coverage status,
pregnancy status, and disability status. The actions in the plan will require federal investment.
Action that can be taken with current funding are underway. Additional funding proposed in
the President’s fiscal year 2023 budget and beyond will enable expansion the implementation
of this Plan.

6
National Research Action Plan on Long COVID
Partner Input
The Plan was developed with engagement from both public and private partners, including
individuals experiencing Long COVID. Meaningful input is critical throughout the research
process, as it can help guide research projects with attention to context and culture, thereby
facilitating translation of research into effective policy and practice. We will continue facilitating
these important conversations with our partners to gain feedback on the Plan, implement the
Plan’s strategies, and iterate the Plan moving forward.
Current U.S. Government Long COVID Research
The Plan builds on ongoing research supported or conducted by the U.S. government and aims
to accelerate and expand it, in addition to calling for enhanced action by the private sector. The
current Long COVID federal research portfolio, ongoing since 2020 and represented in over 100
published articles, demonstrates innovation and early achievements and highlights the
importance of collaboration between the public and private sectors.
The current U.S. government portfolio is described in detail in this report and spans the
following seven areas: characterizing the full clinical spectrum of long COVID and diagnostic
strategies; pathophysiology; surveillance and epidemiology; Long COVID and overall well-
being; therapeutics and other health interventions; human services, supports, and
interventions; and health services and health economics research.
While the U.S. government continues to lead and make advancements in research, much more
must be done to support persons experiencing Long COVID symptoms and to inform care and
support for patients, their families, and caregivers. A national, U.S. government-wide
coordinated, action-oriented approach is urgently needed. By infusing health equity
considerations throughout the Plan, such as having inclusive and diverse participation in
research activities, we are ensuring that our research and resulting policy and programmatic
actions are effective and responsive to the needs of affected populations.
Accelerating Research and Innovation in Long COVID
Fully understanding the implications of Long COVID requires a comprehensive, multi-
disciplinary, effective approach, leveraging the resources of the U.S. research enterprise and
collaborative efforts across the federal government, coupled with strong public and private
partnership. With these core tenets, the Plan includes steps to Accelerate Research and
Innovation in Long COVID.
The Plan provides a detailed call to action across all sectors of research, both public and private,
including academia, to accelerate the delivery of evidence and data. The Plan describes
priorities across the seven research areas, as follows.
1. Characterizing the Full Clinical Spectrum of Long COVID and Diagnostic Strategies.
Build on extensive ongoing research led by National Institutes of Health (NIH), Centers

7
National Research Action Plan on Long COVID
for Disease Control and Prevention (CDC), the Department of Veterans Affairs (VA), and
other agencies, for example the Researching COVID to Enhance Recovery (RECOVER)
Initiative and Innovative Support for Patients with SARS-CoV-2 Infection (INSPIRE), to
convene public and private partners to better align interim definitions of Long COVID for
clinical care, surveillance, and research; lead further studies of the impact of SARS-CoV-2
infection on development and clinical course of new onset chronic disease states (e.g.
diabetes); initiate research that disentangles the broader longer-term effects of the
pandemic on physical and behavioral health (e.g., mental health and substance use
challenges) from those of SARS-CoV-2 infection and re-infection; and further research to
examine Long COVID and other post-infectious illnesses, including dysautonomia, and
myalgic encephalomyelitis and chronic fatigue syndrome (ME/CFS), to identify
commonalities and differences.
2. Pathophysiology. Build on extensive ongoing research, for example the RECOVER
Initiative led by NIH, to achieve a deeper understanding, from the molecular to system
levels, of Long COVID within the broader context of post-infectious chronic conditions
and other diseases that may have infectious origins, including dysautonomia and
ME/CFS.
3. Surveillance and Epidemiology. Build on extensive ongoing research, primarily led by
CDC, such as INSPIRE Study, and the VA, to conduct additional studies of Long COVID
risk factors, health trajectories and outcomes that are inclusive of age, gender, race,
ethnicity, geographic location, socioeconomic status, insurance coverage status,
pregnancy status, and disability status, enabled by new capabilities in data analytics;
and lead studies that can rapidly adapt to examine risk and protective factors as they
emerge (i.e., new variants, vaccinations, repeat infections, and therapies for COVID-19)
on risk of Long COVID.
4. Long COVID and Overall Well-Being. Conduct comprehensive studies of the effects of
Long COVID on educational outcomes in children and youth, with a focus on vulnerable
populations, including racial and ethnic minority groups, those who are economically
disadvantaged, and those in rural communities; and lead research, including qualitative
and mixed methods studies, to understand the impact of Long COVID on health-related
quality of life, behavioral health, employment, disability determinations, education and
development, and the impact on caregivers and family well-being, especially among
disadvantaged groups, expanding upon initial work led by the VA, for example the
COVID-19 Observational Research Collaboratory Long-term Outcomes Study (CORC-
LTO), the Health Resources and Services Administration, for example National Survey of
Children’s Health Longitudinal Cohort Study (NSCH-LC), the Social Security
Administration, and the Department of Labor.
5. Therapeutics and Other Health Interventions. Expand the portfolio of studies, including
the RECOVER Initiative and others, on the effectiveness of therapeutics to prevent and
treat Long COVID to include antivirals, anti-inflammatory, immune modulators, and

8
National Research Action Plan on Long COVID
other existing or new treatments; further studies to subtype Long COVID including
characterization of distinct sub-entities with distinct set of risk factors and outcomes
and evaluation of treatment strategies for these sub-types; and initiate studies of non-
pharmacologic interventions to prevent and treat Long COVID.
6. Human Services, Supports, and Interventions. Lead new work in identifying and
evaluating optimal person-centered models of care for people with various forms of
Long COVID; and lead comprehensive studies of human services and supports
interventions, including disability services and caregiver supports, to ensure individuals
living with Long COVID can fully participate in their communities, building on work by
the Administration for Community Living ACL), the Department of Defense (DOD), and
the SSA.
7. Health Services and Health Economics Research. Lead new studies, including systems
research, to develop, implement, and evaluate models of care delivery that enable
primary care providers to effectively manage Long COVID and associated conditions;
lead new research to address barriers to effective care in underserved communities and
models of care designed to address and eliminate health inequities; conduct rapid and
ongoing synthesis of new evidence to distill existing research into actionable insights
guiding care of people with Long COVID, including cost-effectiveness modeling of
prevention and treatment strategies; and expand work, for example analyses led by the
Office of the Assistant Secretary for Planning and Analysis, in impact of Long COVID on
and economic costs of Long COVID to the health system, human services and supports
programs, and society.
To lead and coordinate the work on Long COVID and in response to the April 5, 2022,
Presidential Memo, Memorandum on Addressing the Long-Term Effects of COVID-
19, HHS
established a U.S. government-wide Long COVID Coordination Council (LCCC), chaired by the
HHS Assistant Secretary for Health. HHS will continue the current approach of the Assistant
Secretary of Health serving as the Long COVID coordinator and formalizing the responsibility
through establishment of a new Office of Long COVID Research and Practice. The Assistant
Secretary for Health, in a role as the Long COVID Coordinator, will implement the National
Research Action Plan on Long COVID and coordinate efforts across the federal government
through the Long COVID Coordination Council. The Coordinator will also establish the
Secretary’s Advisory Committee on Long COVID to continually engage non-governmental
partners and develop and implement a strategic communications plan to share findings from
Long COVID research. This new office, which will need resources and staffing, will ensure
standardization and accountability for the Plan through implementation plans and annual
progress reports.
HHS and federal partner agencies will also work with Congress and government leaders through
continued investment in Long COVID coordination and research, to create and establish new
structures to fulfill the calls to action, such as establishing and maintaining a framework for a
multipronged approach to Long COVID surveillance; effective systems and strategies for rapid

9
National Research Action Plan on Long COVID
implementation of Long COVID clinical trials; considering comprehensive approaches to fund
interdisciplinary research on Long COVID; and engagement of federal agencies to contribute to
a coordinated effort for a national real world evidence approach that would include Long
COVID.
The best prevention of Long COVID and its related impacts remains to avoid infection and re-
infection of SARS-CoV-2 by following basic interventions such as getting vaccinated and
boosted, maintaining social distancing, wearing a mask, and handwashing. At the same time
HHS will work diligently to advance research to prevent, diagnose, treat, and alleviate the
impact of Long COVID for the nation. The Plan affirms the U.S. government’s commitment to
addressing the impacts of Long COVID with federal government resources, in collaboration with
the private sector, and improving our Nation’s health and well-being. The Plan, along with the
accompanying Services Report, represent the federal government’s response to ensure the
acceleration of scientific progress and provide individuals with Long COVID the support and
services they need.

10
National Research Action Plan on Long COVID
Letter from the Secretary of Health and
Human Services
Thank you, President Biden, for the opportunity to coordinate a U.S. government-wide effort to
develop an interagency national research agenda, the National Research Action Plan on Long
COVID.
The COVID-19 pandemic has been burdensome physically, psychologically, and socially. In May
2022, we marked one million American lives lost to COVID-19. The individuals lost represent
loved ones who were taken from their families, friends, and communities.
The pandemic has disproportionately affected racial and ethnic minorities, with Black, Hispanic,
American Indian, and Alaska Native populations experiencing higher rates of COVID-19 and
death compared to White populations. Individuals with intellectual disabilities were also
disproportionately affected and found to be six times more likely to die from COVID-19 than
other members of the population. The challenges associated with COVID-19 exacerbated the
existing health inequities in our country, highlighting social and racial injustices that impede
equitable health outcomes.
As our nation continues to make advancements in addressing this pandemic through enhanced
vaccine availability, research, testing, and treatment, we are also working to address the impact
of Long COVID and associated conditions. For persons with Long COVID, symptoms like fatigue,
shortness of breath, heart palpitations, and joint pain can persist for months, and new chronic
conditions can prevent them from returning to their baseline state of health.
Conducting research in this area is critical to advancing our understanding of Long COVID and
addressing the associated challenges of the pandemic. The U.S. government has been working
on Long COVID research since 2020, with momentum building through 2021 and 2022. The
Memorandum on Addressing the Long-Term Effects of COVID-
19 in April of 2022 highlighted the
Administration’s commitment to harness the full potential of the federal government and to
coordinate with public- and private-sector partners to fully and effectively respond to the
longer-term effects of COVID-19 by developing a National Research Action Plan. The Plan builds
on the research already begun across the federal government to improve our understanding of
the underlying biological causes, epidemiology, and impact of Long COVID; help us understand
the burdens of those affected; foster development of new diagnostics and treatments; inform
decisions related to support services and interventions; develop, implement, and scale
innovative models of care delivery; improve data sharing and transparency among researchers;
and explore the impacts of Long COVID on persons who are underserved by the health care
system and public health infrastructure.

11
National Research Action Plan on Long COVID
Principles guiding the Plan include a commitment to health equity, partner engagement, public-
private collaboration, and prioritization of translational research to inform better health care
delivery and human services.
The challenges posed by Long COVID and associated conditions require that we continue to
work rapidly and share research findings as quickly as possible to drive delivery of effective
health care and supports services. This Plan, along with the companion Services Report,
represent the coordinated, action-oriented, federal government response to ensure the
acceleration of scientific progress and to provide individuals with Long COVID the support and
services they need.
Even as we endeavor to learn more about Long COVID, we already understand that the best
prevention remains to avoid infection and reinfection by following basic prevention
interventions such as getting vaccinated and boosted, maintaining social distancing, wearing a
mask, and handwashing.
I want to thank the staff in the 14 departments and agencies who worked diligently to prepare
this Plan. I also want to thank the Assistant Secretary for Health and the Assistant Secretary for
Planning and Evaluation for their leadership in conceptualizing and driving the production of
this report. Finally, I want to express my sincere gratitude to numerous organizations and
individuals who contributed their time, resources, contacts, and invaluable conversations with
us. This collaboration remains critically important in advancing the science and paving the way
of progress. Those with lived experiences are central to these efforts.
Sincerely,
Xavier Becerra
Secretary of Health and Human Services

12
National Research Action Plan on Long COVID
Chapter 1: Introduction
Recognizing Long COVID: A Brief Timeline
In April 2020, shortly after the beginning of the pandemic, anecdotal reports from patients
started to emerge that previously healthy individuals were experiencing lingering symptoms
and were not fully recovering from an infection with SARS-CoV-2. These patients coalesced
around each other and formed support groups, started to refer to themselves as “Long
Haulers,” and coined the term “Long Covid.” They surveyed their membership and published
the first landmark survey in May 2020 cataloguing the breadth of symptoms experienced by
people with Long COVID. Controlled large-scale reports soon followed in early 2021 which
provided a detailed systematic characterization of the condition. Studies detailing the breadth
of organ dysfunction in people with Long COVID were published in late 2021 and early 2022.
Overall, the early recognition by the patient community, their effort to organize, colloquially
name the condition, and alert the world to study it, galvanized the research community to
pursue research programs in this area, which in a short period of time resulted in substantial
conceptual advances and significant breakthroughs in understanding the condition. The U.S.
government has been conducting research on Long COVID since 2020 and providing care for
individuals with Long COVID within federally supported healthcare systems such as the
Veterans Health Administration, Federally Qualified Community Health Centers, Certified
Community Behavioral Health Clinics, and the Indian Health Service. Milestones in the U.S.
government response include a call for action on Long COVID in the Presidential Health Equity
Task Force, Final Report and Recommendations, released in October 2021, announcement of
the landmark RECOVER study in February 2021, and inclusion of Long COVID in the National
COVID-19 Preparedness Plan in March 2022.
8
6,7
5
4
2,3
1
The Challenges of Long COVID: Public Health,
Economic, and Societal Impacts
In August of 2022, it is now well understood that, amidst the many challenges of the pandemic
are the persistent health effects some individuals face well after initial infection. Recovery from
infection with SARS-CoV-2, the virus that causes COVID-19, can vary from person to person.
Most individuals seem to recover quickly and completely. However, some report symptoms
that persist or emerge weeks or even months after the initial phase of SARS-CoV-2 infection has
passed. These sets of conditions are often referred to as “Long COVID.”
3
Long COVID is a multifaceted disease that can affect nearly every organ system. In total,
about 200 symptoms of Long COVID spanning nearly every organ system have been reported.
Long COVID can manifest as new onset chronic disease, such as heart disease, diabetes, and
1211
5,6,9,10

13
National Research Action Plan on Long COVID
kidney disease; hematologic (blood)
consequences; as well as mental and neurologic
conditions. While estimates of the general risk
of Long COVID vary between 5 and 30%, a recent
large study by CDC suggested that one in five
adult COVID-19 survivors aged 18 to 65 years
and one in four survivors aged 65 years and over
have a health condition related to their previous
COVID-19 illness. Another recent CDC
mathematical modeling study estimated that
millions of U.S. adults have new long-term
symptoms that limit their daily activities after
infection with the virus that causes COVID-19
and that women may be disproportionally impacted. Finally, based on self-reported data from
adults participating in the Census Bureau’s online Household Pulse Survey, more than a third of
those who report having had COVID-19 reported ever having Long COVID symptoms and nearly
one-fifth of those who have had COVID-19 reported currently having Long COVID symptoms.
While estimates of burden of Long COVID vary across studies and settings, the breadth of
symptoms and conditions that are manifestations of Long COVID and the potential scale are
consistent throughout the scientific literature. It is evident that Long COVID is real, that it
already impacts a substantial number of people, and that this number may continue to grow as
new infections occur.
16
15
10
14
13
Reduced health status and complications related to Long COVID are only a small portion of the
consequences of the pandemic. Longer-term disease and disability will have ongoing economic
and societal impacts.
•
For example, in one survey, 44% percent of patients with Long COVID reported not
being able to work at all, compared to their pre-COVID-19 work capacity, and 51% had
reduced their working hours.
17
•
According to one estimate, roughly one million workers may be out of the workforce at
any given time due to Long COVID. This figure equates to approximately $50 billion
dollars annually in lost salary.
17
Health care cost burdens are also expected to increase for treatment of Long COVID as well as
new chronic conditions, such as heart disease, diabetes, and kidney disease, that may be
attributable to COVID-19.
As the United States continues to respond to COVID-19, it will be critical to address how Long
COVID is impacting various communities and populations. While racial disparities in Long COVID
are relatively unexplored, it is well understood that some racial and ethnic minority
communities are disproportionately impacted by COVID-19. Of the 61.4 million COVID-19 cases
recorded in the United States through early 2022, race and ethnicity were recorded for 65%.
Among those cases, Hispanic persons had the largest number of cases, and Black persons
18
“I think it is a mistake to separate Long
COVID and acute COVID. They work
hand in hand. The conversation with
health care providers needs to include
‘If you’ve been diagnosed with COVID-
19, Long COVID symptoms may occur
once you recover.’”
—Representative of a Mental
Health Organization
14
National Research Action Plan on Long COVID
experienced higher COVID-19-related deaths, relative to their population share. When
adjusted for age, the disparities are even more pronounced, with Hispanic persons being 2.4
times, American Indian or Alaskan Native persons being 3.2 times, and Black persons being 2.5
times more likely to be hospitalized with COVID-19 than their White counterparts. Other
analyses also point to non-Hispanic American Indian or Alaska Native persons being at highest
risk for COVID-19 hospitalization, and death.
19
18
18
In addition to higher rates of COVID-19
in certain racial and ethnic minority
groups, the higher burden of co-morbid
chronic disease conditions seen in these
communities can put people at a higher
risk for severe illness and complications
related to COVID-19, including Long
COVID. Finally, outcomes associated
with Long COVID are likely to be
influenced by social determinants of
health such as housing and income—
likely driving even further an uneven
distribution of the toll of Long COVID.
The health care and services
communities will need to understand
and engage with this new disease
process and learn how to use culturally
and linguistically effective services to
provide the highest quality of care
possible.
20
The consequences of Long COVID span
individual health and the health care
system, the economy, and society as a
whole. Pandemics such as influenza and
polio resulted in long-term consequences that persisted for decades. If history is any indicator,
COVID-19 will also have long lasting effects that are only starting to be assessed and
understood.
21
A Foundational Challenge: Understanding Long
COVID and Associated Conditions
The medical, scientific, and public health communities have developed precise terms with
agreed-upon, interim definitions of Long COVID, such as Post-COVID-19 conditions (PCC) and
Post-acute Sequelae of SARS-CoV-2 infection (PASC), to use for communicating about clinical,
research, and public health activities (see text box for an interim public facing definition of Long
Long COVID
Long COVID is broadly defined as signs,
symptoms, and conditions that continue or
develop after initial COVID-19 or SARS-CoV-2
infection. The signs, symptoms, and conditions
• are present four weeks or more after the
initial phase of infection;
• may be multisystemic;
• and may present with a relapsing–remitting
pattern and progression or worsening over
time, with the possibility of severe and life-
threatening events even months or years
after infection.
Long COVID is not one condition. It represents
many potentially overlapping entities, likely with
different biological causes and different sets of
risk factors and outcomes.

15
National Research Action Plan on Long COVID
COVID; see Appendix C for a detailed list of terms in use).This definition was developed by HHS
in collaboration with other departments including with subject matter experts at HHS Office of
the Secretary, CDC, and NIH. The first, Post-COVID-19 conditions, is equivalent to Long COVID, is
broad to optimize sensitivity, and includes both direct and indirect effects of the virus. It is
useful in various clinical setting, assessing the burden to the health care system, and
surveillance. The second is Post-acute Sequalae of SARS-CoV-2 infection, which includes only
the direct effects of the virus. It is used often in clinical contexts and critical to the medical
research community aiming to understand the root causes of Long COVID. In diagnosing Long
COVID, other illnesses need to be excluded. Additional research is underway to sufficiently
separate the various forms and conditions of Long COVID, including from pre-existing ones. The
differences and nuances among the definitions reflect the central challenge of defining,
studying, and addressing the impact of Long COVID. In addition, some scientists and patient
advocate communities point to the importance of conditions associated with Long COVID,
drawing parallels in symptoms and underlying biology to other diseases, such as post-infectious
conditions. The terminology of Long COVID and associated conditions leverages existing
experience and knowledge to recognize commonalities to better treat and care for the many
affected individuals, including the domains of disability and insurance coverage. Although the
definitions will likely change as we learn more, using terms with commonly understood
definitions helps research and public health communities compare findings across studies and
facilitates turning surveillance and research findings into action to improve patient care.
While the Plan uses the term Long COVID throughout, it will be noted if and where specific
research uses a more specific term (i.e., PCC and PASC). Also, it is emphasized that “Long
COVID” is not just one condition, and the use of the term Long COVID in this report should not
be construed as such.
The Need for a National Research Action Plan on Long
COVID
Research into the causes and consequences of Long COVID and associated conditions, the
underlying biological mechanisms, the therapies that work, and services and supports needed
for persons experiencing Long COVID are necessary to develop strategies to prevent, treat, and
support those with Long COVID. As described in detail in Chapter 3, federal agencies are
currently studying the incidence, mechanism, duration, and severity of symptoms following
SARS-CoV-2 infection, as well as risk factors associated with Long COVID. U.S government has a
leading role in Long COVID research through the work it conducts itself, including research in
government-led health systems such as the VA and Indian Health Service; by funding private
research; and by coordinating efforts across public and private entities. Some of U.S.
government efforts include the Researching COVID to Enhance Recovery (RECOVER) Initiative,
led by the NIH. This research initiative brings together patients, caregivers, clinicians,
community leaders, and scientists from across the nation to understand, prevent and treat
Long COVID. The RECOVER Initiative is focusing on enrolling individuals across all ages, genders,
races, ethnicities, and socioeconomic statuses. Enrollment includes pregnant people,
22

16
National Research Action Plan on Long COVID
individuals of reproductive age, individuals with
disabilities, and those from the communities
hardest hit by the pandemic. In addition, CDC is
leading numerous studies, including the
Innovative Support for Patients with SARS-CoV-2
Infections (INSPIRE) Study. It is following nearly
6,000 individuals at select sites across the
country for up to 18 months and conducting
other dedicated research in Tribal and other
hard-hit, high-risk communities. A final
example are studies leveraging the Department
of Veterans Affairs electronic health record data,
which includes over 10 million Veterans, to understand different health impacts of Long COVID
over time as well as evaluating models of care, which has resulted in important early findings
about the clinical manifestations of Long COVID.
24
23
While the U.S. government continues to lead and make advancements in research, much more
must be done to support persons experiencing Long COVID symptoms and to inform care and
support for patients, their families, and caregivers. A national, U.S. government-wide
coordinated, action-oriented approach is urgently needed. The National Research Action Plan
on Long COVID ensures an effective, comprehensive, and equitable research strategy to inform
our national response to Long COVID’s impacts on individuals, communities, and our entire
society.
The Purpose of the National Research Action Plan on
Long COVID
The purpose of the Plan is to advance progress in prevention, diagnosis, and treatment of Long
COVID; and provision of services and supports for individuals, families, and communities
experiencing Long COVID. This includes advancing our understanding of the health and
socioeconomic burdens on individuals with Long COVID, including among subcategories of age,
gender, race and ethnicity, rurality, insurance coverage, economic disadvantage, pregnancy
status, and disability status. The research must identify and provide strategies to inform our
national response to address the pandemic’s impact on these communities and populations.
While the Plan describes several U.S. government Long COVID research initiatives, meeting the
challenge of Long COVID will require deep engagement by private partners including academia,
pharmaceutical industry, professional organizations, and private research organizations,
including the role of organizations that provide the patient data without which research cannot
occur. The Plan also recognizes the centrality of persons affected by Long COVID and their role
as active partners at the core of the research enterprise from initial inception until successful
completion. As such, the U.S. government will lead the effort to convene various stakeholders
“I got COVID-19 and I was embarrassed
that I continued to have symptoms
after the 14-day quarantine. I am 70
years old. I doubted my own symptoms
for so long. I was afraid to even go to
my doctor because I thought they
wouldn’t believe me.”
—Person with Long COVID
17
National Research Action Plan on Long COVID
and partners and facilitate collaborations and partnerships among them to accelerate discovery
and innovation in Long COVID.
Guiding Principles of the National Research Action
Plan on Long COVID
Increasing and enhancing research efforts is imperative to inform and provide equitable
prevention, care, and support to individuals, families, and communities with Long COVID. The
Plan was developed and will be implemented with a coordinated government-wide approach
(see Appendix B for a list of 13 Departments in addition to the Department of Health and
Human Services that participated in the Plan development). The guiding principles of our
coordinated efforts in addressing the impacts of Long COVID are
Orienting the Research Effort to Improve Patient Care and Outcomes. We
will accelerate knowledge synthesis,
dissemination, and implementation of
research findings to promote
coordinated, integrated care models
and expand access to high-quality care
in communities across the country and
drive more effective public health
action.
Health Equity. Issues of access and
utilization of health care are critical
barriers for both COVID-19 and Long
COVID. To achieve our vision, the Plan is guided by health equity principles, building
upon prioritized recommendations made by the Presidential COVID-19 Health Equity
Task Force. These recommendations include, but are not limited to, understanding how
racism, ableism, and discrimination along with provider bias are associated with health
care access, symptom recognition, disease progression, and severity of Long COVID in
communities that are disproportionately disadvantaged and other people who are
underserved, as well as improving data collection, integration, and use so that data can
be disaggregated for these populations who are at higher risk and used to inform
equity-centered response decisions.
25-27
Accelerating and Expanding Current Research. While research on Long COVID is
underway both within and outside of the U.S. government, we must accelerate and
broaden our efforts. The Plan calls for new research partners and data sharing, and new
strategies to extract lessons sooner from existing investments in research. Acceleration
will require rapid short-term trials designed to alleviate the symptoms of those now
living with Long COVID and long-term research on topics such as natural history of
disease and optimal treatments.
“We need to do everything we can now
to learn about Long COVID and how to
help people, because soon we’re going
to have lots of people with this chronic
condition.”
—Person with Chronic
Fatigue Syndrome

18
National Research Action Plan on Long COVID
Partner Engagement. We will continue to work with people living with Long COVID
and other partners on building relationships with communities that are
disproportionately impacted through transparent and ongoing meaningful engagement
to ensure that these efforts address the highest priority needs of persons experiencing
Long COVID.
This Plan is intended for U.S. government agencies and to inform Congress and researchers
both public and private, including academia. This information is also relevant to other groups
such as state policymakers, foundations and other funders of research, health care and service
personnel, public health partners, Long COVID patients and advocacy groups, pharmaceutical
companies, and the general public.
Development of the National Research Action Plan
Workgroup Formation and Report Development
In response to the April 5, 2022 publication of the Memorandum on Addressing the Long-Term
Effects of COVID-
19 , a workgroup was created to draft this Plan. Representatives from
agencies across the U.S. government were brought together. As part of the writing process,
each agency’s workgroup members inventoried current and planned Long COVID research,
shared information about published research articles, and coalesced around future research
topics and priorities.
28
Partner Engagement
An essential part of the Plan, as President Biden highlighted in his Memorandum, is the
engagement of not only U.S. government agencies, but also nongovernmental experts,
organizations, and partners. A coordinated effort within and outside of the U.S. government is
required to adequately address the research challenges we face with Long COVID.
The Plan workgroup gathered relevant information and individual input from a variety of
partners, including persons with Long COVID, through a series of listening sessions. Over a
dozen sessions were held during the drafting on this Plan and the Services Report. Sessions will
continue after publication of the reports.
Participants in these listening sessions included
• Long COVID patients and caregivers, as well as Long COVID advocacy groups
• Patients with disabilities and chronic illness and their caregivers, as well as disability and
chronic illness advocacy groups
• Provider groups and organizations (health care organizations and systems, community
health centers, behavioral health providers, and hospitals)

19
National Research Action Plan on Long COVID
• Researchers, including patient researchers
• Public health partners
• Professional medical organizations and societies
• Mental health advocates
• Children’s advocates
• Organizations representing health and health care for people who are underserved,
such as African Americans or Black persons, Latino persons, Asian and Pacific Islander
persons, American Indian and Alaskan Native persons, and LGBTQIA+ persons.
We also invited participants to share resources and reviewed relevant plans, reports, and
guidelines. These resources highlight the burden of Long COVID and of other post-infectious
conditions, set out strategic goals and research priorities to address these conditions, and
demonstrate skillful approaches to partner engagement.
29-32
Concurrently, we sponsored non-governmental partners to conduct a human-centered research
project, termed Health+, to better understand Long COVIDThis approach works to co-create
solutions with—not for—people impacted by Long COVID. By listening and learning from
patients experiencing Long COVID and associated conditions, caregivers, frontline workers, and
those with lived experience, we will accelerate understanding and breakthroughs together.
Most importantly, the Health+ Long COVID cycle will generate insights with actionable
opportunity areas to improve the quality of care and life.
References
1. Lowenstein F. We Need to Talk About What Coronavirus Recoveries Look Like. The New
York Times. Published April 13, 2020. Accessed June 26, 2022.
https://www.nytimes.com/2020/04/13/opinion/coronavirus-recovery.html
2. Perego E, Callard F, Stras L, Melville-Johannesson B, Pope R, & Alwan NA. Why the
Patient-Made Term 'Long Covid' is needed. Wellcome Open Research. Published
September 24, 2020. Accessed June 26, 2022.
https://wellcomeopenresearch.org/articles/5-224/v1?src=rss
3. Callard F, Perego E. How and why patients made Long Covid. Social Science & Medicine.
2021;268:113426. https://doi.org/10.1016/j.socscimed.2020.113426
4. Patient Led Research Collaborative. Report: What Does COVID-19 Recovery Actually
Look Like? Published 2020. Accessed June 26, 2022.
https://patientresearchcovid19.com/research/report-1/
20
National Research Action Plan on Long COVID
5. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of
COVID-19. Nature. 2021;594(7862):259-264. doi: 10.1038/s41586-021-03553-9
6. Davis HE, Assaf GS, McCorkell L, et al. Characterizing Long COVID in an international
cohort: 7 months of symptoms and their impact. eClinicalMedicine. 2021;38(101019).
https://doi.org/10.1016/j.eclinm.2021.101019
7. Xie Y, Bowe B & Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of
acute infection, demographics and health status. Nat Commun. 2021;12:6571.
https://doi.org/10.1038/s41467-021-26513-3
8. Alwan NA. The road to addressing Long COVID: Reporting, recognizing, and researching
the chronic effects of COVID-19 will help those affected. Science. 2021;373(6554):491-
493. DOI: 10.1126/science.abg7113
9. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-
occurrence, and evolution of long-COVID features: A 6-month retrospective cohort
study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773. doi:
10.1371/journal.pmed.1003773
10. Bull-Otterson L, Baca S, Saydah S, et al. Post–COVID conditions among adult COVID-19
survivors aged 18–64 and ≥65 Years—United States, March 2020–November 2021.
Morbidity and Mortality Weekly Report. 2021;21:713–717.
https://doi.org/10.15585/mmwr.mm7121e1
11. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat
Med. 2022;28:583–590. https://doi.org/10.1038/s41591-022-01689-3
12. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: A cohort study. The
Lancet Diabetes & Endocrinology. 2022;10(5):311-321. https://doi.org/10.1016/S2213-
8587(22)00044-4
13. Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney outcomes in Long COVID. Journal of the American
Society of Nephrology. 2021;32(11):2851-2862.
https://doi.org/10.1681/ASN.2021060734
14. Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with COVID-19: Cohort
study. BMJ. 2022;376:e068993. https://doi.org/10.1136/bmj-2021-068993
15. Tenforde MW, Devine OJ, Reese HE, Silk BJ, Iuliano AD, Threlkel RT, Vu QM, Plumb ID,
Cadwell BL, Rose C, Steele MK, Briggs-Hagen M, Ayoubkhani D, Pawelek P, Nafilyan V,
Saydah SH, Bertolli J. Point Prevalence Estimates of Activity-Limiting Long-Term
Symptoms among U.S. Adults ≥1 Month After Reported SARS-CoV-2 Infection,
November 1, 2021. The Journal of Infectious Diseases. 2022; jiac281.
https://doi.org/10.1093/infdis/jiac281
21
National Research Action Plan on Long COVID
16. Centers for Disease Control and Prevention. (2022). Long COVID: Household Pulse
Survey. Published June 22, 2022. Accessed June 22, 2022.
https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm
17. Cutler DM. The costs of Long COVID. JAMA Health Forum. 2022;3(5):e221809.
doi:10.1001/jamahealthforum.2022.1809
18. Hill L, Artiga S. COVID-19 cases and deaths by race/ethnicity: Current data and changes
over time. kff.org. Published February 22, 2022. Accessed March 15, 2022.
https://www.kff.org/coronavirus-covid-19/issue-brief/covid-19-cases-and-deaths-by-
race-ethnicity-current-data-and-changes-over-time
19. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization,
and death by race/ethnicity. cdc.gov. Updated June 2, 2022. Accessed June 15, 2022.
https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-
discovery/hospitalization-death-by-race-ethnicity.html
20. Berger Z, Altiery De Jesus V, Assoumou SA, Greenhalgh T. Long COVID and health
inequities: The role of primary care. The Milbank Quarterly. 2021;99(2):519–541.
https://doi.org/10.1111/1468-0009.12505
21. Spinney L. Pandemics disable people—the history lesson that policymakers ignore.
Nature. 2022;602(7897):383–385. https://doi.org/10.1038/d41586-022-00414-x
22. National Institutes for Health. RECOVER: Researching COVID to Enhance Recovery.
recovercovid.org. Accessed June 15, 2022. https://recovercovid.org
23. Centers for Disease Control and Prevention. Inspire Covid Study. covidinspire.org.
Accessed June 15, 2022. https://www.covidinspire.org
24. Department of Veterans Affairs, Office of Research and Development. VA research
during the COVID-19 pandemic. research.va.gov. Updated January 27, 2022. Accessed
June 15, 2022. https://www.research.va.gov/covid-19.cfm
25. Centers for Medicare & Medicaid Services. CMS Strategic Plan Pillar: Health Equity.
cms.gov. Accessed June 15, 2022. https://www.cms.gov/sites/default/files/2022-
04/Health%20Equity%20Pillar%20Fact%20Sheet_1.pdf
26. Centers for Medicare & Medicaid Services. CMS framework for health equity 2022–
2032. cms.gov. Published April, 2022. Accessed June 15, 2022.
https://www.cms.gov/files/document/cms-framework-health-equity.pdf
27. Presidential COVID-19 Health Equity Task Force. Final report and recommendations.
minorityhealth.hhs.gov. Published October, 2021. Accessed June 15, 2022.
https://www.minorityhealth.hhs.gov/assets/pdf/HETF_Report_508_102821_9am_508T
eam%20WIP11-compressed.pdf

22
National Research Action Plan on Long COVID
28. The White House. Memorandum on addressing the long-term effects of COVID-19.
whitehouse.gov. Published April 5, 2022. Accessed June 15, 2022.
https://www.whitehouse.gov/briefing-room/presidential-
actions/2022/04/05/memorandum-on-addressing-the-long-term-effects-of-covid-19
29. Albarracin D, Bedford T, Bollyky T, et al. Getting to and sustaining the next normal: A
roadmap for living with COVID. covidroadmap.org. Published March, 2022. Accessed
June 15, 2022.
https://static1.squarespace.com/static/620a6f1b180b6c16f3472588/t/622f9b686fea63
08195cbfb6/1647287147076/Next+Normal+Roadmap+2022.pdf
30. Dimmock M, Joslyn R, Poirier S, Seltzer J, Kanas A. ME/CFS research priorities. NIH
ME/CFS Collaborative Research Centers. Published May 3, 2022. Accessed June 15,
2022. https://cfsformecfs.files.wordpress.com/2022/05/final-public-cac-me_cfs-
research-priorities.pdf
31. MacQueen KM, Harlan SV, Slevin KW, Hannah S, Bass E, Moffett J. Stakeholder
engagement toolkit for HIV prevention trials. FHI 360. Published 2012. Accessed June
15, 2022. https://www.avac.org/sites/default/files/resource-
files/Stakeholder%20EngagementToolkit%20for%20HIV%20Prevention%20Trials.pdf
32. Raj SR, Bourne KM, Stiles LE, et al. Postural orthostatic tachycardia syndrome (POTS):
Priorities for POTS care and research from a 2019 National Institutes of Health Expert
Consensus Meeting - Part 2. Auton Neurosci. 2021;235:102836.
doi:10.1016/j.autneu.2021.102836
23
National Research Action Plan on Long COVID
Chapter 2: Summary of Partner Input
The Long COVID National Research Action
Plan was developed with engagement of
individual public and private interested
persons. The Department of Health and
Human Services’ Office of Intergovernmental
and External Affairs hosted a series of
interactive listening sessions to inform the
development of the Plan. The sessions
included a broad range of participants
including persons with Long COVID,
researchers, health care personnel,
organizations representing health
professionals, public health partners, and
advocacy organizations.
Across listening sessions, some messages
were consistently voiced. These messages
influenced the development of the report
and are reflected in the Plan.
Urgency
An overarching concern among Long COVID
patients, health care personnel, researchers,
and public health partners is the urgent need
for information that can guide health care,
programs, and services. Since the start of the
pandemic, people with Long COVID and
associated conditions have been in need of
symptom relief, health care services, and
financial assistance. Research represents
hope for persons with Long COVID and those
who care for them. To provide relief to those
with Long COVID, legislatures and
governmental and nongovernmental partners must recognize the urgency of this work and act
accordingly.
Partner Engagement throughout the Research Enterprise
An integral way to address research gaps and provide the most insightful and effective results is
to ensure that partners, particularly Long COVID patients are integrated into every stage of the
Summary of Recommendations as
Reflected in the Plan
The Long COVID National Research Action
Plan workgroup considered and
incorporated many individual concerns and
recommendations. The highest priority
recommendations were
• Assuring patient engagement in all
government-sponsored research
throughout the research enterprise
• Inaugurating a central coordinating
office for Long COVID research
• Accelerating therapeutic research
• Engaging researchers and harnessing
prior research on other post-infectious
conditions
• Ensuring transparency in
communications about the research and
findings.
Conversations will continue with a broad
set of partners to gain individual feedback
on the Plan, work to implement the Plan’s
strategies, and iterate the Plan moving
forward.

24
National Research Action Plan on Long COVID
research process. Existing studies and initiatives have engaged patients, but advocacy
organizations indicated that these efforts need to be more robust and occur earlier in the
planning process when research questions and study designs are planned. Long COVID patients’
perspectives are critical to effective, meaningful research.
Research Coordination
Participants in the listening sessions called for cross-governmental coordination to maximize
current and future federal research efforts. The government and private researchers, including
those in academia are conducting a great deal of COVID-19 research. Coordination is required
to ensure that the nation’s research agenda is comprehensive, transparent, and effective.
Standard Definitions
Many inside and outside of government have highlighted an important, yet basic, action of
moving towards a set of standard research, clinical, and surveillance definitions of Long COVID.
Partners indicated a concern over insufficient clarity and consistency in the emerging terms and
definitions, as they might affect the diagnosis of the condition. Others point to the critical
importance of inclusion and the risks with moving too quickly to narrowly define a set of
conditions as yet not sufficiently understood.
Research Gaps and Funding
Participants identified a number of knowledge gaps that should be addressed by research.
Some topics recommended for future research include identification of innovative therapies,
Long COVID in pediatric populations, the impact of reinfection on Long COVID, and the impact
of medical bias on patients with Long COVID. It is particularly important to develop a portfolio
of clinical trials to test as many therapeutics and models of care delivery as possible to enhance
understanding of what works for Long COVID patients. All areas require further research and
funding.
To expedite this research, academic researchers and patient-led research groups requested
faster research funding mechanisms. Due to the highly dynamic nature of Long COVID,
conventional funding mechanisms have limitations to addressing the urgent questions related
to the condition. There was a call for the U.S. government to establish new funding mechanisms
that can help dynamically resource researchers who are working on Long COVID.
Some partners expressed concerns about
current governmental research projects, based
on their personal participation in, or
engagement with them. For example, patient
advocates highlighted the need to integrate
evidence and learnings from prior and current
research on post-infectious conditions, including
dysautonomia and myalgic encephalomyelitis
“A National Research Action Plan must
be explicit and intentional about
including, recruiting, and engaging a
diverse research cohort.”
—Long COVID Researcher

25
National Research Action Plan on Long COVID
and chronic fatigue syndrome (ME/CFS), into ongoing Long COVID research. Some also
expressed concerns with studies that require a positive SARS-CoV-2 test result to be eligible for
enrollment, which excludes persons who were not tested, had false negative results, or those
who were asymptomatic during the initial infection. The importance of relevant control groups
in studies of Long COVID was emphasized. One of the lessons learned from the Centers for
Disease Control and Prevention’s (CDC) longstanding ME/CFS program, echoed by some
individual partners, is that ME/CFS-like illnesses are often unrecognized or overlooked. There is
a need to distinguish the ME/CFS-like (medically unexplained) subgroup of Long COVID from
subgroups with COVID-19-related well-established chronic conditions (such as heart disease,
diabetes, and renal disease) and prioritize research on Long COVID ME/CFS-like illnesses. There
are parallel observations for dysautonomia.
26
National Research Action Plan on Long COVID
Chapter 3: The Current United States
Government Long COVID Research
Portfolio
The National Research Action Plan on Long COVID will build on ongoing efforts across the
federal government. The current federal Long COVID research portfolio demonstrates
innovation and early achievements, as evidenced by a growing body of published studies
funded or conducted by the U.S. government. The portfolio highlights the importance of a
collaboration between the public and private sectors in advancing progress in prevention,
diagnosis, treatment and provision of health care, public health, and human services for
individuals experiencing Long COVID. Ensuring broad representation in research activities of
underrepresented groups remains a challenge, and attention and innovation are required to
specifically address these challenges. The description of the federal research portfolio in this
chapter is intended to be a summary with highlights, and it is not an exhaustive inventory of all
U.S. government supported Long COVID research.
Organization of the Research Portfolio
The federal Long COVID research portfolio described in this chapter is organized into the
following topic areas with subtopics:
1. Characterizing the Full Clinical Spectrum of Long COVID and Diagnostic Strategies
2. Pathophysiology
3. Surveillance and Epidemiology
4. Long COVID and Overall Well-Being
5. Therapeutics and Other Health Interventions
6. Human Services, Supports, and Interventions
7. Health Services and Health Economics Research.
Within each topic area there is a brief synopsis of research, with descriptions of a small
number of the principal research initiatives. Studies may appear in more than one topic
area; for example, a survey of patients with Long COVID can provide information about
impacts of illness as well as access to health care.
Text boxes in each area highlight the core research questions, health equity considerations,
and anticipated impacts of research. Appendix D, the U.S. government Long COVID
Research Portfolio, complements this Chapter by providing an alphabetical list of studies
with basic information and includes more studies than those mentioned here.
27
National Research Action Plan on Long COVID
1. Characterizing the
Full Clinical Spectrum
of Long COVID and
Diagnostic Strategies
It is a public health priority to identify,
understand, and effectively diagnose
conditions across the full clinical spectrum
of Long COVID.
∗
These are necessary first
steps toward the development of optimal
prevention and treatment strategies.
Important research topics include clinical
manifestations of Long COVID and
associated conditions, how these may vary
across the lifespan and across different
demographic groups, how they may
change over time, and how they may be
affected by viral variants, reinfections, and
various interventions such as vaccines.
The observed heterogeneity of Long
COVID thus far strongly suggests that,
rather than being a singular condition,
Long COVID is likely multiple clinical
conditions. Data being collected from
longitudinal studies on Long COVID
include a wide range of data from
laboratory testing, imaging, functional
studies, genomics, mobile health-
wearable devices, participant surveys,
electronic health records, demographic
data, information on social determinants
of health, and other data sources. In
addition, the breadth and complexity of
data being collected in current studies will
necessitate advanced analytics, such as
machine learning, artificial intelligence,
∗
Much of the research described in this section is specific to Post-Acute Sequelae of SARS-CoV-2 Infection (PASC),
but the term “Long COVID” used throughout the report includes PASC.
Core Research Questions
• What is the clinical spectrum of recovery from
acute SARS-CoV-2 infection and how does it
change over time?
• What are the clinical manifestations of Long
COVID? Are there distinct subtypes
(phenotypes) of Long COVID?
• What is the natural history of Long COVID?
How do the clinical manifestations of Long
COVID vary across the lifespan and different
demographic groups? How do they change
over time? How are they affected by various
interventions, such as vaccines?
• Are there distinct phenotypes among those
patients who do not fully recover or who
develop new onset symptoms?
• What are the biologic causes of Long COVID
and how can they be reversed?
• How does SARS-CoV-2 infection initiate or
promote the pathogenesis of conditions or
findings that evolve over time to cause organ
dysfunction or increase the risk of developing
other disorders?
• What are the clinical criteria for diagnosing
Long COVID?
• What diagnostic tests and assays can be used
to accurately and reliably diagnose Long
COVID?
28
National Research Action Plan on Long COVID
natural language processing, or deep learning to extract
meaning and create the needed specialized tools for
prediction, detection, prognosis, diagnosis, and
monitoring treatment response for Long COVID.
Clinical Spectrum of Long COVID
Multiple U.S. government studies are currently
underway investigating important factors of the clinical
spectrum of Long COVID including understanding
different presentations and symptom clusters;
identifying factors that may modify Long COVID such as
the impact of variants and severity of acute illness;
determining the risks for development of other medical
conditions (such as diabetes or cognitive impairment)
after a SARS-CoV-2 infection; and understanding how
Long COVID may impact pre-existing health conditions.
Many of these U.S. government studies are recruiting
diverse adult and pediatric populations and focusing on
specific populations, including Veterans, pregnant
people and their infants, those with pre-existing health
conditions, and persons from medically underserved
populations.
Federal agencies and departments have formed
strategic partnerships that leverage individual missions,
resources, and capabilities to launch collaborative
research efforts and strengthen areas of synergy. For
example, Centers for Disease Control and Prevention
(CDC) and the National Institutes of Health (NIH) are
working together on refining definitions; NIH and the
Patient-Centered Outcomes Research Institute (PCORI)
have worked together on patient-reported outcome
measures; and CDC, the Centers for Medicare &
Medicaid Services (CMS), the Food and Drug
Administration (FDA), and the Department of Health
and Human Services (HHS) are engaged in governance
of the Researching COVID to Enhance Recovery
(RECOVER) Initiative.
Federal departments and agencies recognize that as the
science of Long COVID evolves, discoveries will emerge
and yield new avenues for pursuing research. Many of
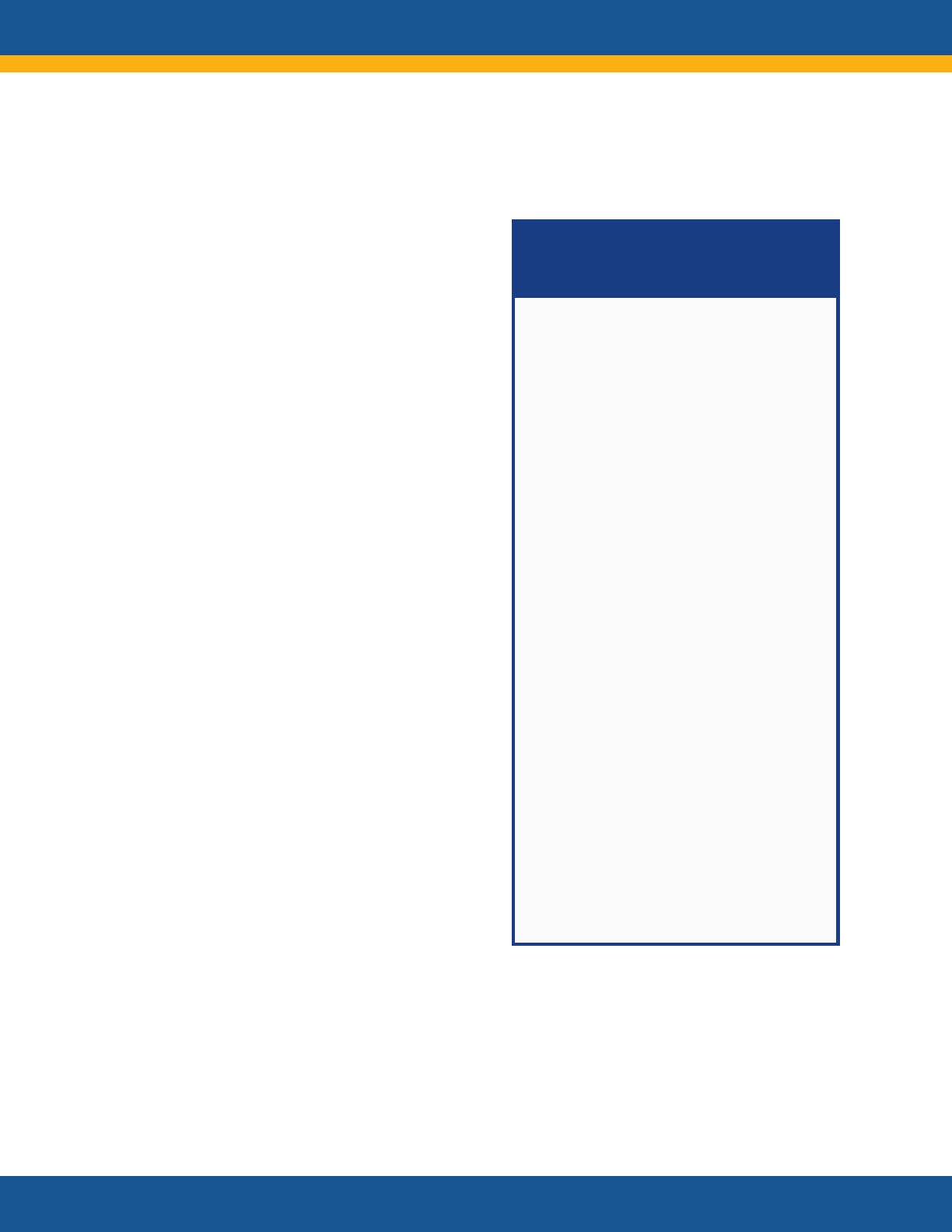
NIH RECOVER Initiative:
Principal Features
RECOVER is a multi-pronged,
multi-disciplinary research effort
of national scale to improve
understanding of Post-Acute
sequelae of SARS CoV-2 infection
(PASC) and to inform the
development of safe and
effective diagnostic, treatment,
and preventive strategies.
Features include
• Clinical cohorts with
thousands of diverse
participants across the
lifespan
• Patient involvement in all
levels of the initiative
• Inclusive, diverse
participation
• Community engagement
• Studies with access to tens of
millions of de-identified
electronic health records and
mechanistic studies of
pathogenesis
• Tissue pathology and autopsy
studies of more than 50 tissue
types
• Clinical trials of promising
treatments
29
National Research Action Plan on Long COVID
the large, U.S. government research initiatives are gathering comprehensive data and
biospecimens with the intent to leverage these in the short and long term.
Principal Research Initiatives
A critical component of the U.S. government
approach to researching the clinical spectrum of
Long COVID is the RECOVER Initiative. NIH
launched RECOVER to improve understanding of
and ability to predict, treat, and prevent PASC.
RECOVER is a multi-study, patient-centered
initiative of national scale with diverse
participation and community engagement.
RECOVER has multiple scientific aims and seeks to
understand the clinical spectrum and the biology
underlying recovery over time, as well as define
distinct subtypes of Long COVID. It includes
multiple sub-studies, such as longitudinal
observational clinical cohort studies with
thousands of diverse participants across the
lifespan; ancillary clinical studies leveraging cohort
data and specimens; pathobiology studies;
analyses of electronic health records; and clinical
trials. RECOVER will also conduct clinical trials to
test promising treatments in subsets of persons
with Long COVID for their ability to reduce
symptom burden and correct or prevent the
abnormal biologic factors that give rise to the
conditions.
Given the variety of organs in the body affected by
Long COVID and the multitude of symptoms
observed, RECOVER will examine a wide array of
body systems. Assessments are tailored to
different populations including adults, children
and their caregivers, pregnant participants and
their newborn infants, and people of reproductive
age. The methodology helps capture different
types of data including radiology, laboratory tests, surveys, immune assays, and genome
analyses. These analyses will be complemented by electronic health record-based studies that
collectively access de-identified health records of tens of millions of adult and pediatric patients
accessible through the National COVID Cohort Collaborative (N3C), the National Pediatric-
Centered Clinical Research Network (PCORnet) and PEDSnet (the Pediatric Learning Health
System consortium of PCORnet). Attention is given to include participants who live in rural
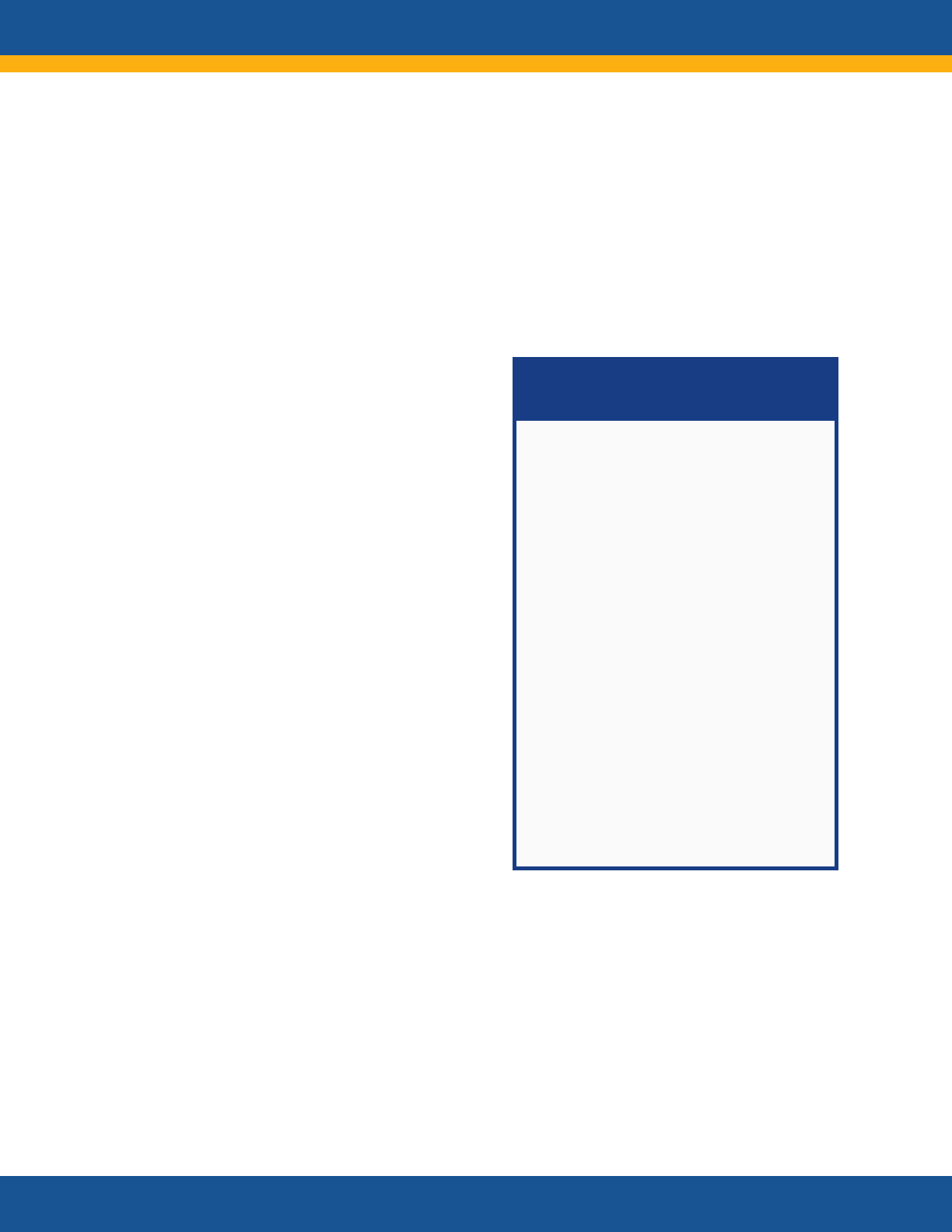
NIH RECOVER Initiative:
Research Questions
Goal: To understand, diagnose, treat,
and prevent post-acute sequelae of
SARS-CoV-2 infection (PASC)
• What are the various forms of
PASC? Characterization of full
clinical spectrum and phenotypes
• How long does PASC last? Can it
have effects later in life? Does it
alter the course of or risk for other
diseases and conditions?
• What are the risk factors for
developing PASC?
• What are the impacts of different
variants and vaccination?
• What are the underlying biologic
mechanisms responsible for PASC?
• What are the underlying
mechanisms responsible for
resilience and resistance to PASC?
• What are specific therapeutic
targets for clinical interventions
(treatment and prevention)?
30
National Research Action Plan on Long COVID
areas, those who are medically underserved, and those who are non-English speaking. The
surveys and research protocols for adults and children are available in multiple languages.
In addition to the RECOVER Initiative, NIH leads or funds additional prospective observational
cohort studies that include adults and children with varying severities of underlying disease,
which will help characterize the clinical sequelae of SARS-CoV-2 over time. Some studies are
characterizing immune responses, while others are focused on specific populations, such as
children and adults who developed diabetes mellitus after SARS-CoV-2 infection, individuals
with dysautonomia, myalgic encephalomyelitis and chronic fatigue syndrome (ME/CFS), and
children with multisystem inflammatory syndrome (MIS-C).
Additional NIH-supported studies are investigating
the persistent neurological and cognitive
symptoms experienced by many patients who
have recovered from SARS-CoV-2 infection. These
studies are recruiting across adult and pediatric
populations and enrolling pregnant people and
their infants, patients with other neurological
diagnoses, and those from historically
underserved backgrounds. The studies are
gathering different types of information and
biospecimens, such as performance on cognitive
measures, Magnetic Resonance Imaging (MRI),
cerebrospinal fluid (CSF), blood specimens, and
autopsy tissue to better understand the
neurological sequelae associated with Long
COVID.
NIH-supported researchers have also been
studying the mental health impacts of Long
COVID. Researchers have developed a publicly
available dataset of COVID-19 patients’ firsthand
accounts of their illness and recovery, including
their anxiety about illness and its lasting effects on
their mental and behavioral health. In addition, researchers supported by RECOVER developed
1
a machine learning model that uses electronic health record data to identify COVID-19 patients
who experienced Long COVID. Thus far, this research has revealed that diagnosis features such
as dyspnea, dyssomnia and other sleep disorders, and malaise are informative for recognizing
potential Long COVID. These conditions and other features of Long COVID can interact to
exacerbate patients’ experiences with new or pre-existing mental health conditions.
2
The Department of Veterans Affairs (VA) is conducting several collaborative studies as part of
the Post COVID Collaborative Merit Program that focus on the clinical spectrum of Long COVID,
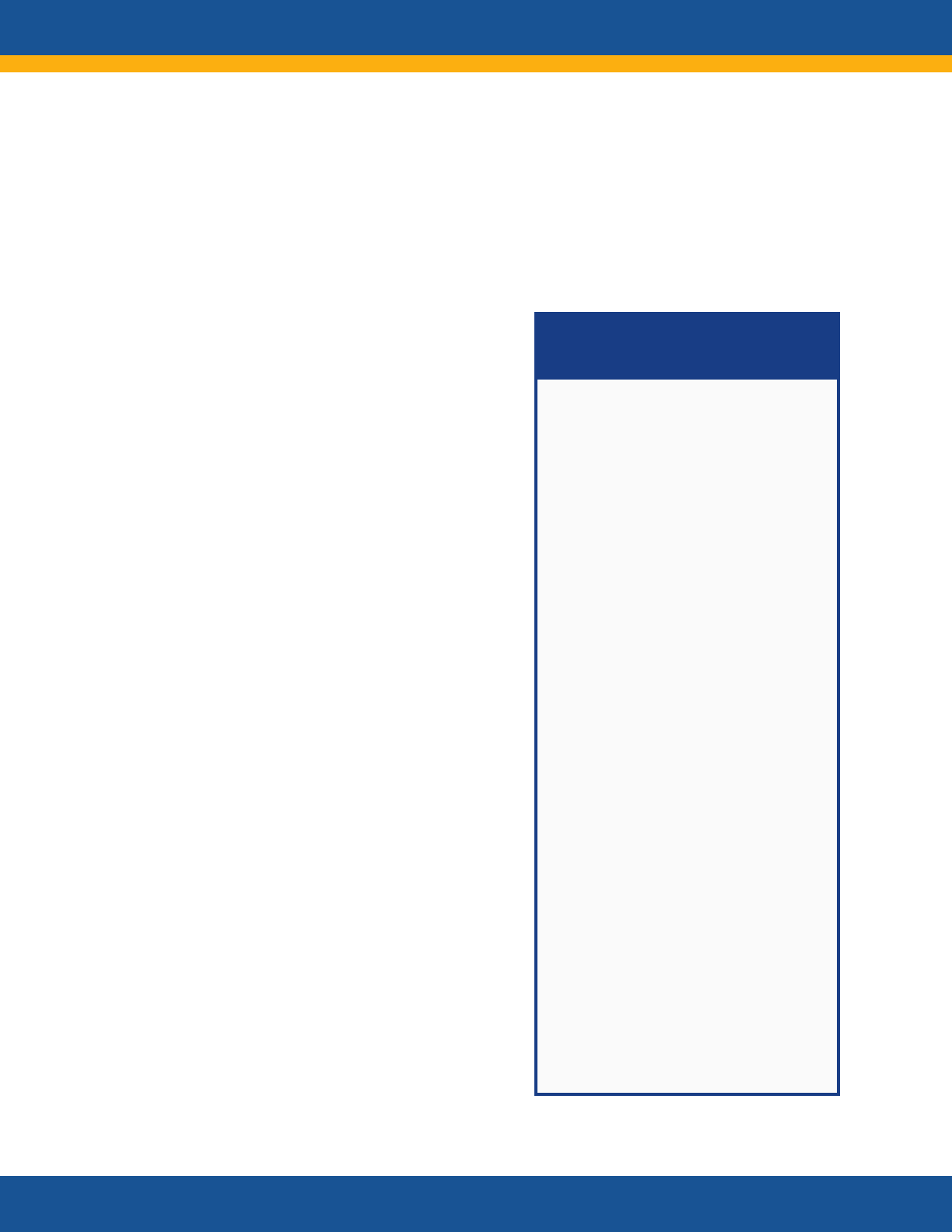
Anticipated Impacts of Research
• Evidence generated by research
will inform researchers, guidelines
developers, health care personnel,
payers, patients, caregivers, and
policy makers. Information will be
disseminated through publications,
professional scientific and medical
society meetings, federal
webpages, and webinars.
• Practitioners will be better able to
diagnose, manage, and monitor
Long COVID.
• The research will inform the
development of new treatment and
prevention strategies.
31
National Research Action Plan on Long COVID
including cognitive impairment, mental health effects, persistent fatigue (as seen in ME/CFS),
functional impairment, sleep disturbances, and recurrent pain symptoms.
CDC has completed and published several studies that have assessed clinical presentation of
Long COVID through both survey and analyses of electronic health record data. Also, CDC’s
National Health Interview Survey (NHIS) contains questions in 2022 and will again in 2023 on
ME/CFS and will provide data to examine the connections between ME/CFS, Long COVID, and
other chronic conditions.
3-5
The Department of Defense (DOD) is conducting the
Epidemiology, Immunology, and Clinical
Characteristics of Emerging Infectious Diseases with
Pandemic Potential (EPICC) study, a prospective
observational cohort study evaluating Long COVID
chronic symptoms and health care encounters after
SARS-CoV-2 infection. EPICC also includes machine
learning approaches to identify Long COVID early
symptom clusters, predict return to pre-illness
health, and identify subtypes of Long COVID.
In conjunction with EPICC, the VA launched the
Epidemiology, Immunology, and Clinical
Characteristics of COVID-19 (EPIC ), which is
following a cohort of Veterans after a positive or
negative test result for the presence of SARS-CoV-2.
The study will describe the clinical trajectory from
acute to Long COVID over time, as well as examine
the role of biomarkers influencing the risk for Long
COVID. At completion of the respective studies
(EPICC and EPIC ), researchers plan to compare
findings from the two participant groups.
3
3
Futhermore, the NIH and the VA are partnering on a
cohort study that aims to define risk factors for
severe COVID-19, with a focus on the role of the
immune system, inflammation, and tissue damage.
This research will aid in our understanding of how
the immune system affects whether an individual
develops severe COVID-19. Results from these
studies will complement evidence generated from
other studies to build a more complete and
comprehensive understanding of Long COVID.
Health Equity Considerations
• Inclusive and diverse
participation in Long COVID
research helps ensure that the
results of the research are
applicable to the wide range of
populations affected
• Meaningful patient and
community engagement is a
guiding principle of RECOVER,
which has intentionally
engaged patients, caregivers,
and individuals from
communities most affected by
SARS-CoV-2 at nearly all levels
of the Initiative. RECOVER
includes a National Community
Engagement Group and, at the
level of enrolling sites,
collaborates with the NIH
Community Engagement
Alliance Against COVID-19
Disparities program
• Research methods must
establish the validity of
instruments in diverse
populations that are the focus
of the studies
32
National Research Action Plan on Long COVID
Diagnosis of Long COVID
The heterogeneity of Long COVID, likely representing multiple clinical conditions, poses
enormous challenges to development of diagnostic strategies for Long COVID. Different
diagnostic tests may be required for different types of Long COVID. Research is just beginning
to establish clinical criteria for diagnoses and to inform laboratory and other test development
and other diagnostic strategies.
Principal Research Initiatives
The NIH RECOVER Initiative is collaborating with the NIH Rapid Acceleration of Diagnostics
(RADx®) Radical (RADx-rad) program, which focuses on rapid detection devices and home-
based testing technologies to address COVID-19 testing needs. The RADx-rad-RECOVER
collaboration is leveraging the National COVID Cohort Collaborative (N3C) to support the
development and validation of data-driven artificial intelligence, including machine learning and
natural language processing (using deep learning software tools) to better understand the
clinical, environmental, social, and behavioral risk factors for developing Long COVID and for
Long COVID prognosis. In addition, a request for information on technologies and approaches
that can identify individuals susceptible to PASC was published in 2021.
Several NIH-supported studies are also investigating specific symptoms. For example, the loss of
smell (anosmia or hyposmia) and its occurrence in Long COVID is one such focus. These projects
aim to develop a quick, inexpensive, self-administered smell tests to screen for COVID-19 at the
individual level and assess prevalence in communities, especially those that have been
traditionally underserved by the health care system and public health infrastructure. For those
with Long COVID, the developed smell tests will facilitate recovery monitoring.
2. Pathophysiology
There is an urgent need to identify the
pathological processes that underpin Long
COVID. It is important to rapidly advance
understanding at the molecular level of the
mechanisms at the root of the clinical
phenotypes and symptoms and other
observed health effects and identify targets
for preventive and therapeutic interventions.
Multiple hypotheses currently exist regarding
underlying biological processes, including
persistence of SARS-CoV-2 or viral antigens,
reactivation of other latent viruses (such as
Epstein-Barr virus), dysregulated
immunological or inflammatory responses,
and central or peripheral neurological
Core Research Questions
• What are the pathophysiologic
mechanisms of Long COVID?
• What potential role do the following
play in the development of Long COVID:
immune dysregulation (including
autoimmunity), persistent SARS-CoV-2
virus, reactivation of endogenous
viruses, and organ injury during acute
infection?
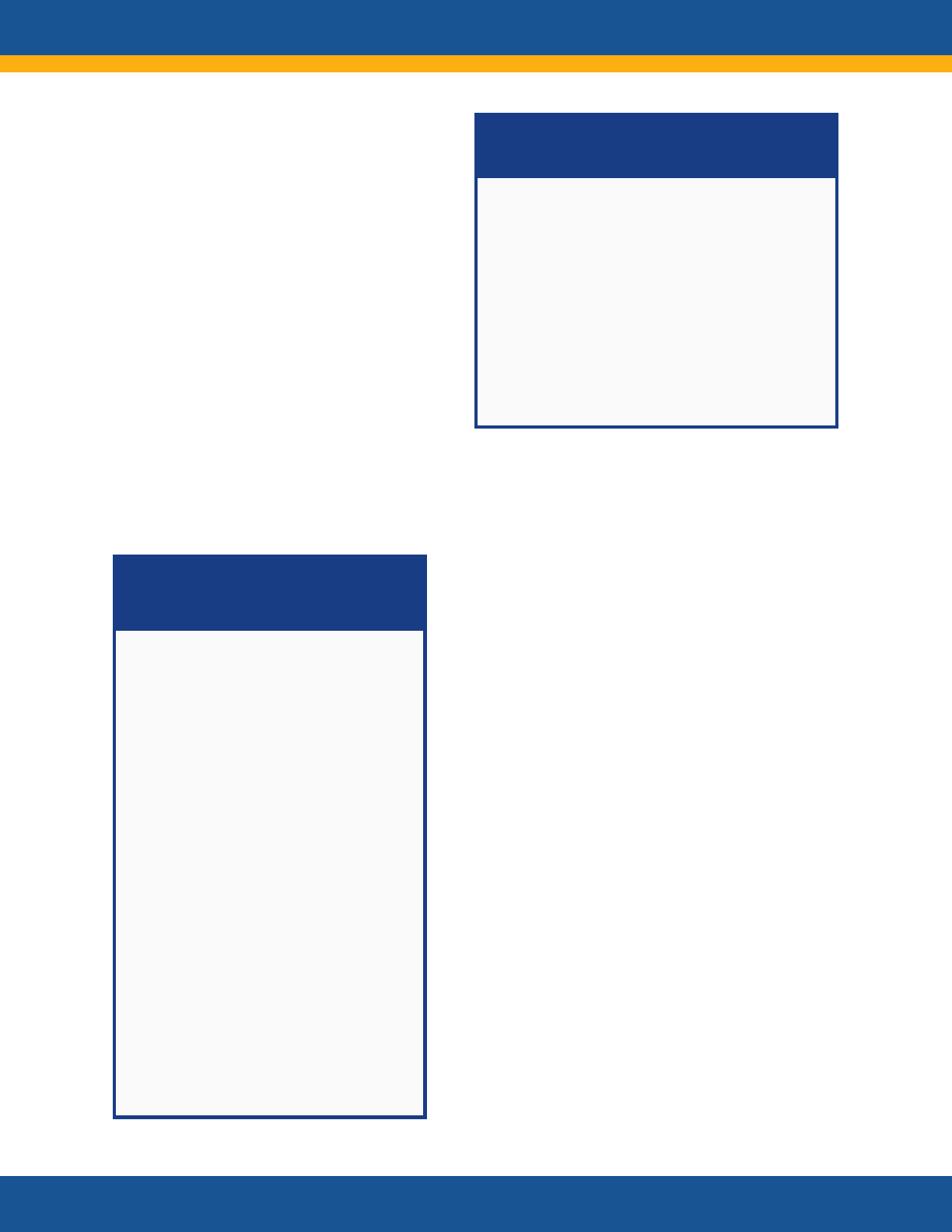
33
National Research Action Plan on Long COVID
damage (including autonomic dysfunction,
and inflammation of blood vessels). As Long
COVID is likely not one singular condition,
there will likely be multiple underlying
pathophysiologic mechanisms at play.
Principal Research Initiatives
Multiple studies across the U.S. government
are examining a broad range of potential
pathophysiological mechanisms of Long
COVID in both adult and pediatric
populations. The DOD’s EPICC study and the
parallel VA EPIC study are focused on
examining the pathophysiology of Long
COVID, including the role of viral and inflammatory markers to inform the Military Health
System clinical care and practice guidelines that aim to improve clinical care and management
of those with COVID-19, as well as inform future disease prevention strategies. These studies
are capturing clinical and laboratory data from patients with SARS-CoV-2 infection to better
understand the disease’s natural history, including
the clinical, virologic, and immunologic
determinants of severe disease. NIH’s RECOVER
Initiative includes a broad suite of pathobiology
studies to identify mechanisms underpinning clinical
phenotypes and symptomatic manifestations,
pathology in multiple organ systems that has led or
will lead to clinically significant health problems,
and potential therapeutic targets. RECOVER also
includes tissue pathology studies conducted at five
centers across the country that utilize a common
protocol with standardized methodologies to
analyze organs and tissue obtained at autopsy,
including the brain. These studies will enable
detailed identification of tissue injury in critical
organs and systems due to SARS-CoV-2 infection, its
sequelae, or both that lead or contribute to Long
COVID.
3
Additional NIH studies seek to understand the
underlying pathobiology of Long COVID. This
includes studies of immunology of both severe and
mild to moderate COVID-19, including longitudinal
in-depth immunophenotyping. Longitudinal cohorts
of both adult and pediatric populations with prior
Health Equity Considerations
Inclusive, representative, and diverse
participation in pathophysiology research
helps ensure that the results of the
research are applicable to the wide range of
populations affected. Ensuring that
interventions for Long COVID are effective,
acceptable, and accessible for diverse
populations contributes to future uptake
and effectiveness.
Anticipated Impacts of
Research
The most important target audience
for this information is other
researchers.
Information will be shared primarily
through publications and
professional meetings.
These studies will contribute to our
understanding of the causes of Long
COVID, help identify biomarkers and
enable risk stratification, contribute
to the development of new
therapeutic targets, and help inform
Long COVID clinical trials.
Understanding Long COVID at the
molecular level is critical to inform
better diagnostics and targeted
interventions for prevention and
treatment.
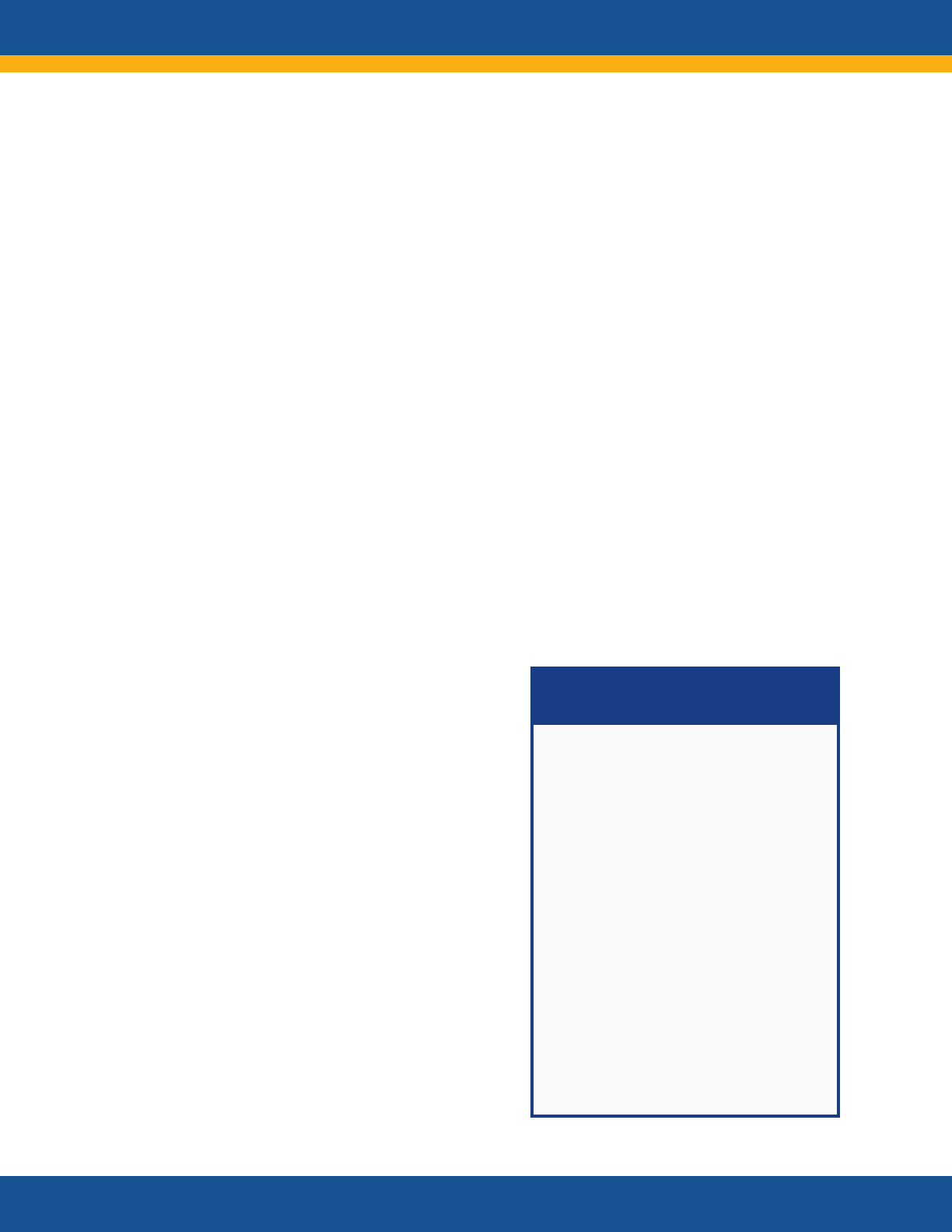
34
National Research Action Plan on Long COVID
SARS-CoV-2 infection include the assessments of immune responses and other investigations
neurological symptoms following a mild to moderate SARS-CoV-2 infection.
NIH’s RECOVER Initiative is also collaborating with the NIH All of Us research program, which is
enabling precision medicine research using five major data types (biometrics, genomics,
electronic health records, surveys, wearable devices) and biospecimens longitudinally obtained
from participants across the United States, with a goal to enroll one million diverse participants.
The collaboration will evaluate the use of biospecimens in potential ancillary studies to analyze
features of the immune system and genomes to further our understanding of the biology that
underpins Long COVID. The VA’s Million Veterans Program genomic program includes over
40,000 patients with documented SARS-CoV-2 infection among 800,000 subjects with genomic
and survey data; surveys are planned to examine the role of genomic markers in the
development of Long COVID symptoms. A principal audience for this information is the broad
community engaged in empirical research to improve the lives of persons living with Long
COVID.
3. Surveillance and Epidemiology
Public health surveillance is the systematic and ongoing collection, analysis, interpretation, and
dissemination of data linked to public health action. Surveillance tracks the occurrence and
burden of Long COVID in the population and can provide insights into groups affected. The
ultimate objectives of surveillance for Long COVID are to inform and guide the planning and
implementation of private and public programs
designed to reduce Long COVID and its
complications, and to evaluate the effectiveness of
public health interventions and policies.
7,8
6
Epidemiologic studies investigate the causes and
risks of health-related events in populations and the
application of new insights to control or mitigate
health problems. The aims of epidemiologic studies
are to provide estimates of the risk of Long COVID by
demographic groups and identify potential risk or
protective factors compared with groups without
history of COVID-19. While surveillance of Long
COVID tracks the burden in the population and can
provide insights into groups impacted, epidemiologic
studies provide estimates of the occurrence of Long
COVID by demographic groups and potential risk or
protective factors.
Core Research Questions
•
What are the population-level
incidence and prevalence of
Long COVID and associated
conditions?
• What are the population-level
incidence and prevalence of
activity limitation and disabilities
associated with Long COVID and
associated conditions?
• What are the factors associated
with incidence and prevalence of
Long COVID?
aims to evaluate the effects of immunotherapies on patients experiencing persistent
aimed at understanding the underlying biology of Long COVID. Further, an NIH-supported study

35
National Research Action Plan on Long COVID
Characterizing the numbers of people living with Long COVID, tracking these numbers over
time, and identifying protective and risk factors will require the use of multiple epidemiologic
studies and surveillance approaches leveraging different sources of information. Federal
agencies have implemented a diverse, layered, research and surveillance portfolio to answer
questions.
9
There are several challenges in surveillance and epidemiologic research for Long COVID given
the wide range of symptoms, effects, and spectrum of severity. These challenges lead to
differing characterizations of Long COVID across studies. Studies vary greatly in how assessment
and measurement are conducted. Studies to date have included patient-reported outcomes,
clinical assessments, laboratory measures, and diagnoses of new conditions and symptoms.
Some studies, but not all, have included measures of Long COVID severity, disability, and
disruption in activities of daily living. Some have focused on hospitalized patients, others only
on ambulatory patients, and others on persons with initial infections that were asymptomatic.
Few studies have included children.
Because many of the individual symptoms and conditions that comprise Long COVID are
common, the inclusion of a comparison group is vital to understand the excess burden of these
symptoms and conditions among persons infected with SARS-CoV-2. Studies currently
underway have approached this in multiple ways, some using participants who test negative
but have COVID-like symptoms, others using historical (pre-pandemic) participants, and others
using participants with no evidence of SARS-CoV-2 infection or COVID-like symptoms.
Diverse Research Portfolio Needed for Long-COVID Surveillance and
Epidemiology
Prospective Cohort Studies: Assess how Long COVID progresses and measure short-term
and longer-term impacts.
Cross Sectional Surveys: Provide point-in-time view of Long COVID in the population by
demographic factors, underlying medical conditions, and other risk factors.
Medical Record Review: Provide insights into common conditions, symptoms, care, and
treatment that patients are receiving in the medical setting.
Modeling studies: Provide population-level estimates of numbers of people affected by
Long COVID when it is not feasible or practical to count all cases.
Electronic Health Care Data: Leverage large numbers of patients to provide estimates of
the incidence of new conditions, symptoms, and health care utilization among persons who
had COVID-19 compared with persons who did not.
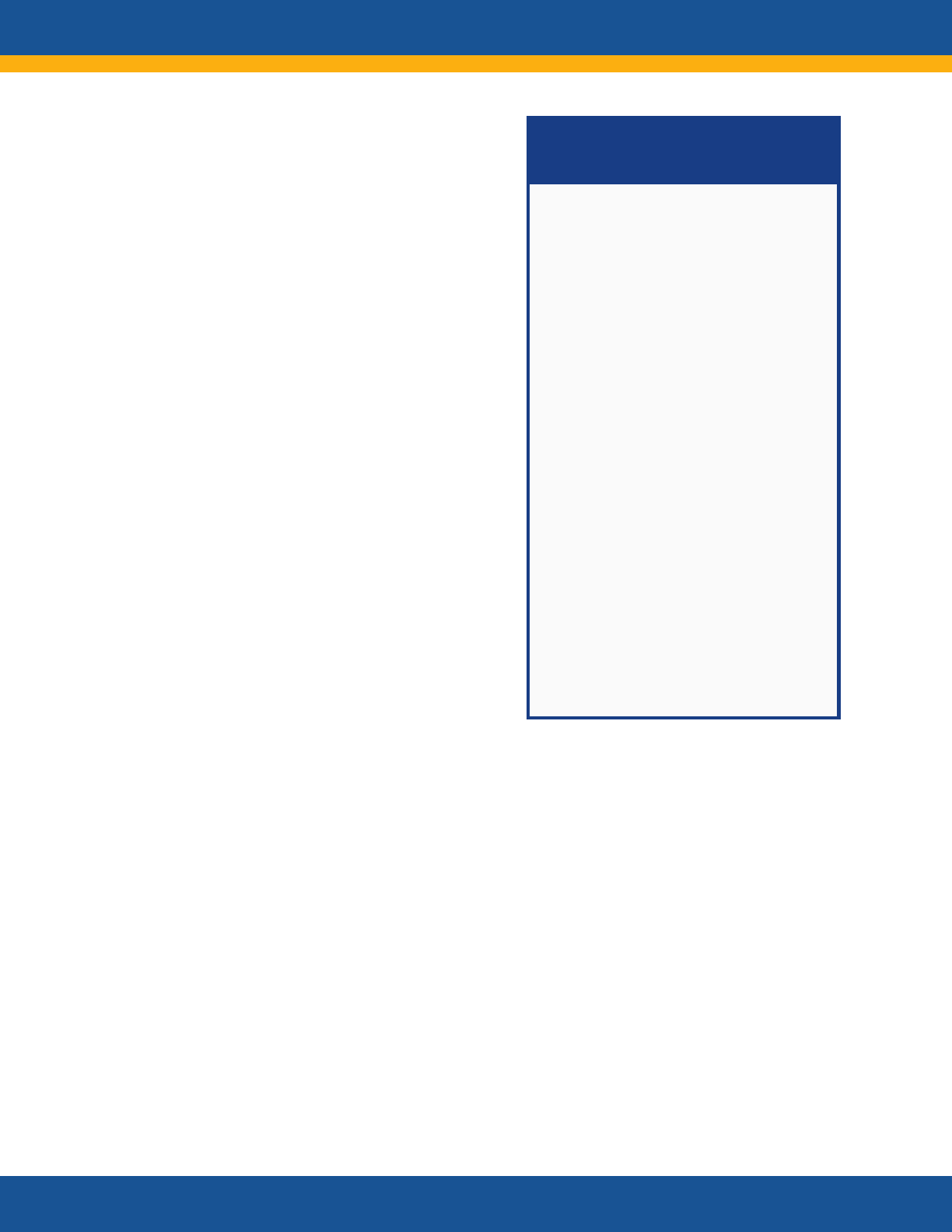
36
National Research Action Plan on Long COVID
Several federal interview surveys harmonize the
Long COVID data collection, including the National
Health Interview Survey, the Household Pulse
Survey, and the Behavioral Risk Factor Surveillance
System. Along with these self-reported interview
data, data from complementary methods such as
cross-sectional health surveys, cohort studies,
analyses of electronic health record data, and
sentinel surveillance are needed to fully characterize
the natural history of post-COVID-19 conditions.
More work needs to be done to align these efforts
across the U.S. government. For all areas, published
studies provide new and important findings that
contribute to the evidence-base for Long COVID
surveillance and epidemiology.
Population-Level Incidence and
Prevalence
There is a growing body of research to provide
measures of population-level incidence (the
proportion of people with new occurrence of Long
COVID among those at risk during a specific period
of time) and prevalence (the proportion of persons
with Long COVID at a specific point in time).
Prospective observational cohort studies, electronic
health care data analysis, and health surveys
currently underway will provide estimates of the incidence and prevalence of Long COVID in the
general population and in specific subpopulations. Building on the existing evidence-base, we
will continue to learn more about estimates of population-level incidence and prevalence, for
specific populations and by subpopulation. Studies from CDC, FDA, HRSA, NIH, VA, and their
partners will help us fill in the gaps in our understanding of incidence and prevalence of Long
COVID.
Principal Research Initiatives
CDC, the Department of Commerce (the Census), FDA, HRSA, NIH, and VA are leading several
studies that will provide estimates of incidence and prevalence (see Appendix D). Select
examples include CDC’s National Health Interview Survey and Behavioral Risk Factor
Surveillance System. Starting in January 2022, CDC’s National Health Interview Survey (NHIS)
added questions to identify the prevalence of Long COVID based on self-report (for adults) and
parent-report (for children). Also, in 2022, CDC’s Behavioral Risk Factor Surveillance System
(BRFSS) added a question to collect information about symptoms of post-COVID-19 conditions
to produce state and territorial-level estimates of burden among adults based on self-report.
Health Equity Considerations
The COVID-19 pandemic has had a
disproportionate impact on some
racial and ethnic communities,
people with lower socio-economic
status, and some people with
disabilities. Understanding risks for
Long COVID and the impact of the
inequities that led to the
disproportionate outcome in
infection, hospitalization, and death
are critical. Groups may be more at
risk for Long COVID due to the
inequities that put them at risk for
COVID-19 or there may be other
factors. Studies of Long COVID must
examine race and ethnicity, socio-
economic status, disability status and
other factors impacted by health
inequities and assess associations
within groups, not just across groups.
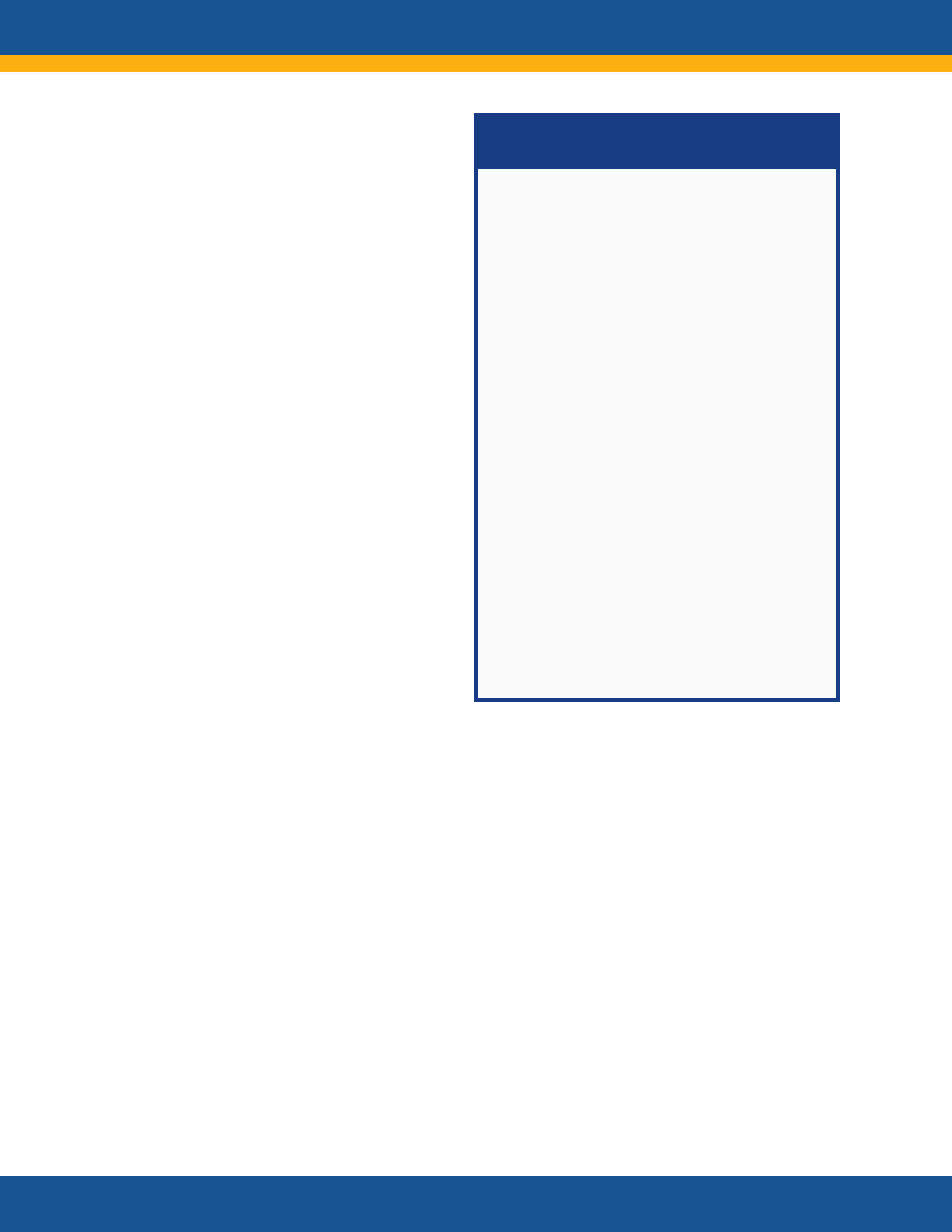
37
National Research Action Plan on Long COVID
Other examples include CDC’s multi-state
Long COVID survey that surveyed adults who
had a positive test result for SARS-CoV-2 for
self-reported acute and post-COVID-19
symptoms lasting four weeks or more to
estimate burden of post-COVID-19 conditions
and identify demographic and disease-
specific risk factors for developing post-
COVID-19 conditions.
Starting in June 2022, questions were added
to the Household Pulse to identify Long
COVID in adults through self-report. Early
results indicate a high level of respondents
stating that they have symptoms of Long
COVID. The Household Pulse Survey, led out
of the Census Bureau, in partnership with
CDC, is one of the first federal data systems
to collect information on Long COVID and will
be a valuable resource to understand
disparities within the U.S. population.
10
The Health Resources and Services
Administration (HRSA) is administering the
National Survey of Children’s Health
Longitudinal Cohort Study (NSCH-LC). By
leveraging the large nationally- and state-
representative sample of the NSCH, the NSCH-LC will be among the only national studies with
pre- and post-pandemic information on children and youth, including racial and ethnic minority
groups and children with special health care needs.
Individual-Level Incidence and Prevalence
Many of these same studies detailed above and in Appendix D also provide estimates of the
individual-level risk of developing Long COVID. Many of these studies include persons without
evidence of SARS-CoV-2 infection as a comparison group. Comparison of the incidence of Long
COVID-19 conditions and symptoms between persons with and without COVID-19 provides an
estimate of both relative and absolute risk. Although each research platform can provide
estimates by subpopulations, the subpopulations included in each varies. The multiple studies
across CDC, CMS, NIH and the VA capture a wide group of populations and subpopulations,
while the longitudinal study at HRSA is focused on children and is expected to provide multiple
independent estimates of the risk of Long COVID as well as risk disaggregated across multiple
factors.
Anticipated Impacts of Research
Evidence generated by surveillance and
epidemiology is useful for the public,
patient groups, health care personnel,
health care systems, patient groups, and
policy makers.
Synthesized information on Long COVID is
being analyzed and shared through peer-
reviewed publications, news media, social
media, webinars, professional associations,
and websites aimed at professional and
general audiences.
Evidence from surveillance and
epidemiology studies is used by the public
to understand individual risk and make
informed decisions, by health care systems
and service providers to project and plan
for human, financial and infrastructure
resources, and by policy makers to similarly
plan for resources and to develop and
promote effective programs and services.

38
National Research Action Plan on Long COVID
Many of these studies will also measure whether COVID-19 can result in new disease states or
exacerbate pre-existing disease. Studies from both the VA and CDC have already documented
increased occurrence of many chronic diseases, such as heart disease, diabetes, and kidney
disease.
Understanding the impact of Long COVID on behavioral health (mental health and
11
substance use), quality of life, activities of daily living and any resulting disability is vital to
understanding the overall impact on society, health care systems, and persons with Long
COVID. Some studies are directly assessing the impact of Long COVID resulting in disabilities
either through detailed abstraction of medical records, or through direct patient reported
outcomes.
Principal Research Initiatives
The VA initiated the COVID-19 Observational Research Collaboratory Long-term Outcomes
Study (CORC-LTO) to investigate outcomes up to 36 months after SARS-CoV-2 infection using a
national cohort of over 200,000 COVID-infected Veterans along with matched controls to
estimate the incidence of and risk factors for Long COVID and the impacts on specific health
outcomes, including functional status and quality of life. The study used a combination of
electronic health record (EHR) data and structured telephone surveys of subsamples from
different time periods to document new diagnoses, symptoms, health-care related quality of
life and health care utilization up to 36 months after infection.
The VA’s Epidemiology, Immunology and Clinical Characteristics of COVID-19 [EPIC ]) is a
3
prospective controlled cohort study of SARS-CoV-2 infection among Veterans recruited from 16
VA medical centers, conducted in partnership with DOD. EPIC will describe the clinical
3
trajectory from acute to Long COVID and examine clinical risk factors (including prior infection
or vaccination) in influencing the risk for Long COVID.
12
Several of CDC’s studies using electronic health care data and patient databases have and will
continue to provide estimates of incidence. For example, CDC is partnering with the American
Board of Family Medicine to analyze electronic health record data from COVID-19 patients seen
at approximately 700 primary care and family medicine practices to assess frequency of new
symptoms and conditions among patients with COVID-19 diagnosis compared with patients
with other respiratory diagnoses. CDC is also conducting analyses of several large health care
data sources to provide estimates of new symptoms and conditions following COVID-19
diagnosis in outpatient and inpatient settings by patient demographic and clinical
characteristics.
Another CDC study focusing on the Navajo and White Mountain Apache communities, COVID-
19 within American Indian Communities at High Risk in the Southwest United States, follows
people who are infected with SARS-CoV-2 for up to 12 months to assess the development and
duration of symptoms, complications from infection, and the immune response to infection.
The DOD established a COVID-19 patient registry in 2020, as a pandemic response capability for
clinical performance improvement in the DOD. Currently, the COVID Registry is capturing and
analyzing data and information on Long COVID, conditions occurring after COVID-19 disease
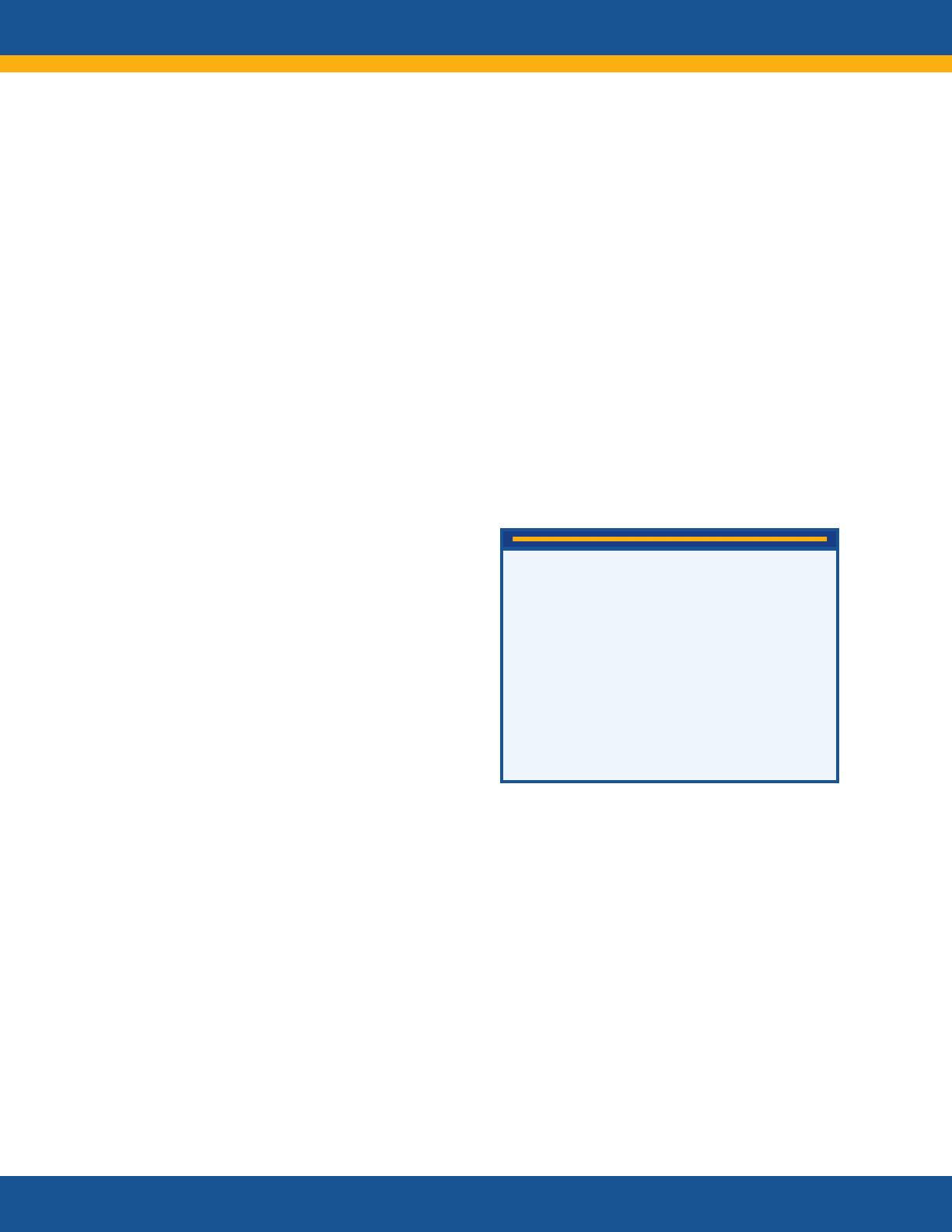
39
National Research Action Plan on Long COVID
and after vaccination. Current analyses will examine Long COVID diagnosis requiring a health
care visit 30-180 days after COVID-19 or vaccination; number of individuals with a diagnosis
associated with Long COVID; and number of individuals with each combination of Long COVID
associated diagnosis (such as fatigue plus headache, plus loss of taste).
Risk Factors
Understanding what risk factors may be associated with occurrence of Long COVID is a critical
research question. Both prospective cohort studies and those using electronic health care data
include data on potential risk factors associated with Long COVID such as age, genders, race,
ethnicity, and underlying medical conditions, including multiple co-morbidities. Those with
more severe illness requiring hospitalization are at highest risk of developing Long COVID.
Most people experiencing Long COVID were never hospitalized and so additional information
on premorbid characteristics, genetics, education level, employment, and social determinants
of health is necessary and is more likely to be captured in prospective cohort studies. A few
electronic health care data sources can also provide residential and facility geographic
information linked to measures of social deprivation or vulnerability or other measures of social
determinants of health.
13,14
Important risk factors for the occurrence of Long
COVID are the SARS-CoV-2 variant associated
with the infection and the vaccination status of
the individual. Studying how different SARS-CoV-
2 variants may be related to Long COVID
symptoms and conditions is important to our
understanding of protective and risk factors.
Because many of these studies are ongoing, they
will include persons infected with SARS-CoV-2 at
different waves of the COVID-19 pandemic and
will capture infections with different variants.
Vaccination has also been observed to decrease the risk of an individual developing Long
COVID.
15
Principal Research Initiatives
CDC’s Immune Response to SARS-CoV-2 among patients in Louisiana follows patients recovering
from COVID-19 to assess longer term clinical and immunity related outcomes up to one year
after the beginning of the illness. The study includes a subgroup of COVID-19 patients
hospitalized for severe COVID-19 illness. Multiple virologic and immunologic specimens are
collected during acute illness, and then patients are followed for one year to determine what
acute factors are correlated with adverse longer-term outcomes.
The longitudinal observational studies of the NIH RECOVER Initiative provide a robust scientific
platform and infrastructure for systematic and ongoing long-term assessments of Long COVID
in highly diverse populations. With its national scale, standardized methodologies, biospecimen
“Like so many other health-related
struggles in the pandemic, Long COVID
illustrates the underlying, fundamental
challenges with our health care
system. More urgent attention and
action to its reform is a priority for
pandemic preparedness."
—State Epidemiologist

40
National Research Action Plan on Long COVID
Vaccination is perhaps the most prominent of protective factors currently under investigation.
Evidence is emerging that vaccinated people who are subsequently infected with SARS-CoV-2
are about less likely to report symptoms of Long COVID than people who received one dose of
COVID-19 vaccine or were unvaccinated in the short-term (four weeks after infection), medium-
term (12 to 20 weeks after infection) and long-term (6 to 12 months after infection).
11,15-18
Vaccine effectiveness studies measure the effectiveness of the COVID-19 vaccine in preventing
infectious, symptomatic illness, hospitalization and death, and the acute outcomes of SARS-
CoV-2 infection. With the recognition of Long COVID as an outcome from SARS-CoV-2
infection, many of these studies are including longer follow-up of participants to estimate the
effectiveness of prior COVID-19 vaccination in reducing Long COVID among persons infected
with SARS-CoV-2. Most studies to date have focused on adults, with only a few including the
adolescent population. In addition, studies have not reported results by subpopulations or
underlying medical conditions or risk factors. In addition to vaccine effectiveness studies, many
of the studies in Appendix D include collection of information on COVID-19 vaccinations and
boosters. Some of this is through available electronic health data, other directly reported by the
patient, and other with linkage to state vaccine information. Some studies will be able to
examine if COVID vaccination prior to SARS-CoV-2 infection reduces the occurrence of Long
COVID, and others if COVID-19 vaccination after SARS-CoV-2 infection reduces on-going Long
COVID symptoms. Importantly, studies will also consider the impact of COVID-19 vaccine dose
and boosters on Long COVID.
19
Principal Research Initiatives
The FDA is collaborating with the VA and CMS to study the safety and effectiveness of vaccines
and Long COVID. The FDA and the VA are describing the main medical outcomes associated
with Long COVID and their severity, estimating the incidence of Long COVID among U.S.
Veterans, selecting among all potential Long COVID outcomes, those more likely to be
collection, comprehensive data, and studies of molecular pathogenesis, RECOVER will be critical
for understanding the risk factors for developing Long COVID and informing development of
diagnostic, treatment, and preventative strategies. RECOVER’s EHR-based studies, which
leverage health records from tens of millions of diverse patients across the lifespan, including
pre-exposure health records, are also ideally suited for identifying risk factors for Long COVID.
In association with CMS, the FDA is using Medicare claims data to study the frequency of
possible Long COVID health outcomes and the risk factors associated with the occurrence of
these outcomes among Medicare Beneficiaries who were discharged from a hospital with a
COVID-19 diagnosis.
Protective Factors, Including Vaccination
Protective factors can help guide public health messaging and efforts to prevent Long COVID.
Potential protective factors under investigation include pre-existing health status, COVID-19
treatments such as Paxlovid
TM
(nirmatrelvir 150 mg and ritonavir 100 mg), and vaccination.
41
National Research Action Plan on Long COVID
associated with prior COVID-19 than with other respiratory infection, and lastly, evaluating
potential risk factors for Long COVID. CDC has developed multiple longitudinal cohort studies
that will assess vaccine effectiveness in adults and children and has worked to incorporate
specific objectives that will assess Long COVID outcomes (including ongoing symptoms, health
status, disability, missed work, and missed school) in similar ways across these studies.
Validation of Operational Definitions of Long COVID in Real-World
Data Sources
The validation of operational definitions of Long COVID, for different purposes, in real-world
data sources considers that there are a wide range of physical and behavioral health
consequences that can follow SARS-CoV-2 infection. To operationalize the definitions, many
studies look for evidence of COVID-19 and SARS-CoV-2 infection determined through either
medical record review or abstractions, confirmation of SARS-CoV-2 test results, or identification
of cases in other electronic health care data, and then look for new or persistent symptoms or
conditions to identify cases of Long COVID. One recent approach to operationalize a definition
has included the use of machine learning using large electronic health care data to identify Long
COVID clinic patients. Since October 2021, there is an ICD-10 code (code U09.9, Post COVID-19
condition, unspecified) for classifying diagnoses and reason for visits in all health care settings.
The ICD-10-CM code for Long COVID is gaining acceptance and also provides a method to assess
and possibly validate Long COVID definitions. Further validation of operational definitions in
real-world data sources will include linkage of patient reported outcomes with electronic health
care data in prospective cohort studies.
21
20
4. Long COVID and Overall Well-Being
The persistent health effects of COVID-19
have broad ramifications. In addition to the
discrete physiologic effects of Long COVID
outlined previously, longer-term symptoms
can have serious impacts on important
aspects of life that are not captured in clinical
diagnoses or self-report of individual
symptoms. These include impacts on quality
of life, education, employment, disability,
mental health and substance use challenges,
and caregiving. Persistent symptoms may
affect quality of life, and patients
experiencing severe fatigue or cognitive
symptoms and impairment (also known as
“brain fog”) may have trouble with schooling or employment and may require caregiving from a
family member or professional. These effects in turn can have severe economic impacts on
individuals with Long COVID and their families. Although outcomes of COVID-19 have been
found to be less severe in young people and current data show that the prevalence of Long
Core Research Questions
What are the impacts of Long COVID on
• Health related quality of life
• Employment, insurance, disability, and
socioeconomic status
• Educational and developmental
outcomes
• Caregivers and families?
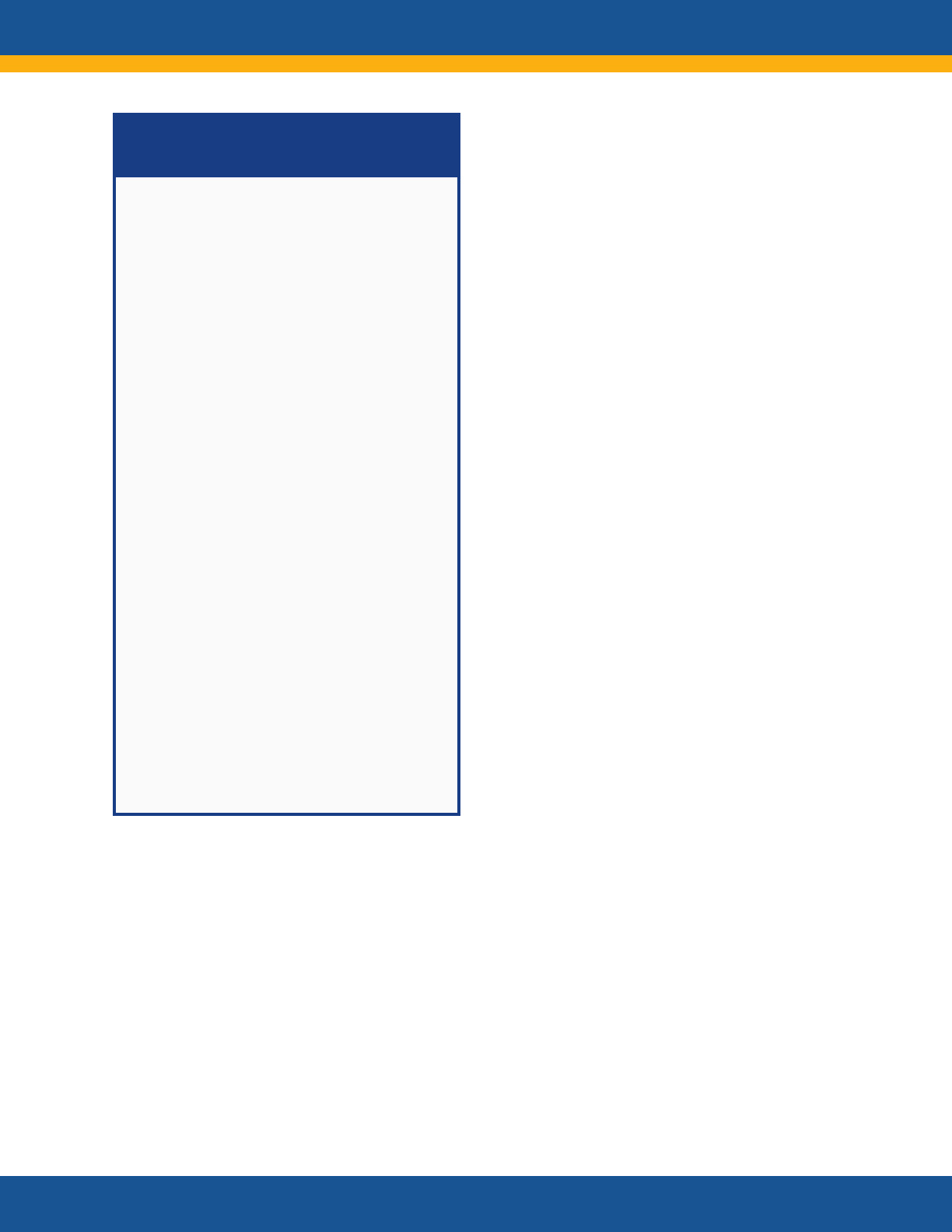
42
National Research Action Plan on Long COVID
COVID is low , Long COVID can occur in
children and may impact normal development;
if not mitigated or otherwise addressed, these
effects may have a long-lasting impact. The
RECOVER Initiative is developing a cohort of
children to study such issues. Family caregivers
of people with Long COVID may be at risk for
secondary effects, including psychological
stress and economic impacts if they must
reduce their paid work. From a societal level, it
is important to capture the magnitude of these
impacts, which may be as important as the
direct health impacts for many individuals and
their families. The COVID-19 pandemic and its
burden of infection has already had detrimental
effects on the physical and behavioral health
and financial well-being of the population.
Overall, the impact of Long COVID will likely
compound these existing repercussions,
reverberating in every dimension of our lives,
as Long COVID does not only impact the health
and well-being of those directly affected with
this disease, but may have consequential
ramifications on labor force participation,
economic productivity, and societal well-being.
22
Efforts to address these questions take a
variety of approaches. Some cohort studies of
affected patients are adding questions related
to employment, schooling, and income. Many
federally sponsored national health surveys are
beginning to include questions on past SARS-
CoV-2 infection. Relevant federal agencies are tracking change in outcomes, such as education
and employment over the pandemic period. There are a few national databases for tracking
child development, education, or employment. Some researchers are attempting to link these
databases to clinical databases to learn more about these factors. Furthermore, policy research
is important to enable and ensure alleviation of impacts based on traditional knowledge gained.
Thus, while pandemic impacts on many of these outcomes have been reported, it can be
difficult to separate out the direct effects of Long COVID from the disruption caused by COVID-
19 or the indirect effects of pandemic disruption to child development, schooling, families, and
employment. Detailed work in survey research is therefore critical.
Why Is This Important?
The wider impacts of Long COVID are
important to patients and families and to
the local, state, and federal organizations
responsible for supporting children’s
health and education, persons with
disabilities, families and caregivers, and
unemployed persons. Such information
will be critical for developing policies
regarding support for patients, students,
and families with Long COVID and for
planning programs to help patients
recover and regain productive schooling
or employment.
Information is developing rapidly on the
medical effects of Long COVID, and large
representative studies from the United
States and other countries are beginning
to allow for population-based estimates.
With respect to effects on development,
educational outcomes, employment and
long-term disability, data from individual
patients has documented the profound
and prolonged effects for some children
and adults, but at this time we lack
representative data to inform guidelines
or policies at this time.
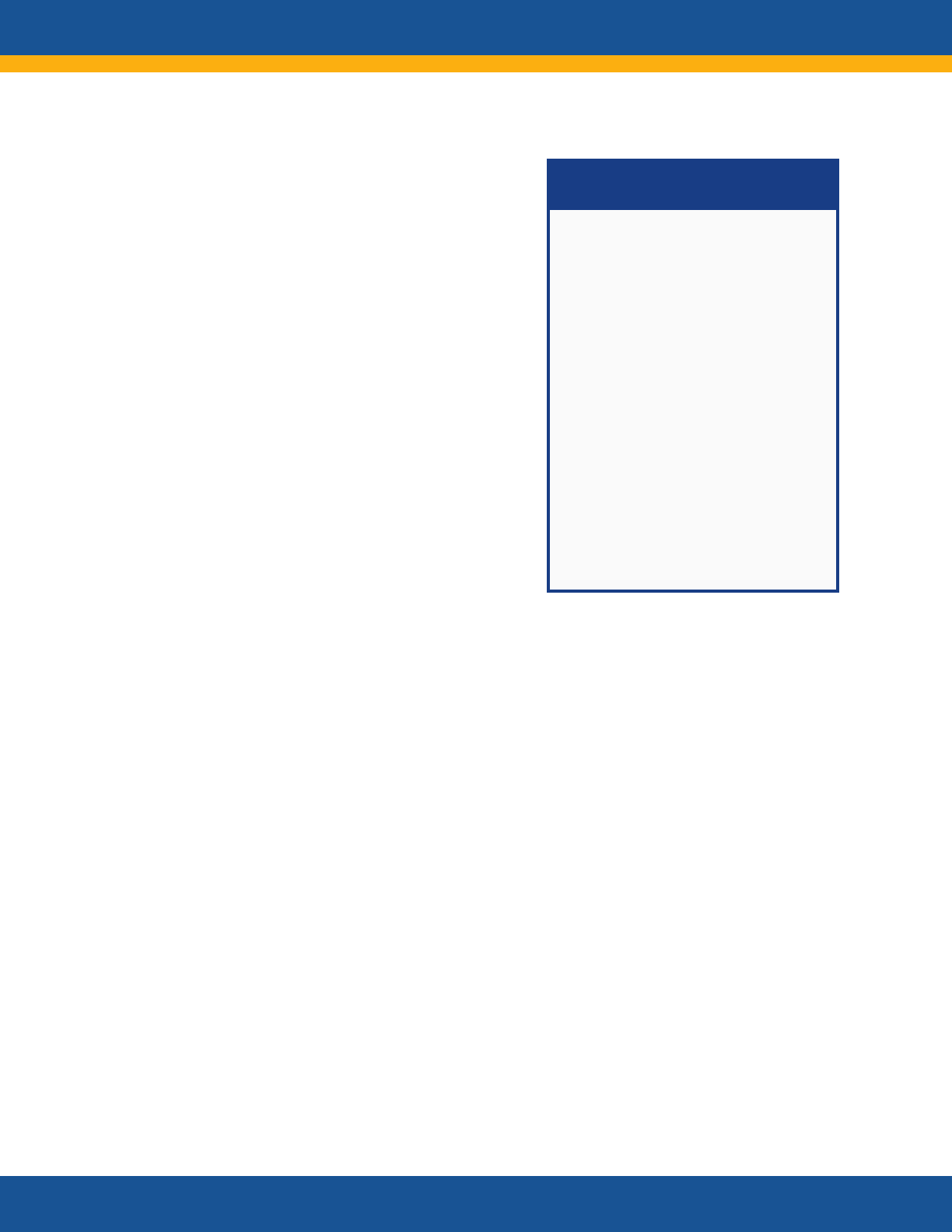
43
National Research Action Plan on Long COVID
Health-Related Quality of Life
Individual symptoms may not adequately capture the
overall impact on patients of an illness like Long
COVID, which may be accompanied by multiple
symptoms affecting many activities. Several federal
research agencies are funding large, longitudinal
cohorts involving either patients with new COVID-19 or
patients meeting some diagnostic criteria for Long
COVID. These include the RECOVER cohorts funded by
NIH, the EPIC cohort of Veterans funded by the VA,
the parallel DOD study of active-duty military, and
multiple studies from CDC that use standardized
instruments for collecting patient-reported outcomes.
All three of these efforts are including validated self-
reported quality of life outcomes in addition to other
more specific outcome measures. Populations included
in these studies include a broad range of geographic
locations, racial and ethnic, and sociodemographic
groups and settings.
3
23
Principal Research Initiatives
The VA has also launched the COVID-19 Observational Research Collaboratory Long-term
Outcomes Study (CORC-LTO) to investigate the longer-term effects of SARS-CoV-2 infection in a
nationally representative sample of SARS-CoV-2 infected Veterans and controls. Patient surveys
will include quality of life measures along with more specific symptom scales. The methods for
identifying the cohorts of persons with Long COVID vary somewhat by study, as does the
presence and nature of any control population. Nonetheless, this portfolio of projects will
provide estimates of health-related quality of life (HRQOL) in Long COVID for a proportion of
the U.S. population in the near term.
Data are much more limited for children. The RECOVER Initiative has as its goal to enroll a
pediatric cohort of approximately 19,000 children. The Pediatric COVID Outcomes Study
(PECOS) led by the Children’s National Hospital in collaboration with NIH’s National Institute of
Allergy and Infectious Diseases (NIAID) will examine the longer-term effects of COVID-19 and
multisystem inflammatory syndrome in children (MIS-C), including health-related quality of life
and social impacts for participants and families and caregivers. Finally, the Maternal and Child
Health Bureau (MCHB) of the Health Resources and Services Administration (HRSA) is
supporting the National Survey of Children’s Health Longitudinal Cohort Study (NSCH-LC), which
aims to produce publicly available data on the longer-term effects of the COVID-19 pandemic
on children and families in the United States, including their quality of life. In 2023, MCHB will
follow up with the parents and caregivers of approximately 60,000 children from birth through
age 17 years, who were originally interviewed right before the pandemic in 2018 to 2019 for
Health Equity Considerations
Factors such as health care access
and use, occupation, and gaps
related to education, income, and
wealth are associated with higher
rates of COVID-19 infection,
hospitalization, and death among
racial and ethnic minority groups.
Implications of Long COVID on
such factors affecting health
equity could further exacerbate
disparities, particularly among
racial and ethnic minority and
socioeconomically disadvantaged
communities who have been
historically underserved.
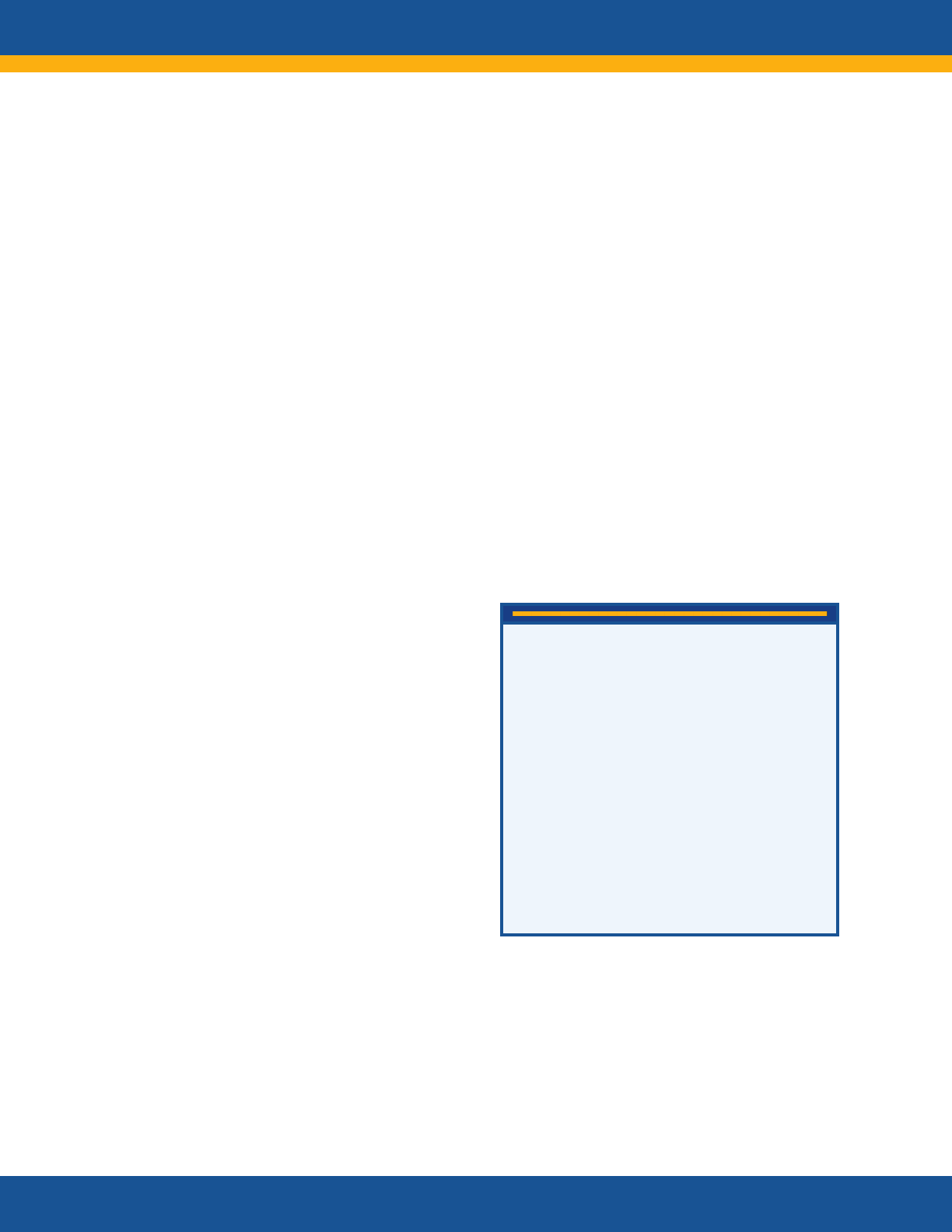
44
National Research Action Plan on Long COVID
the NSCH. As part of the 2023 data collection, the NSCH-LC will collect data on children’s
mental health diagnoses and behavioral problems, impact of children’s health on their quality
of life, children’s education and young adults’ employment outcomes, as well as family-level
employment impacts. The NSCH-LC will also have information on children’s chronic conditions
to assess the impact of Long COVID on children with prior chronic conditions.
Employment, Health Insurance, and Other Economic Impacts
Data on the effects of Long COVID on employment, health care coverage, medical costs, and
loss of personal income are far more limited than data regarding the effects on health
outcomes. Current efforts to assess these impacts include some population-based surveys of
Veterans; a variety of study cohorts of individuals with Long COVID that include questions on
one or more of these outcomes; and some employment datasets that are being augmented or
linked to try to identify patients with Long COVID. Three Department of Labor (DOL) programs
are trying to fill in gaps. Retaining Employment and Talent After Illness/Injury Network (RETAIN)
anticipates enrolling 15,900 working age participants from health care settings with a work
limiting illness or injury including Long COVID with an emphasis on individuals who are
medically underserved. These data may help determine whether early coordinated services will
improve employment outcomes for people experiencing work disabilities from Long COVID. The
DOL Office of Workers Compensation Programs collects information on workers compensation
claims, including for federal employees who contract COVID-19 as part of their duties. Another
DOL Survey of Occupational Injuries and Illnesses
(SOII) estimates nonfatal occupational injuries
and illnesses across 200,000 employers in the
private and public sectors (state and local
government). Although data to date do not
include a specific mention of Long COVID, trends
may be inferred from COVID-related cases that
require days away from work. Also, the NSCH-LC
(previously mentioned) will collect data on the
impact of children’s health on young adults’
employment outcomes, as well as family-level
employment impacts. CDC is funding a study to
evaluate Long COVID in U.S. state workers'
compensation systems. The study is focused on
essential and public-facing workers (such as
health care, public safety, retail industries) in a
detailed population of workers for six states (California, Illinois, Michigan, Ohio, Washington,
Wisconsin) and a broader sample of 27 states through the Workers Compensation Research
Institute (WCRI) for up to a two-year period (January 2020 to 2022).
“People with Long COVID are
experiencing mental health harm as the
world returns to normalcy. It is difficult
for children with Long COVID to return
to schools that no longer have mask
mandates. It is difficult for someone
with Long COVID to return to a
workplace that has removed
accommodations like remote work or
masking.”
—Public Health Administrator

45
National Research Action Plan on Long COVID
Education and Development
The COVID-19 pandemic had profound impacts on education from pre-school through post-
graduate education, due to suspension of in-person classes at the height of the pandemic,
prolonged remote learning, disruptions among families with COVID-19, and illnesses among
teachers and staff. Most of these effects are due to policies imposed because of the pandemic
or to direct effects of illnesses. Little data currently exist on the specific effects of Long COVID
on educational outcomes, with most coming from anecdotal reports from patients. Because the
prevalence of Long COVID in school-age children and adolescents appears low, national
surveys may have a hard time capturing this effect and distinguishing it from other pandemic-
related disruptions.
22,24
Although Long COVID was first recognized in adults, cohort data and national survey data
confirm that children and young people do experience post-COVID-19 conditions. Initial data
indicate children may be less affected than adults, but data are limited. Attributing
symptoms to Long COVID is complicated by the fact that COVID-19 in children may be missed
due to milder or more general symptoms that could be overlooked. Studies have shown that
Long COVID can have a negative effect on quality of life for children and adolescents by limiting
physical activity and creating feelings of distress about symptoms, behavioral health challenges,
and decreased school attendance and participation. Improved study design and methods are
needed to better address these challenges.
25-28
Principal Research Initiatives
Two studies are expected to provide some data on developmental outcomes and impact on
education. The National Survey of Children’s Health Longitudinal Cohort Study (NSCH-LC)
collects a wide range of outcomes from parents and caregivers of children who were ages 0 to
17 years from 2018 to 2019, including development, education, and behavioral concerns. The
Pediatric COVID Outcomes Study (PECOS) will enroll up to 1,000 children and young adults in a
study from Children’s National Hospital in collaboration with the National Institute of Allergy
and Infectious Diseases (NIAID) to examine the longer-term effects of COVID-19 and MIS-C after
these patients have recovered from a COVID-19 infection. Outcomes assessed include social
impacts for participants, families, and caregivers.
29
Disability
The Social Security Administration (SSA) Office of Disability Policy is collecting information on
the effects of COVID-19 and resulting Long COVID through review of disability claims with
reports of COVID-19 and Long COVID, and research on the effects of Long COVID, primarily
through a contract with the National Academies of Science, Engineering, and Medicine
(NASEM), Health and Medicine Division (HMD) to support a Consensus Committee on the Long-
Term Functional Effects of COVID-19. This effort will examine the relationship between Long
COVID and Social Security’s definition of disability, convening a consensus committee to study
Long COVID following an HMD workshop in March 2022. This workshop discussed disparate
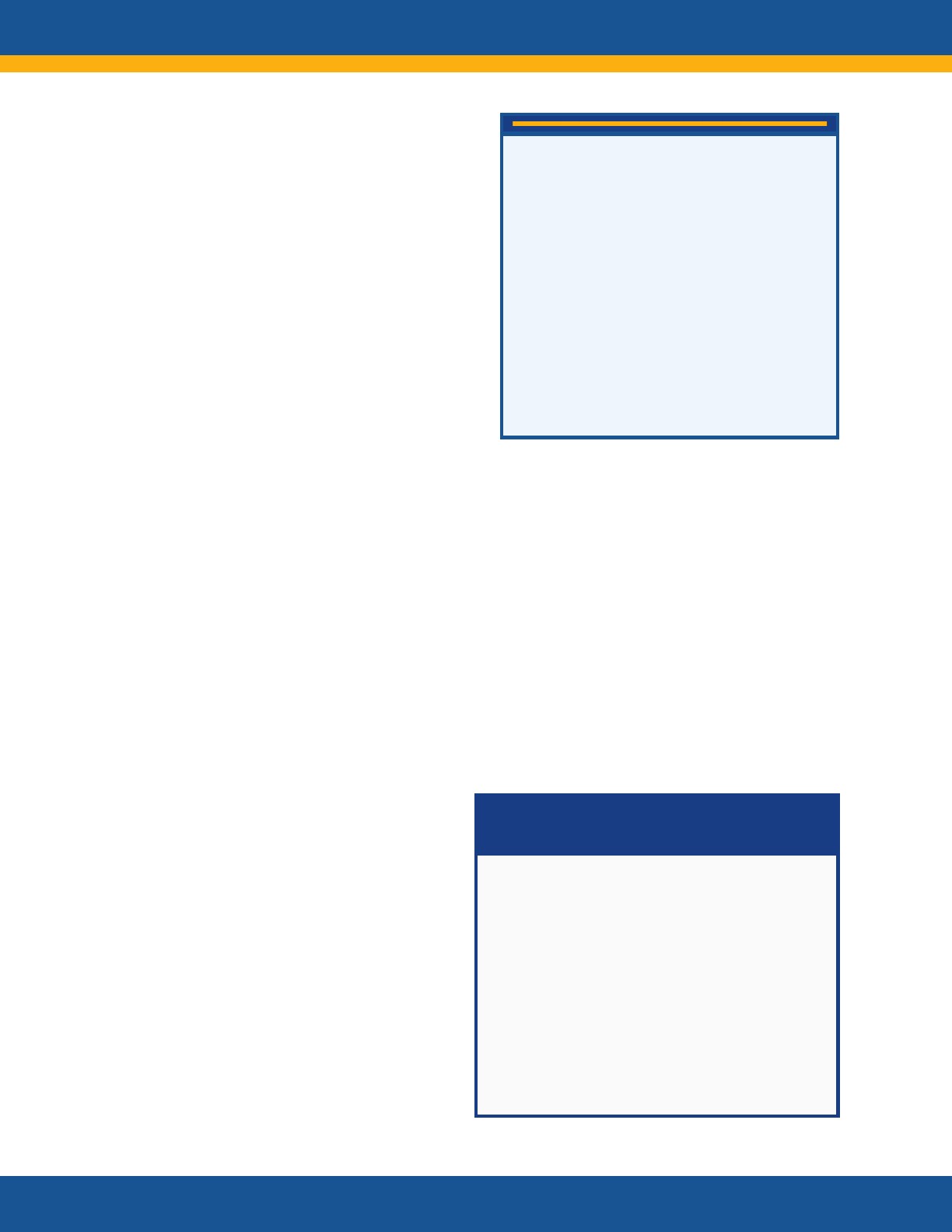
46
National Research Action Plan on Long COVID
impacts of the virus experienced by people
facing barriers and the effects on work-related
functioning due to Long COVID. The Consensus
Committee will study and provide information
about similar topics.
The National Institute on Disability, Independent
Living, and Rehabilitation Research (NIDILRR)
within the Administration for Community Living
is supporting a five-year research grant
examining employment among people with
physical disabilities, including a small qualitative
study of how Long COVID has affected return-to-
work (RTW) efforts for people with disabilities.
NIDLRR also supports a three-year project on
COVID-19 pandemic-related experiences of working-age adults with disabilities. Data collection
launched May, 2022 and includes a newly developed supplement on Long-COVID.
Impact on Caregivers and Family Well-Being
Data are similarly limited on impacts of Long COVID on caregivers and family well-being. The
two studies of children noted earlier, The National Survey of Children’s Health Longitudinal
Cohort Study (NSCH-LC) and the Pediatric COVID Outcomes Study (PECOS), are collecting
information on families and caregivers. The NSCH-LC will also collect family outcomes for
parents of children with Long COVID, including food security, housing, employment, and mental
health of caregivers, but at this time there are no other U.S. government studies focused on
family and caregivers for affected adults.
The companion report to this Plan, the Services and Supports for Longer-Term Impacts of
COVID-19, provides links to U.S. government programs currently available to support those
impacted by Long COVID.
5. Therapeutics and Other
Health Interventions
With potentially millions of Americans
experiencing Long COVID, the development
and testing of strategies to prevent and treat
Long COVID are public health priorities. A
challenge of Long COVID is that it is likely
multiple conditions with a wide range of
symptoms and health effects. Therefore, it is
necessary to test and develop a range of
intervention and prevention strategies to
"Although the pandemic has
highlighted the importance of data,
this is not a new issue. The solutions
are not technically hard, but the
administrative and political complexity
and its resource intensive nature are
formidable. Continued support for
comprehensive, national, public
health-driven data modernization
efforts are needed."
—Hospital Chief Informatics Officer
Core Research Questions
• What treatments are safe and effective
for persons diagnosed with Long
COVID? For which persons are they
effective?
• Which treatments are ineffective or
unsafe?
• What strategies will effectively prevent
Long COVID?
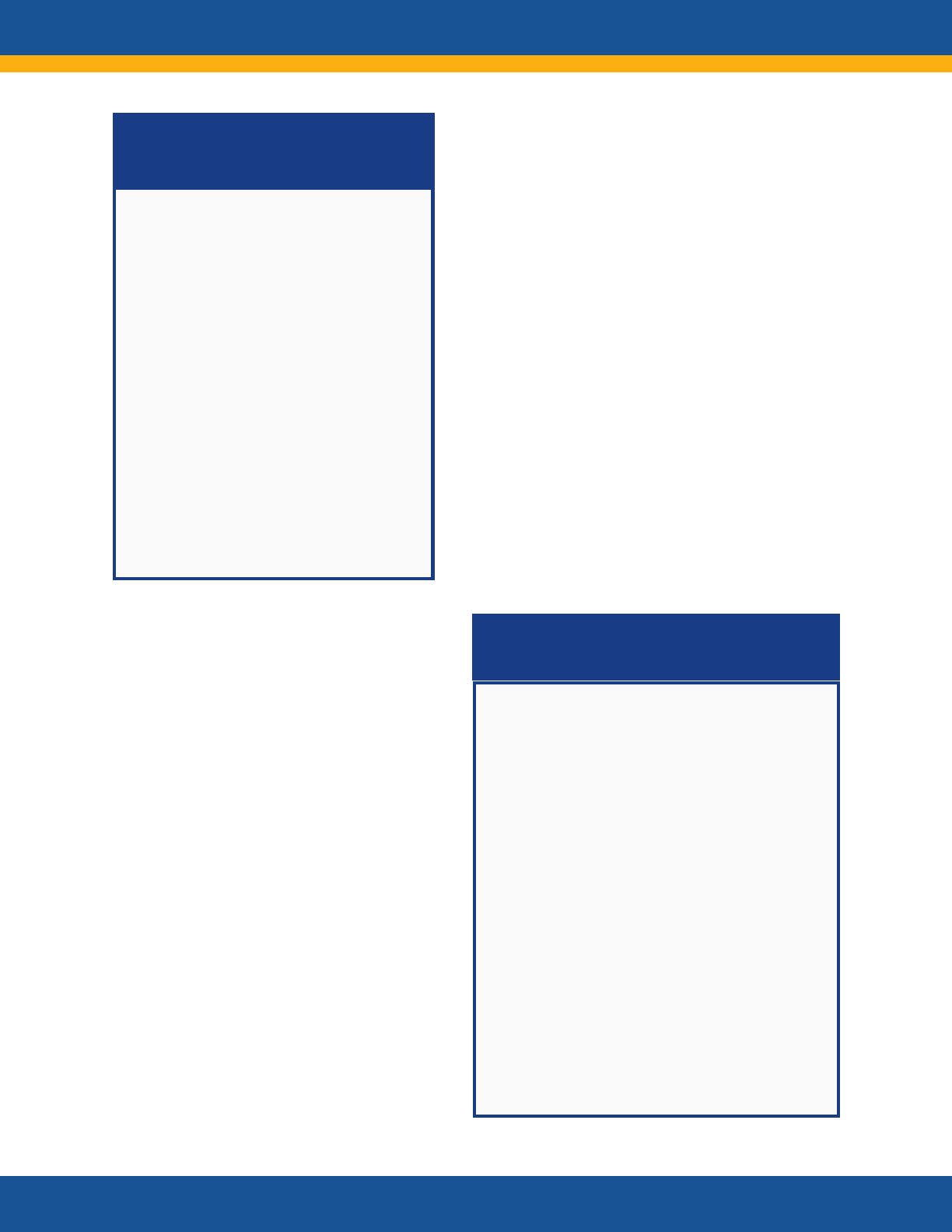
47
National Research Action Plan on Long COVID
address the various symptom clusters and
underlying mechanisms of pathobiology and
improve functioning and quality of life for persons
with Long COVID.
It is critically important that
clinical trials are well-designed and have the scale
and power necessary to generate clear evidence
regarding the safety and effectiveness of strategies
for treating or preventing Long COVID. This will be
especially challenging due to the various
definitions of Long COVID in use and lack of
agreed-upon outcome measures for assessing
meaningful improvements.
Principal Research Initiatives
A major component of the U.S. government efforts
in therapeutics research is the RECOVER Initiative.
In April 2022, NIH issued two research opportunity
announcements: one for a clinical trials data
coordinating center to provide overall project
coordination and support and comprehensive data
management; and the other to solicit trial proposals to identify safe and effective treatments
and preventive strategies for Long COVID in
those ages 18 years and over. The research is
anticipated to begin by the end of 2022. The
focus and design of RECOVER clinical trials
are being informed through a consultative
process that engages patients, clinical
practitioners, and experts from academia
and industry regarding symptom clusters,
outcome measures, and interventions. As is
the case with RECOVER longitudinal
observation studies, RECOVER clinical trials
will include diverse communities and
populations. As our understanding of the
underlying mechanisms of Long COVID
improves through basic and pre-clinical
research, additional candidate interventions
will be evaluated and prioritized for testing.
Two federal initiatives will provide
opportunities to study interventions for Long
COVID in real-world settings. The VA is in the
process of establishing a Long COVID Practice
Health Equity Considerations
Inclusive and diverse participation in
clinical trials, including those typically
underrepresented in biomedical
research and those disproportionately
affected by SARS-CoV-2 infection,
helps ensure that the results of the
research are applicable to the wide
range of populations affected.
Ensuring that interventions for Long
COVID are effective and accessible for
diverse populations enhances future
uptake and effectiveness. This
includes language access and
culturally competent care.
Anticipated Impacts of Research
Information from Long COVID clinical trials
will be important for patients, caregivers,
practitioners, third party insurers, clinical
care guideline developers, and policy
makers.
Information will be shared through
publications and professional meetings,
webinars, and government websites.
Interventions are needed to improve
functioning and quality of life for persons
experiencing Long COVID.
Evidence regarding effective therapies is
needed to inform guidelines for health care
and payers.
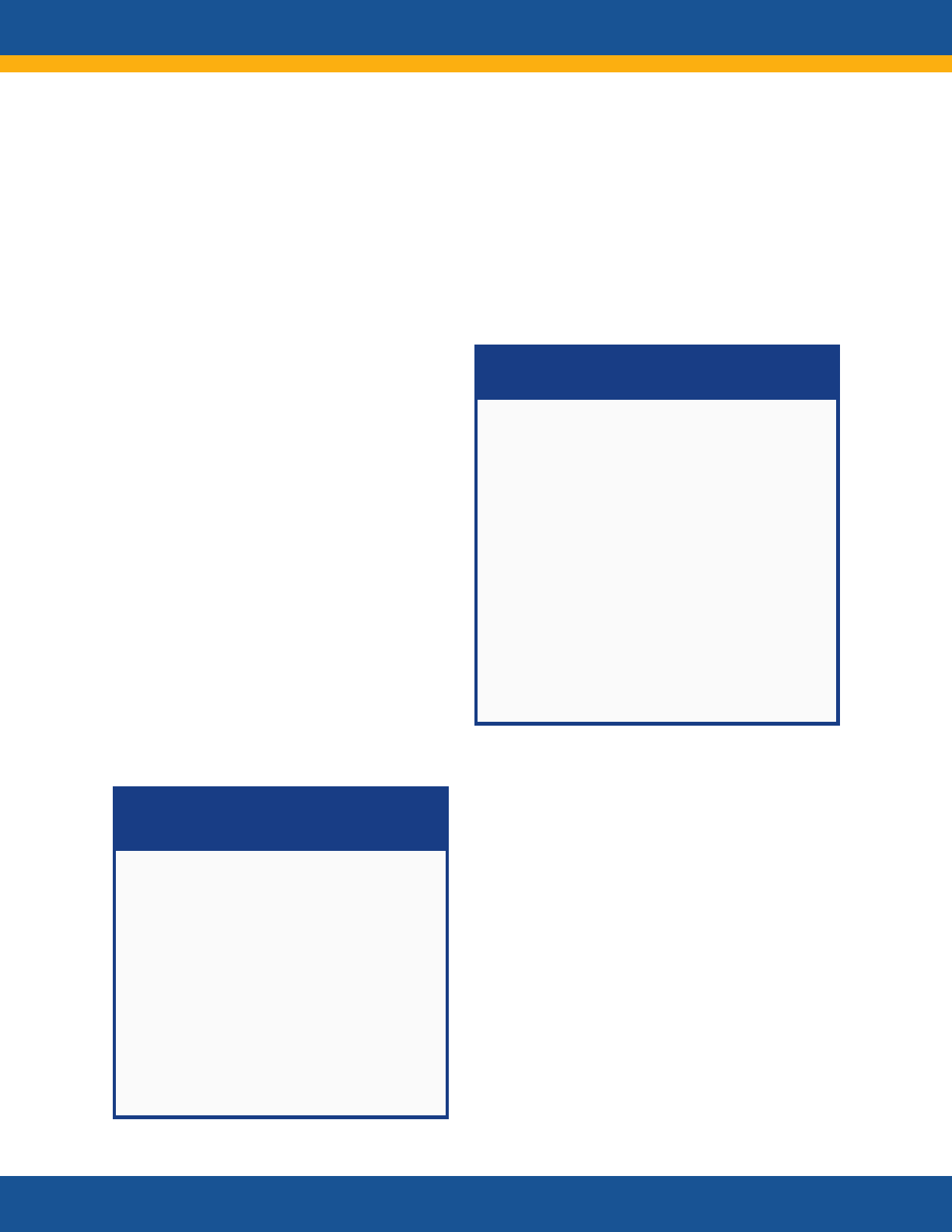
48
National Research Action Plan on Long COVID
Based Research Network to coordinate research in the growing number of VA Long COVID
clinics (currently 22 and growing). This will allow real-world examination of what interventions
patients are undergoing (including self-administered treatments such as supplements) and
whether there are any early signals of effectiveness on safety. In addition, DOD’s EPICC
observational study aims to address numerous gaps in the current understanding of COVID-19
and support the development of treatment and prevention strategies that will benefit
uniformed service personnel and civilian populations.
6. Human Services, Supports, and Interventions
Long COVID impacts a person's ability to
participate fully in society and introduces
complex needs and challenges beyond
individual or community health. The
accompanying Services Report outlines the
services and supports available to individuals
experiencing Long COVID, health care
personnel who work with and treat
individuals experiencing Long COVID,
individuals experiencing longer-term impacts
of COVID-19, including mental health
conditions and substance use challenges, and
individuals dealing with losing a family
member or loved one to COVID-19. In
addition to understanding the landscape of
federally supported services and
mechanisms, researchers must also lay the
groundwork to systematically collect data on the human services, supports, and interventions
to address the unmet needs of individuals with Long COVID. Conceptualizing disability in Long
COVID is essential for this research agenda.
Disability and rehabilitation researchers and
practitioners and individuals with lived
experience are positioned to inform a research
agenda and lead future research on the human
services, supports, and interventions for Long
COVID.
Principal Research Initiatives
Federal agencies have initiated data collection
that may address some domains of the human
services, supports, and interventions for Long
COVID. The only published work thus far has
been related to return to work needs for
Core Research Questions
• What are the human services and
supports needs of individuals with Long
COVID?
• What are the service delivery gaps or
barriers for individuals with Long COVID
in existing human services?
• What interventions can be adapted or
created to meet the needs of individuals
with Long COVID to promote their full
participation in society?
Health Equity Considerations
Individuals may have a disability due to
Long COVID or have a pre-existing
disability in addition to Long COVID.
Individuals from underserved
populations may experience
multifaceted systemic barriers to
accessing human services, supports and
interventions for Long COVID.
49
National Research Action Plan on Long COVID
individuals with Long COVID in a single small qualitative study of rehabilitation professionals on
how Long COVID has affected return-to-work efforts for people with a disability, funded by the
NIDILRR in the Administration for Community Living (ACL). In the area of understanding the
human services and supports need, several efforts are underway by ACL, DOL, and SSA. These
will provide information from national survey data on the experiences of working-age adults
with disabilities and Long COVID as well as information on participants in the RETAIN initiative.
30
The DOL is also conducting internal synthesized literature reviews of existing research on
COVID-19 and Long COVID and developing resources for employers on interventions to meet
the needs of persons with Long COVID including a forthcoming guide for employers to support
individuals with Long COVID. Together this body of work will drive the emerging evidence-base
on the impacts of Long COVID on an individual’s ability to work, the needs and challenges
relating to employment services and supports including disability benefits, and information on
potential interventions to maintain employment. Multiple years are likely needed to produce
and disseminate high-quality, rigorous research.
NIDILRR is funding two grantees collecting data
on Long COVID as part of their larger data
collection efforts. The Rehabilitation Research
and Training Center (RRTC) on Employment for
People with Physical Disabilities completed a
small qualitative study with rehabilitation
professionals on how Long COVID has affected
return-to-work efforts for people with
disabilities. Research participants discussed
challenges in working with “long-haulers” and
recommendations included modifying workplace
policies and providing individualized workplace accommodations. The University of Kansas
Center for Research is administering another iteration of the National Survey on Health and
Disability to document the COVID-19 pandemic-related experiences of working-age adults with
disabilities. This survey includes a Long COVID supplement. Data are being collected from May
to September 2022.
30
Several agencies and programs within the DOL are collecting data on Long COVID through
discretionary grants and other existing data collection mechanisms. The Office of Disability
Employment Policy (ODEP) and the Employment and Training Administration in DOL and
the SSA jointly administer the Retaining Employment and Talent after Injury/Illness Network
(RETAIN) initiative. RETAIN programs in five states (Kansas, Kentucky, Minnesota, Ohio,
Vermont) target individuals with work-related or non-work-related injuries and illnesses,
including Long COVID, who are employed or, at a minimum, in the labor force when the injury
or illness occurs. ODEP collects a comprehensive set of administrative data from each of the
RETAIN states that includes information on individuals’ primary and secondary injury/illness
ICD-10-CM codes (including the ICD-10-CM code for post-COVID-19 conditions), demographics,
socioeconomic indicators, program characteristics and employment outcomes. This data
“Enrollment in Long COVID studies are
often limited to English and Spanish
speakers. Asian American, Native
Hawaiian, and Pacific Islander
communities speak over 100 different
languages. We want to be involved
too.”
—Person with Long COVID
50
National Research Action Plan on Long COVID
collection is underway. Also, ODEP’s Employer Assistance and Resource Network on Disability
Inclusion is conducting meta-analyses of existing research on COVID-19 and Long COVID and
developing resources for employers on how to support individuals with these conditions.
As noted previously, the SSA sponsored the Health and Medicine Division of the National
Academies of Sciences, Medicine, and Engineering (HMD/NASEM) to conduct a public
workshop on the Long-Term Health Effects from COVID-19 and Implications for the SSA in
March 2022. A proceeding of the
presentations and discussions at the
workshop will be prepared.
HHS’s Office of the Assistant Secretary for
Health is sponsoring a human-centered
research project to better understand Long
COVID, termed Health+. The approach works
directly with patients experiencing Long
COVID and associated conditions, caregivers,
frontline workers, and those with lived
experience. The goal of Health+ Long COVID
is to generate insights with actionable
opportunity areas to improve the quality of
care and life.
7. Health Services and
Health Economics
Research
As the world learns more about Long COVID
and discovers ways to diagnose, treat, and
manage its effects, it is also important to
learn how best to provide person-centered
health care for people living with Long COVID
in the context of co-existing illness and risk
factors including social risks. The science of
how care is delivered and how care delivery
can be improved is called health services
research, including mathematical modeling.
Health services research will help the nation
improve the quality, safety, patient-
centeredness, and value of Long COVID care.
It will also allow us to understand if that care
is accessible to all who need it and if not how
to expand access and ensure equity.
Core Research Questions
• What is the current level of access to
care for persons with Long COVID?
• What are the barriers to care for
persons with Long COVID?
• How can the quality of care for persons
with Long COVID be improved?
• What are the best models of care for
Long COVID, and how can those models
be more widely adopted?
• How do we leverage primary care to
diagnose and manage the care of
people with Long COVID in collaborative
care models that provide effective
access to needed specialty services?
• What are the impacts of Long COVID on
health care utilization?
• What are the medical costs of Long
COVID for individuals, families,
communities, the health care system,
states, and the nation?
• How can we improve the financing of
Long COVID care in the United States.?
• What are the broader economic impacts
of Long COVID?
51
National Research Action Plan on Long COVID
For evidence-based health policy, decision
makers must resolve outstanding questions
including: which health services are needed, who
receives Long COVID health care services, how
the services will be financed, and the total cost of
the care? Health economics research can help
address these questions.
Access to Care, Models of Care,
Quality of Care
The U.S. government will do more to examine
access and barriers to care, models of care, and
quality of care for persons with Long COVID.
Health care services include access to acute care,
outpatient services, behavioral health, and
supportive services.
Principal Research Initiatives
The VA has led the nation in thinking
systematically about how best to provide
coordinated care for Veterans living with Long
COVID, which is helping to inform how to treat
the general public. The VA has conducted a
baseline assessment of the Long COVID-19 care
delivery models currently used in different
regions and facilities across the country. Their
initial and on-going evaluations of Long COVID
care delivery models, including the use of Long
COVID multispecialty clinics, may provide valuable lessons for the rest of the nation. With the
establishment of a Long COVID Practice Based Research Network, the Veterans Health
Administration (VHA) will begin to compare the outcomes of different care models within and
between clinics.
CDC is funding the Long COVID and Fatiguing Illness Recovery Program, which aims to improve
the health of patients in underserved communities who have Long COVID and other complex
chronic conditions with similar symptoms, such as myalgic encephalomyelitis and chronic
fatigue syndrome (ME/CFS) and other post-infectious fatiguing illnesses. Embedded in the
program is a randomized trial of online educational interventions for primary care providers in a
Federally Qualified Health Center, to support their management of long COVID and associated
conditions. The Long COVID and Fatiguing Illness Recovery Program will provide guidance on
how online participatory learning and mentoring can make primary care more effective in
treating Long COVID and accelerate the delivery of evidence-based care
Health Equity Considerations
As in all forms of health research, it is
critical to consider equity when working
to understand health care access,
barriers to care, effective models of
care delivery, and the quality and safety
of care. Analysis of health care use,
medical costs, and broader economic
costs must also consider domains of
equity.
Individuals who are medically
underserved and historically
marginalized communities whose
perspectives and needs must be
included in health services and
economics research include but are not
limited to racial and ethnic minority
groups, people living in rural areas,
people with disabilities, people with
limited English proficiency and LGBTQ+
people. In addition, research must
consider Long COVID care for people of
all age groups, people who are or who
want to become pregnant, and all
genders.
52
National Research Action Plan on Long COVID
CDC is conducting an on-line needs assessment of health care professionals to identify
information gaps around Long COVID. This information will be used to inform educational and
outreach materials for health care professionals.
AHRQ, along with other HHS and other
government agencies, is leveraging
health information technology to collect
real-world data to better understand
the impact of Long COVID on health
outcomes in diverse populations. AHRQ
in partnership with NIDDK is developing
and testing three open-source,
Substitutable Medical Applications,
Reusable Technologies (SMART®) on
Fast Health care Interoperability
Resources (FHIR®) electronic care (e-
care) plan applications for clinicians,
patients, and caregivers that include
standardized data elements to study
and manage Long COVID. AHRQ is also
developing living clinical decision
support to build the capacity to readily
bring evolving evidence on Long COVID
to the point of care. This living CDS will
be freely available on AHRQ’s CDS
Connect web-based platform.
Testing and Laboratory
Capability
Laboratory testing in clinical medicine
(for diagnostic purposes) and public
health (for example, needed for
screening and surveillance) is critically
important to render care to patients
and to guide interventions to
communities. As highlighted by the
pandemic, testing outside of the
laboratory in other clinical venues,
pharmacies, and in homes
complements laboratory-based testing
by enhancing access, efficiency, and
speed. Systems and operational
Anticipated Impacts of Research
•
The results of health services research are
relevant to health care professionals, public
and private health care delivery
organizations, payers, public health, and
health services researchers
• Information from this research will be
important for the growing number of
individuals and caregivers seeking to make
informed decisions about services
• Information will be shared through
publications, professional meetings,
professional associations, webinars, and
government websites
• When implemented, the findings from health
services research will guide the expansion of
equitable access to effective, safe, high-
quality, high-value, and patient- and family-
centered Long COVID care
• Care will be inclusive of rehabilitation and
therapies to ensure full participation in
society. Tremendous potential exists for this
research to prevent further marginalization
and lead to more equitable outcomes and
societal participation by individuals who
experience Long COVID
• The findings from health economics research
will allow local, state, and federal policy
makers to make informed decisions about
financing Long COVID care and ensuring
effective support for individuals, families, and
communities with Long COVID

53
National Research Action Plan on Long COVID
research are needed to ensure testing is
available in a timely fashion, that it generates
accurate results based on sufficient and
optimized test performance, and that it delivers
those results primed to aid decision making for
the patient and public health purposes. The
pandemic highlighted some previously
unanticipated systems research questions in
manufacturing, supply chain, test performance
(e.g., balancing test accuracy to diagnosis
infection vs. the need to determine
contagiousness), results reporting, and
integration of data across systems.
Health Care Utilization, Medical Costs, Financing Models, and Broader
Economic Impacts
Although there is ongoing research on the economic impacts of COVID-19, much work remains
to examine health care use, medical costs, options for financing Long COVID care, and the
broader economic impacts of Long COVID. As data and coding systems advance, many types of
health economics research will become easier and more accurate.
Principal Research Initiatives
CMS, in partnership with the HHS Office of the Assistant Secretary for Planning and Evaluation
are conducting analyses to document health services use by Medicare beneficiaries who have
been diagnosed with COVID-19.
Prior to the pandemic, the Health Resources and Services Administration (HRSA) began a
longitudinal, observational cohort study that enrolled approximately 60,000 children and their
families. By collecting information on COVID-19 infections and the emergence of Long COVID in
these families, along with information about health care use, health care expenses, and
experience of care, this study may provide some important information on pediatric health care
utilization for Long COVID, costs to families, and the accessibility and experience of Long COVID
care for families.
The VA is conducting a large, population-based study of the longer-term impacts of COVID-19
using a group of matched cases and controls to examine a range of clinical outcomes, health
care utilization, and health care costs. Although not focused on Long COVID, the study will
permit comparison of a subset with longer-term symptoms to patients with resolved symptoms
and controls who were never infected. VHA is also establishing a practice-based research
network involving Long COVID clinics (currently 22 in the VA) that will collect data on the care
received and costs of patients presenting with Long COVID symptoms.
“Where should you tell someone with
Long COVID to go? What if they don’t
have a doctor that they regularly see?
What if they don’t have health
insurance? Even when there is a Long
COVID expert in the area, there is a
wait time of at least four months to be
seen.”
—Community Health Care Worker
54
National Research Action Plan on Long COVID
References
1. Thomason ME, Werchan D, Hendrix CL. COVID-19 patient accounts of illness severity,
treatments and lasting symptoms. Sci Data. 2022;9(1):2. Published January 10, 2022.
doi:10.1038/s41597-021-01103-6
2. Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: A
machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532-e541.
doi:10.1016/S2589-7500(22)00048-6
3. Wanga V, Chevinsky JR, Dimitrov LV, et al. Long-term symptoms among adults tested for
SARS-CoV-2 — United States, January 2020–April 2021. MMWR Morb Mortal Wkly Rep.
2021;70:1235–1241. DOI: http://dx.doi.org/10.15585/mmwr.mm7036a1.
4. Hernandez-Romieu AC, Leung S, Mbanya A, et al. Health care utilization and clinical
characteristics of nonhospitalized adults in an integrated health care system 28–180
days after COVID-19 diagnosis — Georgia, May 2020–March 2021. MMWR Morb Mortal
Wkly Rep. 2021;70:644-650. DOI: http://dx.doi.org/10.15585/mmwr.mm7017e3.
5. Chevinsky JR, Tao G, Lavery AM et al. Late conditions diagnosed 1–4 months following
an initial coronavirus disease 2019 (COVID-19) encounter: A matched-cohort study using
inpatient and outpatient administrative data—United States, 1 March–30 June 2020.
Clinical Infectious Diseases. 2021;73(1):S5–S16. https://doi.org/10.1093/cid/ciab338.
6. Centers for Disease Control and Prevention. Updated guidelines for evaluating public
health surveillance systems: Recommendations from the working group. MMWR Morb
Mortal Wkly Rep. 2001;50:1-36.
https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5013a1.htm
7. Centers for Disease Control and Prevention. Updated Guidelines for Evaluating Public
Health Surveillance Systems: Recommendations from Guidelines Working Group.
MMWR. 2001;50(RR 13):1-35. https://www.cdc.gov/mmwr/pdf/rr/rr5013.pdf
8. Centers for Disease Control and Prevention. CDCs vision for public health surveillance in
the 21st Century. MMWR. 2012;61(Suppl):1-44.
https://www.cdc.gov/mmwr/pdf/other/su6103.pdf
9. Saydah S, Brooks J, Jackson B. Surveillance for post-COVID conditions is necessary:
Addressing the challenges with multiple approaches. J Gen Intern Med. 2022;37:1786-
1788. https://doi.org/10.1007/s11606-022-07446-z
10. Centers for Disease Control and Prevention. Long COVID: Household pulse survey.
Published June 22, 2022. Accessed July 22, 2022.
https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm.
55
National Research Action Plan on Long COVID
11. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of
COVID-19. Nature. 2021;594:259-264.
12. U.S. Department of Veterans Affairs. VA.gov | Veterans Affairs. Seattle Epidemiologic
Research and Information Center (ERIC). Accessed July 22, 2022.
https://www.seattle.eric.research.va.gov/research/CSP-2028-EPIC3/home.asp
13. Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of
COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw
Open. 2021;4(9):e2127403. doi:10.1001/jamanetworkopen.2021.27403
14. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-
occurrence, and evolution of long-COVID features: A 6-month retrospective cohort
study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773.
https://doi.org/10.1371/journal.pmed.1003773
15. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med.
2022. https://doi.org/10.1038/s41591-022-01840-0
16. Mohr NM, Plumb ID, Harland KK, et al. Presence of symptoms 6 weeks after COVID-19
among vaccinated and unvaccinated U.S. health care personnel. medRxiv: Pre-Print.
2022. https://doi.org/10.1101/2022.02.16.22271092
17. UK Health Security Agency. The effectiveness of vaccination against long COVID: A rapid
evidence briefing. Published February, 2022. Accessed June 21, 2022.
https://ukhsa.koha-ptfs.co.uk/cgi-bin/koha/opac-retrieve-
file.pl?id=fe4f10cd3cd509fe045ad4f72ae0dfff
18. Zisis S, Durieux J, Mouchati C, Perez J, McComsey G. The protective effect of COVID-19
vaccination on post-acute sequelae of COVID-19 (PASC): A multicenter study from a
large national health research network. Open Forum Infect Dis.
2022;fac228. https://doi.org/10.1093/ofid/ofac228
19. Centers for Disease Control and Prevention (CDC). COVID-19 Vaccine Effectiveness. CDC
Website. Updated December 23, 2021. Accessed July 20, 2022.
https://www.cdc.gov/coronavirus/2019-
ncov/vaccines/effectiveness/index.html?msclkid=8c0ea00cd10d11ecadbf4a719825d1a0
20. Pfaff ER, Girvin AT, Bennett TD, et al. Who has long-COVID? A big data approach.
MedRxiv. 2021;10.18.21265168. DOI: https://doi.org/10.1101/2021.10.18.21265168
21. Pfaff ER, Madlock-Brown C, Baratta JM, et al. Coding Long COVID: Characterizing a new
disease through an ICD-10 lens. MedRxiv. 2022.04.18.22273968. DOI:
https://doi.org/10.1101/2022.04.18.22273968
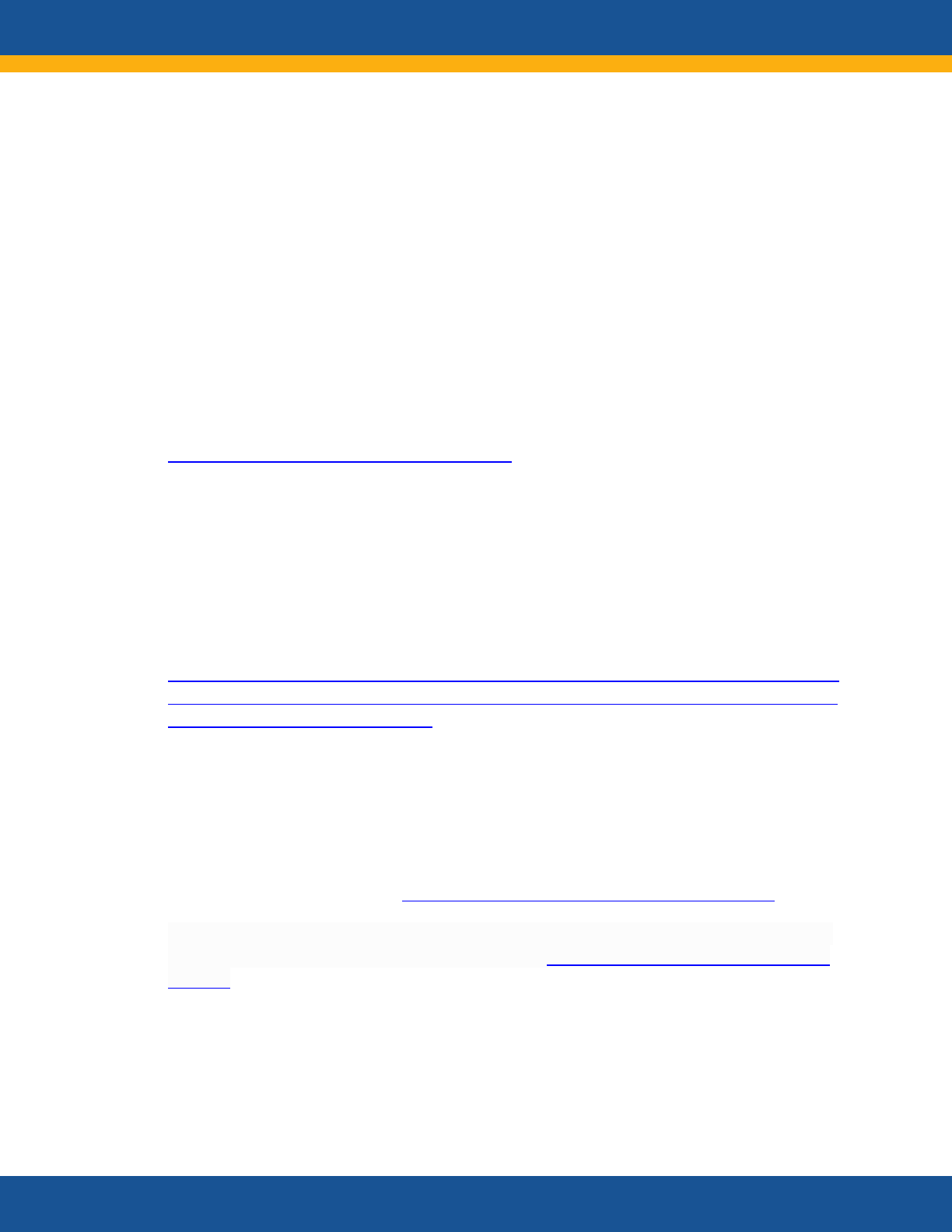
56
National Research Action Plan on Long COVID
22. Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2
infection in children and adolescents [published online ahead of print, 2021 Jul
15]. JAMA. 2021; 326(9):869-871. doi:10.1001/jama.2021.11880
23. Malik P, Patel K, Pinto C, et al. Post-acute COVID-19 syndrome (PCS) and health-related
quality of life (HRQoL)—A systematic review and meta-analysis. J Med Virol. 2022;
Jan;94(1):253-262. DOI: 10.1002/jmv.27309
24. Osmanov IM, Spiridonova E, Bobkova P, et al. Risk factors for post-COVID-19 condition
in previously hospitalised children using the ISARIC Global follow-up protocol: a
prospective cohort study. Eur Respir J. 2022 Feb 3;59(2):2101341. DOI:
10.1183/13993003.01341-2021
25. Borch L, Holm M, Knudsen M et al. Long COVID symptoms and duration in SARS-CoV-2
positive children—a nationwide cohort study. Eur J Pediatr. 2022;181:1597–1607.
https://doi.org/10.1007/s00431-021-04345-z.
26. Berg SK, Palm P, Nygaard U, et al. Long COVID symptoms in SARS-CoV-2-positive
children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): A
national, cross-sectional study. The Lancet. 2022. https://doi.org/10.1016/ S2352-
4642(22)00154-7.
27. Office for National Statistics. COVID-19 Schools Infection Survey, England: mental health
and long COVID, November to December 2021. Updated February 28, 2022. Accessed
June 15, 2022.
https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditio
nsanddiseases/bulletins/covid19schoolsinfectionsurveyengland/mentalhealthandlongco
vidnovembertodecember2021#toc.
28. Hernandez-Romieu AC, Carton TW, Saydah S, et al. Prevalence of select new symptoms
and conditions among persons aged younger than 20 years and 20 years or older at 31
to 150 days after testing positive or negative for SARS-CoV-2. JAMA Netw Open.
2022;5(2):e2147053. Published 2022 Feb 1. doi:10.1001/jamanetworkopen.2021.47053.
29. National Institute of Health. Pediatric COVID Outcomes Study. Updated January 14,
2022. Accessed June 15, 2022. https://clinicaltrials.gov/ct2/show/NCT04830852
30. Wong J, Ezeife N, Kudla A et al. Employment consequences of COVID-19 for people with
disabilities and employers. J Occup Rehabil. 2022. https://doi.org/10.1007/s10926-021-
10012-9

57
National Research Action Plan on Long COVID
Chapter 4: Accelerating Research and
Innovation in Long COVID
The end of the declared Public Health Emergency will not signal the end of the COVID-19
pandemic. Long after the more immediate effects of the pandemic, the long-term impacts on
the health of the nation will continue for years to come. The scale of Long COVID morbidity and
the breadth of its clinical manifestations represent an unprecedented, but not insurmountable,
challenge.
1
Federal agencies have already launched ambitious and significant research efforts to
understand Long COVID and ameliorate its devastating impacts, as demonstrated in Chapter 3
and Appendix D. The current Long COVID research enterprise demonstrates solid scientific
foundations, dedicated researchers, and sound partnerships. However, we acknowledge that
conventional research endeavors are not sufficient to address the serious and urgent
challenges posed by post-infectious illnesses of pandemic proportion. The current lag in
research being translated into practice is simply not acceptable in the current environment. We
must aggressively innovate how we do research and accelerate the pace of research to meet
the challenge of the moment. We must collaborate with private research entities and the
pharmaceutical industry to focus our efforts on the highest priority research questions. We
need to rethink how we disseminate research and translate findings into practical solutions
more quickly.
Long COVID demands a comprehensive, multi-disciplinary, effective approach, leveraging the
resources of the U.S. research enterprise and collaborative efforts across the U.S. government
coupled with strong public and private partnership. Beyond the U.S. research enterprise, the
global health research community and international health organizations play an important
role. This chapter is outlined in three sections. The first section, Research Priorities, describes
priority research areas for both public and private research, including academia. The second
section, Strategic Actions, describes actions that the U.S. government will take to not only drive
public-sector research but also accelerate private-sector research, through funding,
collaboration, and data sharing; disseminate research; and translate research into policy and
program. The final section describes Implementation.
Research Priorities for Long COVID
The following are the research questions and topics that we deemed to be of highest
importance and with the greatest potential to yield early, safe, and effective benefit for persons
with Long COVID. This Plan is a call to action to enhance research efforts as well as to rapidly
deliver evidence and data in these areas. Addressing the challenge of Long COVID and the most
pressing research priorities will require an urgent and coordinated all-hands-on-deck whole-of-
government approach, including robust public-private partnership. Research questions that can

58
National Research Action Plan on Long COVID
be investigated with current funding are underway. Additional federal investment will be
necessary in fiscal year 2023 and beyond as well as commitment from partners in the private
sphere include academia, the pharmaceutical industry, private research organizations, public
health entities, and medical professional organizations. To most efficiently and effectively
accelerate research on Long COVID, it is particularly important to leverage knowledge and
expertise (of affected individuals and researchers) gained from research of conditions with
similarities to Long COVID including dysautonomia, myalgic encephalomyelitis and chronic
fatigue syndrome (ME/CFS), and other post-infectious illnesses.
While this is a call to action to the U.S. research enterprise, this Plan also lays out the U.S.
government commitment in these areas. Appendix E describes new and enhanced activities
that the U.S. government intends to take within the areas, which will build on the portfolio of
ongoing research described in Chapter 3 and Appendix D, and documented in over 100
published U.S. government Long COVID research articles.
1. Characterizing the Full Clinical Spectrum of Long COVID and
Diagnostic Strategies
a. Convening public and private partners to better align interim definitions of Long
COVID for clinical, surveillance, and research
b. Further study of the impact of SARS-CoV-2 infection on development and clinical
course of new onset chronic disease states including diabetes mellitus, kidney
disease, heart disease, gastrointestinal disease, neurologic disease, hematological
(blood) illnesses, and other potential long-term consequences
c. New research that disentangles the broader longer-term effects of the pandemic on
physical and behavioral health (i.e., mental health and substance use challenges)
from those of SARS-CoV-2 infection, for example, distinguishing the neurocognitive
and behavioral effects on children and adolescents of infection with SARS-CoV-2
from the cumulative effects of loss of access to in-person early childhood education
and learning environments
d. Leveraging of existing research on post-infectious chronic illness to support
identification of commonalities and differences with Long COVID, accelerate
research and innovation, as well as prepare for the long-term consequences of
future pandemics
e. Further research to identify clinical screening tools and diagnostic tests and assays

59
National Research Action Plan on Long COVID
2. Pathophysiology
a. Deeper understanding, from the molecular to system levels, of Long COVID within
the broader context of post-infectious chronic conditions and other diseases that
may have infectious origins, including dysautonomia and ME/CFS
3. Surveillance and Epidemiology
a. Coordinated and enhanced surveillance to characterize and understand Long COVID,
the potential health impacts, and provide information for public health and health
care planning
b. Additional studies of Long COVID risk factors, health trajectories and outcomes that
are inclusive of age, gender, race, ethnicity, geographic location, socioeconomic
status, insurance coverage status, pregnancy status, and disability status, enabled by
new capabilities in data analytics
c. Studies that can rapidly adapt to examine risk and protective factors as they emerge,
including new variants, vaccinations, repeat infections, and therapies for COVID-19
on risk of Long COVID
4. Long COVID and Overall Well-Being
a. Comprehensive studies of the effects of Long COVID on educational outcomes in
children and youth, with a focus on vulnerable populations, including racial and
ethnic minority groups, those who are economically disadvantaged, and those in
rural communities
b. Research, including qualitative and mixed methods studies, to understand the
impact of Long COVID on health-related quality of life, behavioral health,
employment, disability determinations, education and development, and the impact
on caregivers and family well-being, especially among disadvantaged groups
5. Therapeutics and Other Health Interventions
a. Expanded and expansive portfolio of studies on the effectiveness of therapeutics to
prevent and treat Long COVID to include antivirals, anti-inflammatory, immune
modulators, and other existing or new treatments, based on molecular level
understanding and disaggregated by age, gender, race, ethnicity, and pregnancy
status
b. Further studies to subtype Long COVID including characterization of distinct sub-
entities with distinct set of risk factors and outcomes and evaluation of treatment
strategies for these sub-types

60
National Research Action Plan on Long COVID
c. New studies of non-pharmacologic interventions to prevent and treat Long COVID
6. Human Services, Supports, and Interventions
a. New work in identifying and evaluating optimal person-centered models of care for
people with various forms of Long COVID in the context of co-existing illness, risk
factors, and social and community circumstances
b. Comprehensive studies of human services and supports interventions, including
disability services and caregiver supports, to ensure individuals living with Long
COVID can fully participate in their communities
7. Health Services and Health Economics Research
a. New studies, including systems research, to develop, implement, and evaluate
models of care delivery to enable primary care providers to effectively manage Long
COVID and associated conditions in partnership with specialty care when needed
and community service organizations
b. Research to address barriers to effective care in underserved communities and
models of care designed to address and eliminate health inequities
c. New work in identifying and evaluating optimal models of care for people with
various forms of Long COVID and associated conditions
d. Rapid and ongoing synthesis of new evidence to distill existing research into
actionable insights guiding care of people with Long COVID, including cost-
effectiveness modeling of prevention and treatment strategies
e. Expanded work in impact of Long COVID on and economic costs of Long COVID to
the health system, human services and supports programs, and society
Strategic U.S. Government Actions for Long COVID
Research
The following are the structures and processes to be created within the U.S. government
specifically to drive the research described above. Innovation and comprehensive approaches
will drive an accelerated and enhanced research portfolio. To address the urgency of this
moment and the monumental challenges of Long COVID, HHS will need to work with private
partners and with Congress to fund and support action on these items.
Establish the HHS Office of Long COVID Research and Practice to lead efforts across the U.S.
government and with private partners. On the basis of the April 5, 2022 Memorandum, the
HHS Secretary directed the Assistant Secretary for Health to lead a U.S. government response,
who, in turn, formed a Long COVID Coordination Council with two workgroups. To formalize
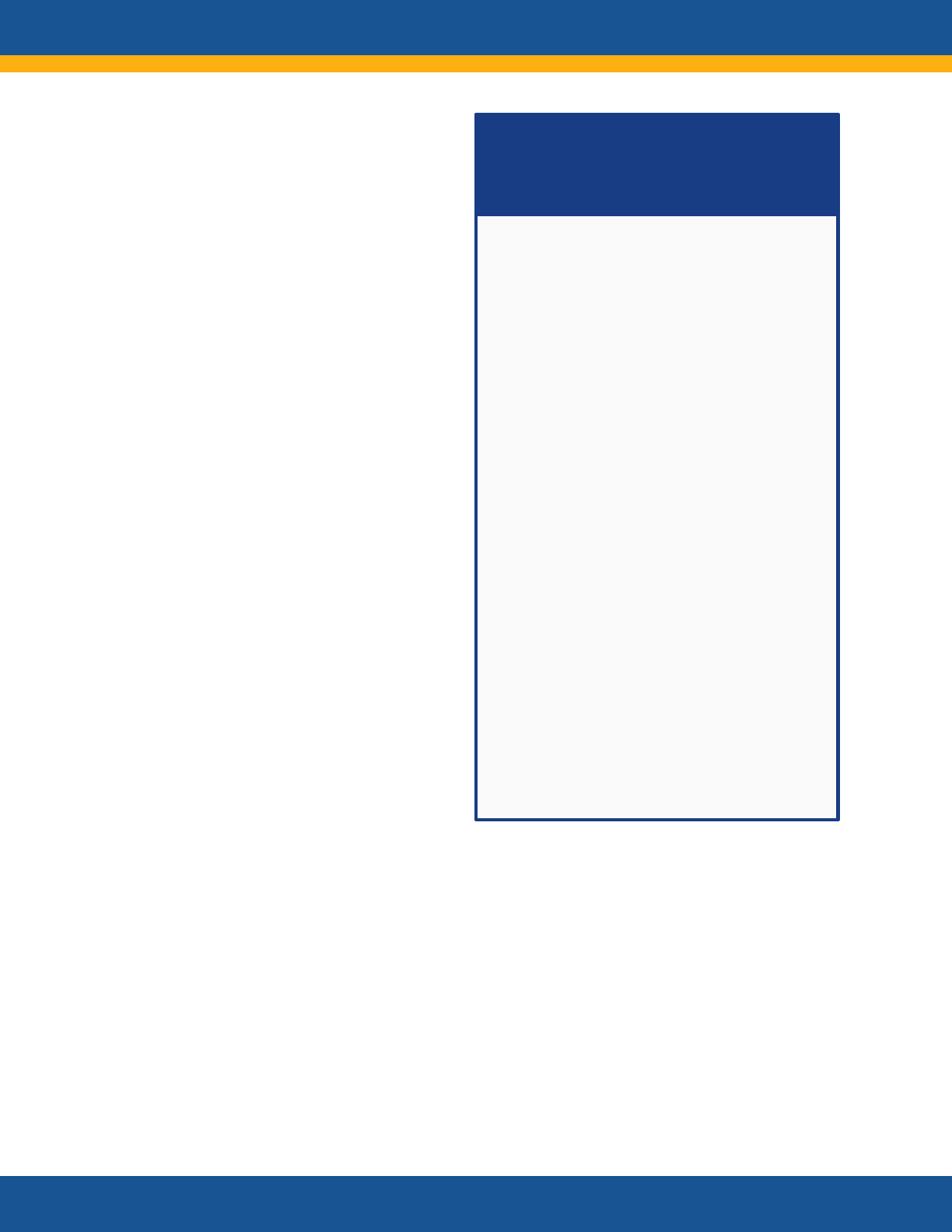
61
National Research Action Plan on Long COVID
and further the work, HHS will transition to
more permanent high-level leadership by
designating the Assistant Secretary as the
Long COVID Coordinator to oversee the
newly created HHS Office of Long COVID
Research and Practice. The Office will be
charged with the implementation of the
National Research Action Plan on Long COVID
and the Services and Supports for Longer-
Term Impacts of COVID-19 Report. The Office
will be responsible to update the Plan on a
regular basis. To stand up and maintain the
Office and ensure the needed long-term
focus on Long COVID dedicated funding for
permanent staff and operations will be
necessary.
The Coordinator will maintain the Long
COVID Coordination Council (established in
May 2022 in response to the Presidential
Memorandum on Addressing the Long-Term
Effects of COVID-19) as a mechanism for
coordination and communication across the
government. The Coordinator will also
establish the Secretary’s Advisory Committee
on Long COVID. The Coordinator and the
Office will establish methods to ensure
standardization and accountability across all
U.S. government conducted or sponsored
research, including the following
Harmonize an approach in U.S.
government-sponsored Long COVID cohort research to include measurement of the
broader impacts of the disease (such as development, education, employment,
disability, and health-related quality of life)
Incorporate the voices of patients and others with lived experience into research from
inception to completion and including dissemination. The Coordinator will
comprehensively place the lived experiences of patients at the core of the research
enterprise to inform development of research priorities, research design, execution,
interpretation, and dissemination. Building on existing model patient engagement
strategies, the Secretary’s Advisory Committee on Long COVID will establish principles
for patient and stakeholder inclusion in research and identify and implement strategies
to address barriers to engagement of underrepresented groups and to prioritize the
Federal Government Efforts That
Will Improve Identification of Long
COVID and Connect Patients to Care
• Convene public and private partners to
better align the definitions of Long
COVID and develop agreed upon
terminology for clinical care,
surveillance, and research
• Develop comprehensive and equitable
Long COVID diagnosis, care, and
treatment guidance
• Make available tools to help individuals
recognize symptoms of Long COVID and
to discuss their symptoms with their
health care providers
• Advance research that helps separate
the broader effects of the pandemic on
an individual’s physical and mental
health from those of the initial SARS-
CoV-2 infection and re-infections
• Use findings from Health+ Long COVID
patient-centered research to improve
the way individuals access and receive
health care and services.

62
National Research Action Plan on Long COVID
voices of patients disproportionately affected by COVID-19 and Long COVID. The
Coordinator will be responsible for implementation and monitoring of these strategies.
Implement strategies to measure and mitigate inequities in Long COVID. This includes
continuing efforts to collect detailed information on age, gender, race, ethnicity,
rurality, economic disadvantage, insurance coverage, pregnancy status, and disability
status in all U.S. government-conducted and sponsored research in Long COVID, in line
with Office of Management and Budget (OMB) and other U.S. government policies, and
ensuring diverse representation in all research activities including clinical trials, with
focused efforts to identify and address barriers to participation. This also includes
ensuring access to U.S. government-funded clinical trials and proposed Centers of
Excellence.
Identify strategic collaborations between public and private entities needed to
accelerate Long COVID diagnostic and therapeutic research and identify and address
barriers to collaborations. Host convenings of research partners, including government,
public health, academia, pharmaceutical industry, national pharmacy chains, and health
systems to initiate collaborations.
Further harmonization of and consensus on clinical care, surveillance, and research
definitions of Long COVID, described above. The definitions will evolve as our
understanding of Long COVID progresses, and while multiple definitions for different
purposes will be needed, developing consensus on evidence-based definitions will
facilitate clinical care, surveillance, and research, including clinical trials, epidemiology,
and health services research.
Translate research into evidence and guidance. Work with partners to rapidly develop
comprehensive and equitable Long COVID diagnosis, care, and treatment guidance, and
compile existing guidance. Develop mechanisms to synthesize new data about therapies
(such as innovative drugs, rehabilitation), models of care, and services and supports so
that national guidelines can be developed and updated quickly.
Develop and implement a strategic communications plan to share findings from Long
COVID research, including evidence-based guidelines, with the broader research
community, including public and private research institutions, federal and state policy
makers, health care, rehabilitation, payers, industry, patients, care givers, and
advocates. An accelerated phase of generating knowledge must be accompanied by an
intentional strategy to share information in rapid fashion, combat misinformation,
generate and maintain momentum in implementing a shared research agenda, promote
stakeholder engagement, and facilitate translation of evidence into practice. The
communications plan will include strategies to ensure materials are culturally and
linguistically effective and include digital resources, such as a public reporting portal and
website. Simultaneously, develop and implement a plan specifically for health care
personnel, services and supports providers, and policy makers to drive adoption of

63
National Research Action Plan on Long COVID
effective interventions. Implementation strategies may include expanding and tailoring
existing state-of-the-art tele-mentoring and practice facilitation.
Broadly share U.S. government research on Long COVID. Leverage existing platforms
and resources for clinical trial, genetic data, and literature (e.g., ClinicalTrials.gov,
GenBank, Sequence Read Archive, and PubMed Central) to provide broad access to
information about U.S. Long COVID research and the results as a foundational
component of the communications plan.
Establish a framework for a multipronged approach to Long COVID surveillance. Given the
medical and public health complexity of Long COVID, surveillance is best achieved through
integrated approaches leveraging other data modernization initiatives, which requires
collaboration with public health, health care, and developers of data collection systems. The
approach needs to build on established methods and approaches to implement sustainable
surveillance to monitor incidence and prevalence. Progress will be parallel to other approaches
depending on innovation in electronic health record surveillance and serve as a model for
surveillance of post-infectious sequelae in future pandemics. In the interim, prompt analyses of
varied estimates of incidence, prevalence, and risk factors (including vaccination status) in the
overall U.S. population and in various demographic groups will provide up-to-date estimates of
the burden of Long COVID that can be shared publicly to guide policy-making and resource
allocation Additional funding is being requested in FY23 to support these efforts.
Build on existing programs and infrastructure to improve systems and strategies for rapid
implementation of clinical trials to test existing and new diagnostic tests and therapies for
Long COVID. The U.S. government, in partnership with academia, patients, health care systems,
and pharmaceutical companies, developed tests, vaccines, and therapeutics for COVID-19 in
record time. Continue this level of urgency, ingenuity, and flexibility in developing therapies for
Long COVID; testing whether existing therapeutic interventions (both pharmacologic and non-
pharmacologic) can be repurposed; and developing new therapies. Long COVID may also be
considered as an area of focus for the newly formed Advanced Research Projects Agency for
Health (ARPA-H). Additional funding has been requested in FY23 to support these efforts.
Engage U.S. government agencies to contribute
to a coordinated effort for a national real world
evidence approach, which would include Long
COVID. Engage private partners (such as
industry, health care systems, payers, state
governmental public health and other
stakeholders) to develop a prompt data sharing
and harmonization approaches to address urgent
knowledge gaps in Long COVID. The COVID-19
pandemic demonstrated well that coordinated, real-time, real-world evidence collection
“Once there is agreement on evaluation
tools and outcome measures, we need
to create a model for linkages to care.
This is a critical component of anything
we do and the data we are collecting.”
—Long COVID Researcher

64
National Research Action Plan on Long COVID
systems need to be in place and ready for major emerging issues. The standing up of a true
real-world evidence center will require coordination of EHR Vendors and large health care
systems, resources, and time. Long COVID should be part of broader efforts supported by the
U.S. government. For example, this effort shall leverage the computing prowess and new
computational mechanisms of the Department of Energy, CDC’s Data Modernization Initiative,
and engagement of biomedical, clinical, and social science researchers to analyze data and
generate evidence. The strategic priorities will be to understand the longer-term health
consequences of SARS-CoV-2 infection and accelerate the discovery of therapeutics of Long
COVID.
Develop a network of Long COVID Centers of Excellence to integrate gold-standard patient-
engaged care delivery and research. Establish and engage a network of Long COVID Centers of
Excellence that will advance implementation research in care delivery models that include
state-of the-art and evidence-based rehabilitation services, behavioral health, and linkages to
human services and supports programs and to primary care. Funding has been requested in
FY23 to grow this network.
Establish primary care networks to develop, implement, evaluate, and scale person-centered
models of care to enable primary care to prevent, diagnose, and manage Long COVID. These
primary care networks will work in partnership with diverse communities to determine the
most effective strategies for improving access, quality, equity, and outcomes of care for Long
COVID, integrating behavioral health and primary care, implementing stepped care and
collaborative models with specialty care and Long COVID clinics, and building linkages to public
health. The Long COVID Centers of Excellence and primary care networks will collaborate to
help assure that all persons with Long COVID receive the level of care they need in a timely
fashion and make it easier to navigate the health system. Funding has been requested in FY23
to grow this network.
Continue to prioritize the recruitment and retention of investigators tackling interdisciplinary
Long COVID research. Support a diverse body of researchers and research portfolios to solve
the interdisciplinary challenges facing people with Long COVID.
Overall, we must build upon lessons learned in addressing other public health crises, such as
HIV, to drive innovation in terms of how research is conceived, funded, conducted, and
disseminated. Research should facilitate data integration, harmonization, and novel approaches
of developing synthesized and linked data that safeguard privacy and facilitates rapid research.
Improved methods to synthesize findings will assist in the rapid dissemination and
implementation of important research.

65
National Research Action Plan on Long COVID
Implementing the National Research Action Plan on
Long COVID
The National Long COVID Coordinator, supported by the Long COVID Coordination Council and
in consultation with the Secretary’s Advisory Committee on Long COVID, will be responsible for
coordinating the implementation of this Plan. HHS will develop regular implementation plans,
including performance and progress metrics, to execute the strategic priorities and coordinate
the activities of federal agencies and private partners to expedite research on Long COVID (see
Appendix E for an Interim Implementation Plan). Regular reports shall be produced
documenting progress made and outlining any new strategic directions and priorities that
should be incorporated into the strategic plan.
Implementation of this Plan will maintain focus on the principles set forth in this report,
including a commitment to health equity, partner engagement, and orientation of the research
effort to improve patient care and outcomes. Strategic plans and implementation plans must
reflect the recommendations of, and contribute to, the overall U.S. government’s
implementation of the Presidential COVID-19 Health Equity Task Force. These plans must push
the needle in measuring and responding to health inequities in Long COVID.
While there is reason to be optimistic and an opportunity to seize the incredible momentum of
the moment, there are important challenges to acknowledge and address. The COVID-19
pandemic is not over, and other threats to our nation’s health unrelated to SARS-CoV-2
continue to demand our attention. Challenges specific to Long COVID include various research
approaches, complicated by distinct agency missions; the need to elevate the patient voice
across the research portfolio; persistent challenges in engaging underrepresented groups into
clinical research; and, most importantly, the challenge of implementing a comprehensive and
coordinated research portfolio, from basic science all the way to services delivery, for a set of
symptoms and conditions with emerging understanding. Nevertheless, with this Plan, the U.S.
government affirms its commitment to leverage existing and future resources to implement the
necessary steps to address the still-unfolding pandemic sequelae of SARS-CoV-2 infection.
References
1. Office of the Assistant Secretary for Preparedness & Response. Renewal of Determination
that a Public Health Emergency Exists. Published April 12, 2022. Accessed June 28, 2022.
https://aspr.hhs.gov/legal/PHE/Pages/COVID19-12Apr2022.aspx

66
National Research Action Plan on Long COVID
Appendix A: Report Abbreviations
ACL
Administration for Community Living, HHS
CDC Centers for Disease Control and Prevention, HHS
CMS
Centers for Medicare & Medicaid Services, HHS
COCA
Clinician Outreach and Community Activity
CORC-LTO
COVID-19 Observational Research Collaboratory Long-term
Outcomes
COVID-19
Coronavirus disease of 2019
CSF
Cerebrospinal fluid
DOD
Department of Defense
DOL
Department of Labor
EPIC
3
Epidemiology, Immunology, and Clinical Characteristics of
COVID-19
EPICC
Epidemiology, Immunology, and Clinical Characteristics of
Emerging Infectious Diseases with Pandemic Potential
FDA
Food and Drug Administration, HHS
HHS
Department of Health and Human Services
HMD
Health and Medicine Division
HRQOL
Health-related quality of life
HRSA
Health Resources and Services Administration, HHS
ICD-10-CM
10th revision of the International Statistical Classification of
Diseases and Related Health Problems
INSPIRE
Innovated Support for Patients with SARS-CoV-2 Infections
LGBTQI+
Lesbian, gay, bisexual, transgender, queer, intersex
MCHB
Maternal and Child Health Bureau, HRSA, HHS
ME/CFS
Myalgic encephalomyelitis and chronic fatigue syndrome
MIS-C
Multisystem Inflammatory Syndrome—Children

67
National Research Action Plan on Long COVID
MMWR
Morbidity and Mortality Weekly Report
MRI
Magnetic resonance imaging
N3C
National COVID Cohort Collaborative
NASEM
National Academies of Science, Engineering, and Medicine
NHIS
National Health Interview Survey
NIAID
National Institute of Allergy and Infectious Diseases, NIH, HHS
NIDILRR
National Institute on Disability, Independent Living, and
Rehabilitation Research, NIH, HHS
NIH
National Institutes of Health, HHS
NSCH-LC
National Survey of Children’s Health Longitudinal Cohort
ODEP
Office of Disability Employment Policy, DOL
PASC
Post-acute sequelae of SARS-CoV-2 infection
PCC
Post-COVID-19 Conditions
PCORnet
National Patient-Centered Clinical Research Network
PECOS
Pediatric COVID Outcomes Study
RECOVER
Researching COVID to Enhance Recovery (NIH Initiative)
RETAIN
Retaining Employment and Talent After Illness Injury Network
RRTC
Rehabilitation Research and Training
RTW
Return-to-work
SARS-COV-2
Severe acute respiratory syndrome coronavirus 2
SOII
Survey of Occupational Injuries and Illnesses
SSA
Social Security Administration
VA
Department of Veterans Affairs
VHA
Veterans Health Administration, VA
68
National Research Action Plan on Long COVID
Appendix B: Contributing U.S.
Government Departments
Departments
Department of Defense (DOD)
Department of Labor (DOL)
Department of Education (ED)
Department of the Treasury
(DOT)
Department of Energy (DOE)
Department of Veterans Affairs
(VA)
Department of Health and
Human Services (HHS)*
Equal Employment Opportunity
Commission (EEOC)
Department of Homeland
Security (DHS)
Federal Emergency
M
anagement Agency (FEMA)
National Council on Disability
(NCD)
Department of Housing and
Urban Development (HUD)
Office of Personnel
Management (OPM)
Department of Justice (DOJ)
Social Security Administration
(SSA)
HHS Divisions*
Administration for Children and Families (ACF)
Administration for Community Living (ACL)
Agency for Healthcare Research and Quality (AHRQ)
Centers for Disease Control and Prevention (CDC)
Centers for Medicare & Medicaid Services (CMS)
Food and Drug Administration (FDA)
Health Resources and Services Administration (HRSA)
Indian Health Service (IHS)
National Institutes of Health (NIH)
Office for Civil Rights (OCR)
Office of Global Affairs (OGA)
Office of Intergovernmental and External Affairs (IEA)
Office of the Assistant Secretary for Administration (ASA)
Office of the Assistant Secretary for Health (OASH)
Office of the Assistant Secretary for Planning and
Evaluation (ASPE)
Office of the Assistant Secretary for Preparedness and
R
esponse (ASPR)
Office of the Secretary (IOS)
Office of the Surgeon General (OSG)
Substance Abuse and Mental Health Services
A
dministration (SAMHSA)

69
National Research Action Plan on Long COVID
Appendix C: Terminology and
Definitions
“Long COVID” is the term used in the National Research Action Plan and reflects the voice of
patients. Several terms for Long COVID exist, such as Post-COVID-19 Conditions (PCC), Post-
Acute Sequelae of SARS-CoV-2 (PASC) infection, and persistent symptoms, or COVID-19
consequences. While there has been progress towards a common understanding of the two
technical terms (i.e., PCC and PASC) differences in understanding and usage remain. The table
below provides historic information regarding some terms used, the primary source for the
term along with an online reference and definition. The list is not exhaustive but demonstrates
the challenges with terminology for a new, emerging set of complex, inter-related conditions
and the need to promote consensus for research, clinical care, and surveillance.
Source Term Definition Reference
Patients and
people with lived
experience;
patient-
researchers
Long COVID
Can be broadly defined as signs, symptoms, and
sequelae that continue or develop after acute
COVID-19 or SARS-CoV-2 infection for any period
of time; are generally multisystemic; might
present with a relapsing–remitting pattern and a
progression or worsening over time, with the
possibility of severe and life-threatening events
even months or years after infection
How and why patients
made Long Covid,
ScienceDirect
Centers for
Disease Control
and Prevention
(CDC)
Post-COVID-
19
conditions
(plural)
Umbrella term for the wide range of physical and
mental health consequences experienced by
some patients that are present four or more
weeks after SARS-CoV-2 infection, including by
patients who had initial mild or asymptomatic
acute infection; equivalent to the lay term, “Long
COVID”
Long COVID or Post-
COVID Conditions |
CDCl
Department of
Veterans Affairs
(VA)
Post-COVID
Conditions
Post-COVID conditions are symptoms that last or
start weeks or months after a person was infected
with the SARS-CoV-2 virus. This is the virus that
causes COVID-19. This can happen even if you
didn’t know you had the virus. You may hear
these conditions called long COVID, post-acute
COVID, chronic COVID, or other terms. The
symptoms can include tiredness, headaches, loss
of taste and smell, trouble breathing, and
dizziness
About Post-COVID
Conditions (Long
COVID, Chronic COVID)
| Veterans Affairs
(va.gov)
70
National Research Action Plan on Long COVID
National Institutes
of Health (NIH)
Post-acute
Sequelae of
SARS CoV-2
infection
Ongoing, relapsing, or new symptoms, or other
health effects occurring after the acute phase of
SARS-CoV-2 infection (i.e., present four or more
weeks after the acute infection). The definition
will be revised in an iterative manner based on
existing and new data, medical literature, and
feedback from the scientific community
Understanding the
Long-term Impact of
COVID-19 in Adults
(recovercovid.org)
Food and Drug
Administration
(FDA)
Post-COVID
conditions
(plural)
While most people with COVID-19 have resolution
of their symptoms within weeks of their illness,
some people experience post-COVID conditions.
Post-COVID conditions are new, returning, or
ongoing health problems people can experience
four or more weeks after initial infection with the
SARS-CoV-2 virus. These conditions have also
been termed long COVID, long-haul COVID, post-
acute sequelae of COVID-19, long-term effects of
COVID, or chronic COVID. Post-COVID conditions
have been observed in people with mild to severe
COVID-19 infection, and can present with
localized and systemic symptoms impacting
nearly all organ systems.
FDA CERSI Lecture on
Long COVID: Risk
factors, Symptomology
and Patient Reported
Outcomes Captured
Through a Novel Digital
Platform by Dr. Erica
Spatz & Dr. Kelli
O’Laughlin -
11/09/2021 -
11/09/2021 | FDA
World Health
Organization
(WHO)
Post-COVID-19
Condition
(singular)
Post-COVID-19 condition occurs in individuals
with a history of probable or confirmed SARS-
CoV-2 infection, usually 3 months from the onset
of COVID-19 with symptoms that last for at least 2
months and cannot be explained by an alternative
diagnosis; common symptoms include fatigue,
shortness of breath, and cognitive dysfunction,
and generally have an impact on everyday
functioning; symptoms might be new onset after
initial recovery from an acute COVID-19 episode
or persist from the initial illness; symptoms might
also fluctuate or relapse over time; a separate
definition might be applicable for children;
recognize “Long COVID”
A clinical case
definition of post-
COVID-19 condition by
a Delphi consensus -
The Lancet Infectious
Diseases
Broader
Research
Community
Persistent
symptoms or
COVID-19
consequences
Persistent signs and symptoms that continue or
develop after acute COVID-19 for any period of
time
Persistent symptoms
following SARS-CoV-2
infection amongst
children and young
people: A meta-
analysis of controlled
and uncontrolled
studies - ScienceDirect
71
National Research Action Plan on Long COVID
American
Academy of
Physical Medicine
and
Rehabilitation
Post-acute
sequelae of
SARS CoV-2
infection
(equates it
with Long
COVID)
Post-Acute Sequelae of SARS-CoV-2 infection
(PASC) or Long COVID is a condition that occurs
in individuals who have had COVID-19 and report
at least one persistent symptom after acute
illness. Long COVID encompasses a constellation
of varied and ongoing symptoms – even in the
same patient across time – and may include
neurological challenges, cognitive symptoms
such as brain fog, cardiovascular and respiratory
issues, fatigue, pain and mobility issues, among
others.
PASC Guidance
(aapmr.org)
American College
of Cardiology
Post-acute
sequelae of
SARS-CoV-2
Infection
PASC encompasses a constellation of symptoms
that emerge or persist weeks to months after
recovery from COVID-19 [referencing CDC,
WHO]. Although evidence guiding the care of
these patients continues to evolve, there is a
need to develop common taxonomies and
approaches to care that can be updated
iteratively as new data become available.
2022 ACC Expert
Consensus Decision
Pathway on
Cardiovascular
Sequelae of COVID-19
in Adults: Myocarditis
and Other Myocardial
Involvement, Post-
Acute Sequelae of
SARS-CoV-2 Infection,
and Return to Play: A
Report of the American
College of Cardiology
Solution Set Oversight
Committee | Journal of
the American College of
Cardiology (jacc.org)
American
Thoracic Society
Long COVID
The term that is often used to describe these
persistent symptoms. You are considered to
have ‘Long COVID’ when you are still having
symptoms at least 4 weeks after the initial
infection. Long COVID may also be referred to by
other names such as post-COVID conditions,
PASC (post-acute sequelae of SARS-CoV-2
infection) or long-haul COVID.
Long COVID Patient
Fact Sheet
(thoracic.org)
Humana Military Long COVID
While most people with COVID-19 get better
within weeks, some continue to have
symptoms—or develop new ones—after their
initial recovery. The technical term for this is
post-acute sequelae of SARS-CoV-2 infection
(PASC), or simply “long COVID.” People with long
COVID are often called “longhaulers.” A person
of any age who has had COVID-19 can later
develop a post-virus condition.
Long COVID resource
guide (humana.com)
Infectious Disease
Society of
America
Post-COVID-
19 Conditions
(plural)
References other definitions, Long COVID, CDC,
and WHO
Post COVID Conditions
(e.g., Long COVID)
(idsociety.org)
72
National Research Action Plan on Long COVID
Wikipedia Long COVID
Long COVID is a condition characterized by long-
term consequences persisting or appearing after
the typical convalescence period of COVID-19. It
is also known as post-COVID-19 syndrome, post-
COVID-19 condition, post-acute sequelae of
SARS-CoV-2 infection (PASC), or chronic COVID
syndrome (CCS). Long COVID can affect nearly
every organ system, with sequelae including
respiratory system disorders, nervous system
and neurocognitive disorders, mental health
disorders, metabolic disorders, cardiovascular
disorders, gastrointestinal disorders,
musculoskeletal pain, and anemia. A wide range
of symptoms are commonly reported, including
fatigue, malaise, headaches, shortness of breath,
anosmia (loss of smell), parosmia (distorted
smell), muscle weakness, low fever and cognitive
dysfunction.
Long COVID - Wikipedia
International
Classification of
Diseases (ICD)-10-
CM code
U09.9 Post-
COVID
condition,
unspecified
No definition is given, but the following are
noted:
• This code enables establishment of a link
with COVID-19.
• This code is not to be used in cases that are
still presenting with active COVID-19.
However, an exception is made in cases of
reinfection with COVID-19, occurring with a
condition related to prior COVID-19.
•
Post-acute sequelae of COVID-19
2022 ICD-10-CM
Diagnosis Code U09.9:
Post COVID-19
condition, unspecified
(icd10data.com)
National Institute
for Health and
Care Excellence
(NICE)
*
Ongoing
symptomatic
COVID-19
Signs and symptoms of COVID-19 from 4 weeks
up to 12 weeks
Guideline COVID-19
rapid guideline:
managing the long-
term effects of COVID-
19 (nice.org.uk)
National Institute
for Health and
Care Excellence
(NICE)
**
Po
st-COVID-
19 syndrome
Signs and symptoms that develop during or after
an infection consistent with COVID-19, continue
for more than 12 weeks and are not explained by
an alternative diagnosis; it usually presents with
clusters of symptoms, often overlapping, which
can fluctuate and change over time and can
affect any system in the body; post-COVID-19
syndrome might be considered before 12 weeks
while the possibility of an alternative underlying
disease is also being assessed
Guideline COVID-19
rapid guideline:
managing the long-
term effects of COVID-
19 (nice.org.uk)
Note: Adapted from the Table titled, “Commonly used terminology in the research of COVID-19 sequelae” in Munblit
D, O’Hara M, Akrami A et al. Long COVID: aiming for a consensus. Lancet Respir Med. 2022; S2213-2600(22)00135-
7. https://www.thelancet.com/action/showPdf?pii=S2213-2600%2822%2900135-7. Accessed June 21, 2022.
*United Kingdom National Institute for Health and Care Excellence (NICE)

73
National Research Action Plan on Long COVID
**NICE also states that: “In addition to the clinical case definitions, the term ‘long COVID’ is commonly used to
describe signs and symptoms that continue or develop after acute COVID-19. It includes both ongoing
symptomatic COVID-19 (from 4 to 12 weeks) and post-COVID-19 syndrome (12 weeks or more).”
74
National Research Action Plan on Long COVID
Appendix D: Highlights of Current U.S.
Government Research Portfolio on Long
COVID
The U.S. government is currently conducting and supporting many studies that will contribute
to the successes of the National Research Action Plan. This table contains a comprehensive list
of the most prominent projects in the Long COVID federal research portfolio, including the
research described in Chapter 3. This is not an exhaustive list of all Long COVID research or U.S.
government supported research. The list is in alphabetical order based on the study title.
Agencies that are leading and collaborating in the research are also listed. Finally, for each
research study, research areas corresponding to the topic areas highlighted in Chapter 3 of this
report are listed.
Study Title Agency* Research Areas
Advancing the epidemiological and
mechanistic understanding of the
neurological complications of COVID-19
NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Surveillance and Epidemiology
All of US Research Program NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Pathophysiology
Applied Research to Address the
Coronavirus (COVID-19) Emerging Public
Health Emergency
CDC
Surveillance and Epidemiology
Long COVID and Overall Well-Being
Behavioral Risk Factor Surveillance System
(BRFSS)
CDC
Su
rveillance and Epidemiology
Characterization of host immune responses
to SARS-CoV-2
NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Pathophysiology

75
National Research Action Plan on Long COVID
Cognitive sequelae of the biological effects
of COVID-19 on the nervous system in a
health disparity population
NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Cohort study of effects of SARS-CoV-2
infection, vaccination, and immunology on
gut tissue and clinical symptoms
NIH
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Pathophysiology
COVID-19 Neuro Databank Biobank NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Surveillance and Epidemiology
COVID-19 Observational Research
Collaboratory Long-term Outcomes Study
(CORC-LTO)
VA
I
mpacts of Illness
Surveillance and Epidemiology
COVID-19 patient registry DOD
C
linical Spectrum of Long COVID and Diagnostic
Strategies
COVID-19: Predictive Immune and Airway
Monitoring in Healthcare workers and
Hospitalized COVID-19 patients
NIH, VA
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Pathophysiology
Surveillance and Epidemiology
COVID–Standardized Evaluation of Long-
term Effects (COVID-SELECT)
CDC Surveillance and Epidemiology
COVID-19: Understanding the Post-Viral
Phase (COVID-UPP)
CDC
Su
rveillance and Epidemiology
Clinical Spectrum of Long COVID and Diagnostic
Strategies
COVID-19 within American Indian
Communities at High Risk in the Southwest
U.S.
CDC
Su
rveillance and Epidemiology
CSP #2028 (Epidemiology, Immunology and
Clinical Characteristics of COVID-19 [EPIC3])
VA
Surveillance and Epidemiology
Clinical Spectrum of Long COVID and Diagnostic
Strategies

76
National Research Action Plan on Long COVID
Descriptive and Analytical Study of Post-
acute Sequelae of COVID-19 (Long COVID)
among U.S. Veterans
FDA, VA
Su
rveillance and Epidemiology
Effects of Long COVID on inflammatory
processes in the central nervous system
NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Pathophysiology
Effects of Long-COVID on Maintenance and
Incidence of ME/CFS
NIH
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Surveillance and Epidemiology
Pathophysiology
Effects of Long COVID on Topological
Mapping of Immune, Microbiota,
Metabolomic and Clinical Phenotypes to
Reveal ME/CFS Disease
NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Employer Assistance and Resource Network
on Disability Inclusion
ODEP Human Services, Supports, and Interventions
Epidemiology, Immunology, and Clinical
Characteristics of Emerging Infectious
Diseases with Pandemic Potential (EPICC)
DOD
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Exploratory Analysis of Long COVID service
use among Medicare beneficiaries
CMS, ASPE Health Services and Health Economics Research
FindCOVID CDC
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Formative Evaluation of Long COVID care
delivery models in the VA
VA
H
ealth Services and Health Economics Research
Health+ HHS, OASH Long COVID and Overall Well-Being

77
National Research Action Plan on Long COVID
Household Pulse Survey
CDC,
Commerce
Surveillance and Epidemiology
Identifying Long COVID algorithms to
operationalize for use in the FDA Sentinel
system and other administrative claims or
electronic health records data sources.
FDA, CDER Therapeutics
Immune Response to SARS-CoV-2 among
patients in Louisiana
CDC Surveillance and Epidemiology
Immunophenotyping in a COVID-19 Cohort
(IMPACC)
NIH
Surveillance and Epidemiology
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Pathophysiology
Immunotherapy for Neurological Post-Acute
Sequelae of SARS-CoV-2 (IN-PASC)
NIH
P
athophysiology
Therapeutics
Impact of Long COVID on return-to-work
efforts for people with disability
ACL Human Services, Supports, and Interventions
Innovative Support for Patients With SARS-
CoV-2 Infections (INSPIRE)
CDC
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Leveraging the power of VA data to
understand Long COVID
VA Surveillance and Epidemiology
Long COVID and Fatiguing Illness Recovery
Program (LC&FIRP)
CDC Surveillance and Epidemiology
Long COVID Needs Assessment of Health
Professionals (2022 DocStyles Survey)
CDC Health Services and Health Economics Research
Long COVID Practice Based Research
Network
VA Therapeutics
Long COVID supplement to the National
Survey on Health and Disability
ACL
H
ealth Services and Health Economics Research

78
National Research Action Plan on Long COVID
Longitudinal Study of COVID-19 Antibody
Response in Children in Seattle, WA
CDC Surveillance and Epidemiology
Longitudinal At Home Smell Testing to
Detect Infection by SARS-CoV-2
NIH
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Longitudinal Study of COVID-19 Sequelae
and Immunity
NIH
Su
rveillance and Epidemiology
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Pathophysiology
Longitudinal study of immune memory in
COVID-19 patients with mild to moderate
symptoms
NIH
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Pathophysiology
Multi-site Study of Post-COVID Conditions
(MPCC)
CDC
Surveillance and Epidemiology
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Multi-state Long COVID Survey CDC Surveillance and Epidemiology
National COVID Cohort Collaborative (N3C) NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Surveillance and Epidemiology
Long COVID and Overall Well-Being
Pathophysiology
Therapeutics and Other Health Interventions
National Health Interview Survey CDC Surveillance and Epidemiology
79
National Research Action Plan on Long COVID
National Patient-Centered Clinical Research
Network (PCORnet)
PCORI
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Surveillance and Epidemiology
Long COVID and Overall Well-Being
Pathophysiology
Therapeutics and Other Health Interventions
National Survey of Children’s Health
Longitudinal Cohort Study (NSCH-LC)
HRSA,
Su
rveillance and Epidemiology
Health Services and Health Economics Research
NIH Epidemiologic Study of Diabetes
Incidence and Severity and Its Potential
Association with COVID-19
NIH
Su
rveillance and Epidemiology
Occurrence of post-COVID-19 conditions and
symptoms among adults seen in primary
care practices by sociodemographic
factors—American Board of Family Medicine
PRIME Registry, American Family Cohort
CDC
Su
rveillance and Epidemiology
Office of Workers Compensation Program DOL Impacts of Illness
Pediatric COVID Outcomes Study (PECOS) NIH
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Surveillance and Epidemiology
Pathophysiology
Impacts of Illness
Pediatric Research Immune Network on
SARS-CoV-2 and MIS-C (PRISM)
NIH
Su
rveillance and Epidemiology
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Pathophysiology

80
National Research Action Plan on Long COVID
Pediatric Learning Health System
consortium of PCORnet (PEDSnet
consortium)
PCORI
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Surveillance and Epidemiology
Long COVID and Overall Well-Being
Therapeutics and Other Health Interventions
Planning workshop: Long-Term Health
Effects from COVID-19 and Implications for
the SSA
SSA
H
uman Services, Supports, and Interventions
Population-based study of long-term
impacts of COVID-19 infection (including
subset of Veterans with Long COVID)
VA
H
ealth Services and Health Economics Research
Post COVID Collaborative Merit Program VA
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Project ECHO (Extension for Community
Healthcare Outcomes)
AHRQ Health Services and Health Economics Research
RADx Radical program NIH
C
linical Spectrum of Long COVID and Diagnostic
Strategies
Rapid olfactory tools for telemedicine-
friendly COVID-19 screening and
surveillance
NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Researching COVID to Enhance Recovery
(RECOVER)
NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Surveillance and Epidemiology
Long COVID and Overall Well-Being
Pathophysiology
Therapeutics and Other Health Interventions
Research on COVID Long-Term Illness,
Effects, and Risk Factors (COVID-RELIEF)
CDC Surveillance and Epidemiology
Retaining Employment and Talent After
Illness/Injury Network (RETAIN)
DOL
I
mpacts of Illness

81
National Research Action Plan on Long COVID
Risk Factor Assessment Study for Post-Acute
Sequelae of COVID-19 (Long COVID) in the
Medicare Population
FDA, CBER,
CMS
Surveillance and Epidemiology
Risk of developing post-COVID-19 conditions
among younger and older adult COVID-19
survivors—Cerner Real World Data
CDC Surveillance and Epidemiology
SCENTinel: A Rapid Smell Test for COVID-19
Surveillance
NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Survey of Occupational Injuries and Illnesses
(SOII)
DOL Impacts of Illness
TLC Trial (Treatment of Long COVID).
FDA, CDER,
NIH,
NCATS
Therapeutics and Other Health Interventions
Understanding the Pathophysiology and
Clinical Course of New-Onset Diabetes
Following COVID-19
NIH
Clinical Spectrum of Long COVID and Diagnostic
Strategies
Pathophysiology
VA COVID-19 Observational Research
Collaboratory Long-term Outcomes Study
(CORC-LTO)
VA Surveillance and Epidemiology
Qualitative study Long COVID-19 impact on
return-to-work efforts for people with
disability
ACL Human Services, Supports, and Interventions
COVID-19 pandemic-related experiences of
working-age adults with disabilities
ACL Human Services, Supports, and Interventions
Analysis of disability claims with report of
Long COVID
SSA Human Services, Supports, and Interventions
*Agency abbreviations, in alphabetical order
A
CL: Administration for Community Living
AHRQ: Agency for Healthcare Research and Quality
ASPE: Office of the Assistant Secretary for Planning and Evaluation
CBER: Center for Biologics Evaluation and Research
CDC: Centers for Disease Control and Prevention

82
National Research Action Plan on Long COVID
CDER: Center for Drug Evaluation and Research
CMS: Centers for Medicare & Medicaid Services
DOD: Department of Defense
DOL:
Department of Labor
FDA: Food and Drug Administration
HHS: Department of Health and Human Services
HRSA: Health Resources and Services Administration
NCATS: National Center for Advancing Translational Sciences
NIH: National Institutes of Health
OASH: Office of the Assistant Secretary for Health
ODEP: Office of Disability Employment Policy
PCORI: Patient-Centered Outcomes Research Institute
SSA: Social Security Administration
VA: Department of Veterans Affairs

83
National Research Action Plan on Long COVID
Appendix E: U.S. Government Long
COVID Research, Interim
Implementation Plan
As the U.S. government is standing up the new Office of Long COVID Research and Practice,
there is a need for an interim implementation plan. The following tables demonstrate the
actions that the U.S. government will take over the next six months to implement the strategies
and priorities of this Plan. At a high level, this interim plan will guide federal efforts until the
Department of Health and Human Services develops the first annual implementation plan,
including performance and progress metrics, to execute the strategic priorities and coordinate
the activities of federal agencies and private partners. Efforts described in these tables are new
or enhanced activities, above and beyond the research described in Chapter 3 and Appendix
D, almost all of which is ongoing. These two tables correspond to the two main sections in
Chapter 4. The first table below, U.S. Government Research Priorities for Long COVID, describes
specific research that U.S. government will undertake to respond to the broad call to action
described in Chapter 4 for the U.S. government as well as the private research community. The
second table below, Strategic U.S. Government Actions, are the new structures and processes
in U.S. government to drive the research.
U.S. Government Research Priorities for Long COVID
Characterizing the Full Clinical Spectrum of Long COVID and Diagnostic
Strategies
Host a convening of public and private partners to better align interim definitions of
Long COVID for clinical, surveillance, and research
Augment use of existing chronic disease initiatives (e.g., the Serological Sciences
Network (SeroNet)) to better understand the increased risk of chronic disease and
clinical course among persons with Long COVID
Surveillance and Epidemiology
Establish a framework for a multipronged approach to Long COVID surveillance to
coordinate and enhance existing efforts in the context of broader data modernization
efforts
Leverage existing research on post-infectious chronic illness to support identification of
commonalities and differences with Long COVID, accelerate research and innovation, as
well as prepare for the long-term consequences of future pandemics

84
National Research Action Plan on Long COVID
Long COVID and Overall Well-Being
Publish findings from Health+ Long COVID patient-centered research, qualitative
studies of patients experiencing Long COVID and associated conditions, caregivers,
frontline workers, and those with lived experience
Assess existing cohort studies as well as studies conducting qualitative interviews with
persons with Long COVID, to expand consideration of outcomes such as behavioral
health, employment, education, caregiving, care utilization, and experience with care,
especially among groups who are disproportionately affected
Therapeutics and Other Health Interventions
Implement additional strategies to accelerate and enhance enrollment into RECOVER
studies
Human Services, Supports, and Interventions
Identify existing national surveys related to human services, supports and interventions
that could be amended to include modules for data collection about Long COVID
Health Services and Health Economics Research
Build on current studies to evaluate the economic costs and outcomes of Long COVID,
across the full range of economic costs and outcomes, and at all levels-- patient and
family, health system, state, federal (including Medicare and Medicaid), and national
Strategic U.S. Government Actions
Establish an Office of Long COVID Research and Practice
Support ongoing meetings of the Long COVID Coordination Council
Develop first annual implementation plan
Establish the Secretary’s Advisory Committee on Long COVID
Develop and contribute to best practices to collect detailed information on age, gender, race,
ethnicity, rurality, economic disadvantage, insurance coverage, pregnancy status, and
disability status in all USG-conducted and sponsored research on Long COVID, in line with
OMB and other U.S. government policies
Facilitate development of comprehensive and equitable Long COVID diagnosis, care and
treatment guidance and compile existing guidance

85
National Research Action Plan on Long COVID
Develop and immediately begin implementation of a strategic communications plan to share
findings from Long COVID research widely with partners and stakeholders to coordinate and
enhance current efforts
Leverage existing platforms and resources for clinical trial, genetic data, and literature
(e.g., , GenBank, Sequence Read Archive, and PubMed Central) to provide
broad access to information about U.S. Long COVID research and the results.
ClinicalTrials.gov
Host a convening of research partners, including government, academia, public health,
pharmaceutical industry, national pharmacy chains, and health systems to initiate
collaborations.
